What is Major Histocompatibility Complex (MHC Molecules)?
- The major histocompatibility complex (MHC) is a cluster of genes that play a crucial role in intercellular recognition and the discrimination between self and non-self. The term “histo” refers to tissue, while “compatibility” denotes getting along or being agreeable. The term “complex” indicates that the genes are localized to a large genetic region containing multiple loci. These genes encode for antigens that determine the compatibility of transplanted tissues. Compatible tissues are accepted by the immune system, while histo-incompatible ones are rejected, leading to an immune response against the cell surface molecules.
- The concept of the major histocompatibility complex was first identified by Peter Gorer and George Snell. The primary function of MHC molecules is to present antigens on the cell surface for recognition by T cells. In humans, the genes coding for MHC molecules are found on the short arm of chromosome 6.
- The major histocompatibility complex is a critical locus on the DNA of vertebrates that contains a set of closely linked polymorphic genes. These genes code for cell surface proteins known as MHC molecules, which are essential for the adaptive immune system. The name “major histocompatibility complex” originates from the study of tissue compatibility in transplantation. However, it was later discovered that MHC molecules have a broader role beyond tissue rejection. They bind antigens derived from self-proteins or pathogens and present them on the cell surface for recognition by T cells. MHC molecules facilitate the interactions of leukocytes, also known as white blood cells, with other leukocytes or body cells. They determine the compatibility of organ transplants and influence susceptibility to autoimmune diseases.
- Within a cell, protein molecules of the host’s own phenotype or other biological entities are continuously synthesized and degraded. Each MHC molecule on the cell surface displays a small peptide called an epitope, which is a fraction of a protein. The presentation of self-antigens prevents the immune system from targeting the host’s own cells. Conversely, the presentation of pathogen-derived proteins leads to the elimination of infected cells by the immune system.
- The diversity of self-antigen presentation mediated by MHC self-antigens is achieved through several mechanisms. First, an organism’s MHC repertoire is polygenic, meaning it involves multiple interacting genes. Second, MHC expression is codominant, meaning it occurs from both sets of inherited alleles. Lastly, MHC gene variants are highly polymorphic, varying diversely from organism to organism within a species. Sexual selection has been observed in male mice, with individuals choosing to mate with females possessing different MHCs. Additionally, evidence of antigenic peptide splicing has been found, particularly in MHC I presentation. This process combines peptides from different proteins, significantly increasing antigen diversity.
Definition of Major Histocompatibility Complex (MHC Molecules)
The major histocompatibility complex (MHC) molecules are a set of cell surface proteins encoded by a cluster of genes that play a crucial role in intercellular recognition and immune responses. They present antigens derived from self-proteins or pathogens on the cell surface, allowing T cells to recognize and respond to them. MHC molecules determine tissue compatibility for transplantation and influence susceptibility to autoimmune diseases. They contribute to the diversity of self-antigen presentation through polygenic, codominant, and highly polymorphic characteristics.
Nomenclature and Inheritance of Major Histocompatibility Complex (MHC)
- The HLA specificities are defined by a letter and a number (A1, B5, etc.), whereas the haplotypes are identified by the specificities of each individual (e.g., A1, B7, Cw4, DP5, DQ10, DR8).
- Genomic analysis (PCR)-defined specificities are denoted by a letter for the locus and a four-digit number (e.g., A0101, B0701, C0401, etc.).
Inheritance of Major Histocompatibility Complex (MHC)
- Histocompatibility genes are passed down as a group (haplotype) from each father. Thus, MHC genes are expressed codominantly in each individual.
- A heterozygous individual receives one paternal and one maternal haplotype, with each haplotype comprising three Class-I (B, C, and A) and three Class II (DP, DQ, and DR) sites.
- Each individual can inherit up to two alleles per locus.
Characteristics of MHC Molecules
MHC (Major Histocompatibility Complex) molecules possess several characteristic features that contribute to their crucial role in antigen recognition and immune responses. These characteristics include:
- Tissue Rejection: MHC molecules, also known as transplantation antigens, are responsible for the rejection of grafts between genetically dissimilar individuals. They encode glycoprotein molecules involved in tissue compatibility.
- Antigen Presentation: MHC molecules bind peptide antigens processed by Antigen-presenting Cells (APCs) and present them to T cells. They facilitate antigen recognition by the T cell receptors (TCRs).
- Specificity for TCR Binding: Unlike B cell receptors, TCRs interact with MHC molecules to receive and bind processed antigens presented in the form of peptides. Thus, TCRs are specific for MHC molecules.
- Human Leukocyte Antigen (HLA): In humans, the MHC is referred to as the Human Leukocyte Antigen (HLA) complex. It encompasses the MHC genes responsible for tissue compatibility and antigen presentation.
- Class I, II, and III MHC Proteins: MHC molecules are classified into three major classes: class I, class II, and class III MHC proteins. Each class has distinct functions in antigen presentation and immune responses.
- Genetic Variability: MHC genes exhibit genetic variability, and the MHC is polygenic, meaning it has several genes for each class. This genetic variability contributes to the diversity of MHC molecules and their ability to recognize a wide range of antigens.
- Polymorphism: MHC molecules are highly polymorphic, with numerous alleles existing in the population for each MHC gene. Individuals inherit a limited set of alleles from their parents, and these alleles tend to be inherited as a block or haplotype.
- Structure: MHC class I molecules have two domains, consisting of two parallel α helices on top of a β-pleated sheet platform. The overall structure resembles a cleft, with the α helices forming the sides and the β sheet forming the floor. This structure provides a binding site for peptide antigens.
- Broad Specificity for Peptide Antigens: MHC molecules have a broad specificity for peptide antigens, enabling them to present various peptides. However, each MHC allele can bind and present only one peptide at a time.
- Role in T Cell Function: The interaction between T cells and MHC molecules is essential for immune responses. CD8+ cytotoxic T lymphocytes (Tc cells) recognize peptide antigens presented by class I MHC molecules, while CD4+ Helper T cells (Th cells) recognize peptide antigens associated with class II MHC molecules.
In summary, MHC molecules exhibit genetic variability, polymorphism, and specificity for antigen recognition. They play a crucial role in tissue rejection, antigen presentation, and immune responses, with different MHC classes and alleles contributing to the diversity and specificity of immune recognition.
What is HLA Complex?
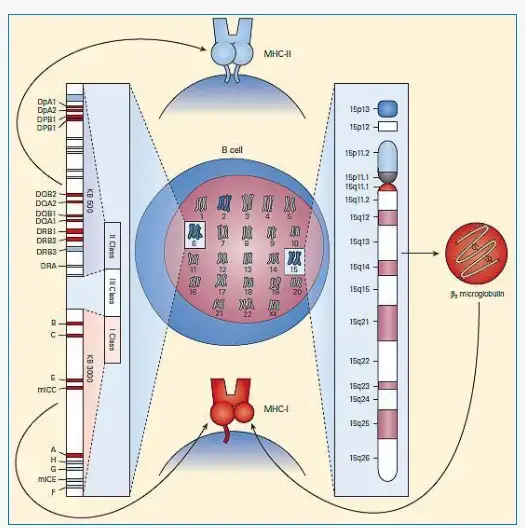
The HLA (Human Leukocyte Antigen) complex refers to the MHC (Major Histocompatibility Complex) in the human genome, specifically located on the short arm of chromosome 6. The HLA complex is a crucial genetic region containing several genes that play a critical role in immune function.
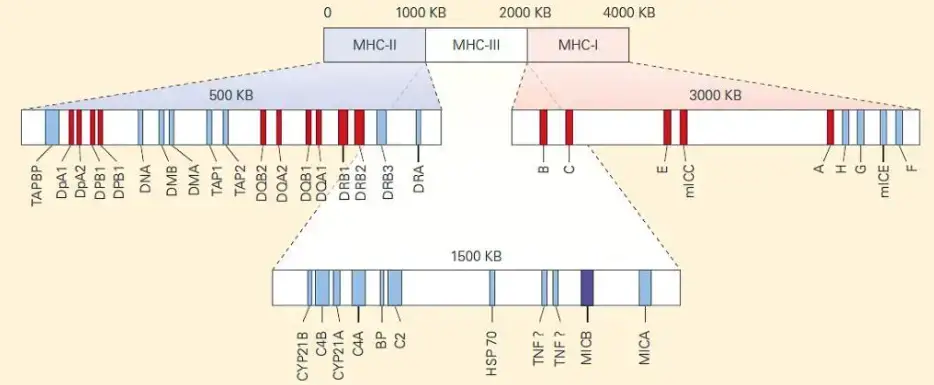
The HLA complex is divided into three classes: class I, class II, and class III.
- Class I: The class I region includes the genes HLA-A, HLA-B, and HLA-C. These genes encode human MHC class I α chains, which are involved in antigen presentation to cytotoxic T cells (CD8+ T cells). Class I MHC molecules present antigens derived from intracellular pathogens or abnormal host proteins.
- Class II: The class II region consists of the genes HLA-DR, HLA-DQ, and HLA-DP. These genes are located within the HLA-D region. Class II MHC molecules play a role in antigen presentation to helper T cells (CD4+ T cells). They present antigens derived from extracellular pathogens to initiate immune responses.
- Class III: The class III region contains genes encoding complement system components such as C2, C4, and factor B. It also includes genes for cytokines such as TNF-alpha and TNF-beta. The class III region is involved in various immune processes and inflammatory responses.
The HLA complex spans approximately 3,500 kb on chromosome 6 and consists of 12 main sections. While each section contains multiple genes, only a few of them are functional and directly involved in antigen presentation. The HLA complex also includes MHC class Ib and IIb proteins, which are structurally similar to class I and class II molecules but have distinct functions unrelated to antigen presentation.
The class III region of the HLA complex contains genes involved in immunological responses, including those encoding complement components, heat shock proteins (HSPs), and cytokines such as TNF and lymphotoxin (LT). However, this region is not known to encode peptide-binding presentation molecules.
In summary, the HLA complex in humans is a genetic region on chromosome 6 that encompasses several genes critical to immune function. It includes class I, class II, and class III regions, each with specific genes and functions related to antigen presentation, complement system components, and immune responses.
HLA Typing
HLA typing refers to the process of determining the specific human leukocyte antigen (HLA) alleles present in an individual. HLA antigens are found on almost all tissues in the body and are crucial for immune recognition and compatibility in transplantation.
HLA typing is particularly important in allogenic (distinct) transplantation, where a donor organ or tissue is transplanted into a recipient. Achieving a close HLA match between the donor and recipient is desirable to minimize the risk of rejection and improve transplant success.
There are two main classes of HLA typing procedures:
- Class I Typing: Class I HLA typing involves identifying the alleles at the HLA-A, HLA-B, and HLA-C loci. The primary methods for class I typing include:
- Microcytotoxicity: This method uses antibodies specific to HLA antigens and tests the compatibility between donor and recipient cells. It can determine the presence or absence of specific HLA antigens.
- Cellular approaches (such as complement-mediated lymphocytotoxicity, CML): These techniques involve mixing donor and recipient cells and observing cell lysis (death) mediated by complement activation in the presence of HLA antibodies. CML can be used for HLA-DPw typing.
- Class II Typing: Class II HLA typing focuses on identifying alleles at the HLA-DR and HLA-DQ loci. The methods used for class II typing include:
- Cellular techniques like mixed lymphocyte reaction (MLR) or mixed lymphocyte culture (MLC): These methods assess the compatibility of donor and recipient lymphocytes by measuring the proliferation of T cells in response to HLA differences. MLR/MLC can be used for HLA-DR typing.
- Molecular procedures like polymerase chain reaction (PCR) and direct sequencing: These techniques involve amplifying specific HLA genes using PCR and then sequencing the amplified DNA to determine the exact HLA alleles present. PCR and direct sequencing are commonly used for HLA-DR and HLA-DQ typing.
By performing HLA typing, healthcare professionals can assess the compatibility between potential organ or tissue donors and recipients, helping to minimize the risk of graft rejection and improve the success of transplantation procedures.
Significance of HLA Typing
HLA typing, or the identification of human leukocyte antigen (HLA) alleles in individuals, holds significant importance in various fields and applications:
- Anthropology: HLA typing has contributed to the field of anthropology by providing insights into population relationships and migration patterns. The variations in HLA types among different ethnic populations have helped establish or support connections between populations. For example, the prevalence of specific HLA types, such as HLA-A34 in Australian Aborigines, can indicate distinct genetic lineages or migration histories.
- Paternity Testing: HLA typing can be used in paternity testing scenarios. If a man and a child share an HLA haplotype, it suggests a potential biological relationship between the man and the child. However, HLA typing alone cannot conclusively determine paternity. If the man and child do not share an HLA haplotype, it indicates that the man is not the biological father.
- Transplantation: HLA typing plays a critical role in transplantation medicine. Prior to organ transplantation, histocompatibility testing is performed to assess the compatibility between the donor and recipient. Matching HLA types between the donor and recipient increases the chances of successful organ engraftment and reduces the risk of graft rejection. Living donors who are closely related and share one or both HLA haplotypes with the recipient generally yield better transplantation outcomes compared to unrelated cadaveric donors.
- Transfusion: HLA typing is also relevant in the context of blood transfusion. HLA compatibility is considered when matching blood donors and recipients to minimize the risk of adverse reactions, such as transfusion-related complications or immune-mediated responses.
- Forensic Science: HLA typing has been used in forensic investigations to aid in the identification of individuals or to establish associations between individuals and biological evidence. By analyzing the HLA types present in samples, forensic scientists can provide evidence that supports or refutes the involvement of specific individuals in criminal cases.
- Disease Association: HLA typing has revealed associations between certain HLA alleles and various diseases. Certain HLA alleles have been linked to an increased susceptibility or resistance to certain autoimmune disorders, infectious diseases, and other conditions. Understanding these associations can aid in disease diagnosis, prognosis, and treatment.
Disease Associations with HLA
HLA (human leukocyte antigen) associations with certain diseases have been identified, indicating that specific MHC haplotypes are more prevalent in individuals with these conditions. Here are some disease associations with HLA:
- Ankylosing Spondylitis: Ankylosing spondylitis, a chronic inflammatory disease affecting the spine and joints, is strongly associated with the HLA-B27 haplotype. Individuals with the HLA-B27 gene have an increased risk of developing this condition.
- Celiac Disease: Celiac disease, an autoimmune disorder triggered by gluten consumption, has a strong association with the HLA-DQ2 and HLA-DQ8 haplotypes. These HLA alleles are commonly found in individuals with celiac disease.
- Reiter’s Syndrome: Reiter’s syndrome, a reactive arthritis typically occurring after an infection, is also strongly associated with the HLA-B27 haplotype.
- Acute Anterior Uveitis: Acute anterior uveitis, an inflammation of the eye’s iris and surrounding tissues, is associated with the HLA-B27 haplotype.
- Psoriasis Vulgaris: Psoriasis vulgaris, a chronic skin condition characterized by red, scaly patches, has an association with the HLA-Cw6 haplotype.
Disease Associations with Class II HLA:
- Hashimoto’s Disease: Hashimoto’s disease, an autoimmune thyroid disorder, is associated with the HLA-DR5 haplotype.
- Primary Myxedema: Primary myxedema, a severe form of hypothyroidism, has an association with the HLA-DR3 haplotype.
- Graves’ Thyrotoxicosis: Graves’ thyrotoxicosis, an autoimmune condition causing overactive thyroid function, is associated with the HLA-DR3 haplotype.
- Insulin-Dependent Diabetes: Insulin-dependent diabetes, also known as type 1 diabetes, is strongly associated with the HLA-DQ2 and HLA-DQ8 haplotypes.
- Addison’s Disease: Addison’s disease, an autoimmune disorder affecting the adrenal glands, has an association with the HLA-DR3 haplotype.
- Goodpasture’s Syndrome: Goodpasture’s syndrome, a rare autoimmune disease affecting the kidneys and lungs, is associated with the HLA-DR2 haplotype.
- Rheumatoid Arthritis: Rheumatoid arthritis, a chronic inflammatory joint disease, has an association with the HLA-DR4 haplotype.
- Juvenile Rheumatoid Arthritis: Juvenile rheumatoid arthritis, a form of arthritis that occurs in children, has an association with the HLA-DR8 haplotype.
- Sjögren’s Syndrome: Sjögren’s syndrome, an autoimmune disorder affecting the moisture-producing glands, is associated with the HLA-DR3 haplotype.
The exact mechanisms underlying these disease associations with HLA are not fully understood. However, theories include the presence of antigenic similarities between pathogens and MHC molecules, as well as the modulation of immune responses mediated by class II HLA genes. Further research is needed to elucidate the precise mechanisms involved in these associations.
Gene Products of HLA complex
The HLA complex, also known as the MHC complex, consists of genes that encode various gene products involved in immune function. Here are the gene products of the HLA complex:
- Class I MHC Gene Products: The genes within the class I region of the HLA complex encode glycoproteins that are expressed on the surface of nearly all nucleated cells in the body. The major function of these class I gene products is to present endogenous peptide antigens to CD8+ T cells. They bind and present peptides derived from intracellular proteins, allowing CD8+ T cells to recognize and respond to infected or abnormal cells.
- Class II MHC Gene Products: The genes within the class II region of the HLA complex encode glycoproteins that are predominantly expressed on antigen-presenting cells (APCs) such as macrophages, dendritic cells, and B cells. Class II gene products primarily function in presenting exogenous antigenic peptides to CD4+ T cells. They capture and present peptides derived from extracellular proteins, including those from pathogens, to CD4+ T cells, which play a crucial role in coordinating immune responses.
- Class III MHC Gene Products: The genes within the class III region of the HLA complex encode several different proteins, some of which have immune functions. These gene products include components of the complement system, which is an important part of the immune response involved in the clearance of pathogens and immune complex formation. Additionally, molecules involved in inflammation, such as cytokines, are encoded by class III genes. These proteins contribute to the regulation and coordination of immune responses.
Nomenclature of HLA
- Historically, the HLA nomenclature evolved from the original serological identifiers. Antibody response patterns were originally used to define protein polymorphisms. Modern definitions incorporate DNA sequences to define alleles. During the Tenth International Histocompatibility Workshop in 1987, the current nomenclature was recommended, with minor adjustments added in 1990.
- Each individual possesses two copies of each chromosome; hence, a normal tissue type will include twelve HLA antigens (three HLA class I loci [A, B, and C] from each parent and three class II loci [DR, DQ, and DP] from each parent).
- HLA-DM and HLA-DO are neither extremely polymorphic nor typed. These twelve antigens are co-dominantly inherited.
- A person’s MHC phenotype specifies the alleles they have without reference to heredity. A person may be typed as HLA-A1, -A3; B7, B8; Cw2, Cw4; DR15, DR4, DQ3, DQ6, DP4, DP4; or DR15, DR4, DQ3, DQ6, DP4, DP4.
- A haplotype is the collection of HLA antigens that are inherited from a single parent. For instance, the mother of the individual whose HLA type is shown above may have HLA-A3, -A69; B7, B45; Cw4, Cw9; DR15, DR17; DQ6, DQ2; DP2, DP4; or DR15, DR17. Therefore, the A3, B7, Cw4, DR15, DQ6, and DP4 genes were transmitted from the mother to the child in the preceding sentence. This set of antigens is known as a haplotype. Despite the vast number of alleles at each expressed locus, the number of haplotypes observed in the population is substantially lower than what would be predicted theoretically. This is because specific alleles tend to co-occur on the same haplotype, as opposed to segregating at random. The term for this is linkage disequilibrium.
Rules that dictate the nomenclature of HLA
- HLA is prepended to all antigens and alleles. A capital letter signifies a specific site (A, B, C, or D).
- All genes in region D are prefixed with the letter D and a second letter signifying the subregion of D. (DR, DQ, DP, DM, or DO). Next, loci encoding specific class II peptide chains are discovered (A1, A2, B1, and B2).
- Greek letters are used for protein designations while Latin capital letters are used for gene/allele designations, e.g. DRp1 as opposed to DRB1.
- Specific alleles are denoted by a “*” followed by a two-digit number indicating the most closely connected serologic specificity, and then a two-digit number defining the unique allele.
- For instance, the serologically determined HLA-A2 specificity consists of seventy-seven different alleles. These alleles are currently designated as HLA-A*02:01 to *02:99.
- Some alleles carry a third two-digit number (HLA-B*35:01:01 and B*35:01:02), which indicates that the two variants differ by a silent nucleotide change but do not differ in amino acid sequence 6.
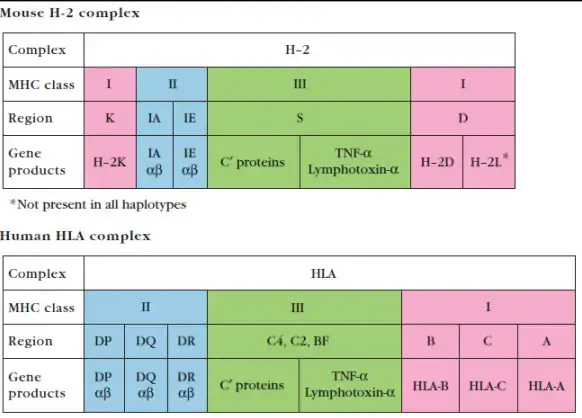
H-2 Complex
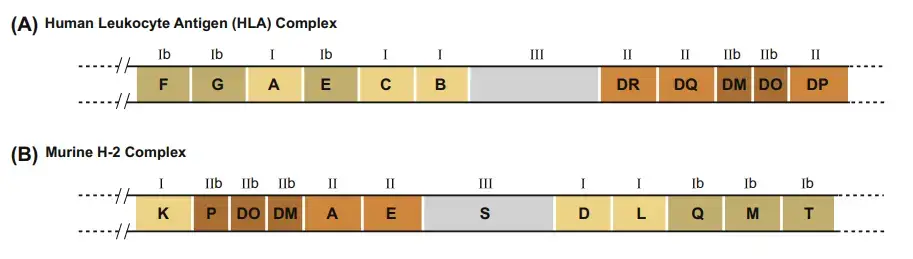
- The MHC in the mouse genome is referred to as the H-2 complex. On chromosome 17, the H-2 complex spans 3000 kb and has 12 main areas.
- The K, D, and L sections each contain a single gene encoding the mouse MHC class I α chain.
- Each of the A and E sections encodes a single functional gene for an MHC class II α chain and one or more functional genes for an MHC class II β chain.
- The S section of the H-2 complex encodes MHC class III proteins, including again complement proteins, HSPs, TNF, and LT.
- The Q, T, and M sections of the H-2 complex contain genes for class Ib proteins, while the P, DO, and DM regions encode class IIb proteins.
Distribution of MHC
MHC I Molecules
- The classical class I MHC molecules are expressed on the majority of nucleated cells, however the level of expression varies between cell types.
- Class I molecules are expressed at the highest quantities in lymphocytes, where they account for approximately 1% of all plasma membrane proteins, or 5×105 molecules per cell.
- In contrast, fibroblasts, muscle cells, hepatocytes of the liver, and brain cells express negligible amounts of class I MHC molecules.
- The low amount on liver cells may contribute to the high success rate of liver transplants by decreasing the probability of graft identification by the recipient’s Tc. A few cell types (such as neurons and sperm cells at specific stages of development) appear to be devoid of class I MHC molecules entirely.
- As stated previously, a single MHC molecule can bind many peptides. Since the MHC alleles are codominantly expressed, a heterozygous individual expresses the gene products at each MHC locus that are encoded by both alleles.
- On each of its nucleated cells, an F1 mouse expresses the K, D, and L from each parent (six distinct class I MHC molecules). In humans, the A, B, and C alleles from each parent (six distinct class I MHC molecules) are expressed on the membrane of each nucleated cell by a heterozygous individual.
- Due to the production of so many class I MHC molecules, each cell is able to present a high number of peptides in the peptide-binding clefts of its MHC molecules.
- In normal, healthy cells, class I molecules will exhibit self-peptides as a result of the natural turnover of self proteins.
- In virus-infected cells, both viral peptides and selfpeptides will be expressed. On the membrane of a single virus-infected cell, there are many class I molecules displaying diverse sets of viral peptides.
- Due to individual allelic variances in the peptide-binding clefts of class I MHC molecules, different people within a species will be able to bind distinct sets of viral peptides.
MHC II Molecules
- In contrast to class I MHC molecules, class II molecules are expressed constitutively only by antigen-presenting cells, primarily macrophages, dendritic cells, and B cells; however, thymic epithelial cells and other cell types can be induced to express class II molecules and to function as antigen-presenting cells under certain conditions and in response to stimulation by certain cytokines.
- Among the numerous cell types that express class II MHC molecules, there have been reported to be significant expression discrepancies.
- In some instances, class II expression is dependent on the stage of cell development. Class II molecules, for instance, cannot be found on pre-B cells but are constitutively produced on the membrane of mature B cells.
- Until they are triggered by contact with an antigen, monocytes and macrophages express only modest quantities of class II molecules. α
- Due to the fact that each of the classical class II MHC molecules consists of two distinct polypeptide chains that are encoded by different loci, a heterozygous individual expresses not only the parental class II molecules, but also molecules including α and β chains from different chromosomes. For instance, an H-2k mouse expresses class II IAk and IEk molecules; an H-2d mice expresses class II IAd and IEd molecules.
- Four parental class II molecules and four molecules having one parent’s chain and the other parent’s chain are expressed in the F1 offspring of mice with these two haplotypes.
- Since the human MHC contains three classical class II genes (DP, DQ, and DR), an individual who is heterozygous expresses six parental class II molecules and six molecules containing α and β chain combinations from any parent.
- The existence of numerous β-chain genes in mice and humans, and multiple α-chain genes in humans, increases the number of various class II molecules expressed by one individual.
- This diversity likely increases the amount of antigenic peptides that can be presented, which is helpful for the organism.
What is Linkage Disequilibrium?
- Linkage disequilibrium is a genetic phenomena in which two alleles are more frequently observed together than would be predicted.
- It is the relationship between alleles at distinct loci that is not random. For instance, if 16% of the population has a particular HLA-A antigen (A1) and 10% of the population has a certain HLA-B antigen (B8), the probability of discovering A1 genetically connected to B8 on the same chromosome is given by the product of their gene frequencies (16% x 10% = 6%).
- In actuality, this is not always the case. Certain combinations of A and B specificities occur more frequently than would be predicted by chance alone. The frequency of the combination A1 and B8 in human populations is 8.8 percent, compared to the expected frequency of 1.6 percent. These coupled specificities are referred to as being in linkage disequilibrium.
- Haplotypes HLA-A1, B8, DR3 (DRB1*0301), and DQ2 (DQB1*0201) are highly preserved in the Caucasian population.
- This effect is so prevalent in HLA class II that the existence of specific HLA-DR alleles can be used to accurately predict the HLA-DQ allele prior to testing.
- The order of the HLA alleles on chromosome 6 is DP-DQ-DR-B-Cw-A. Typically, linkage disequilibrium is strongest between alleles that are physically nearest to one another. It is plausible that certain haplotypes are favourable from an immunological standpoint, giving them a selective advantage.
Types of Major Histocompatibility Complex (MHC)
The major histocompatibility complex is a group of genes arranged along a lengthy stretch of DNA on chromosome 6 in humans and chromosome 17 in mice. Humans and mice, the species in which these regions have been researched the most, refer to the MHC as the human leukocyte antigen (HLA) complex and H-2 complex, respectively. Although the order of genes in the two species differs slightly, the MHC genes in both cases are grouped into sections encoding three types of molecules.
- Class I MHC genes: Class I MHC genes encode glycoproteins expressed on nearly all nucleated cell surfaces; the primary function of class I gene products is presentation of endogenous peptide antigens to CD8+ T lymphocytes.
- Class II MHC genes: Class II MHC genes encode glycoproteins that are principally expressed on APCs (macrophages, dendritic cells, and B cells), where they present exogenous antigenic peptides to CD4+ T lymphocytes.
- Class III MHC genes: Class III MHC genes encode several proteins, some of which have immunological roles, such as complement components and molecules implicated in inflammation.
| Class | Encoding | Expression |
| I | (1) peptide-binding proteins, which select short sequences of amino acids for antigen presentation, as well as (2) molecules aiding antigen-processing (such as TAP and tapasin). | One chain, called α, whose ligands are the CD8 receptor—borne notably by cytotoxic T cells—and inhibitory receptors borne by NK cells |
| II | (1) peptide-binding proteins and (2) proteins assisting antigen loading onto MHC class II’s peptide-binding proteins (such as MHC II DM, MHC II DQ, MHC II DR, and MHC II DP). | Two chains, called α & β, whose ligands are the CD4 receptors borne by helper T cells. |
| III | Other immune proteins, outside antigen processing and presentation, such as components of the complement cascade (e.g., C2, C4, factor B), the cytokines of immune signaling (e.g., TNF-α), and heat shock proteins buffering cells from stresses | Various |
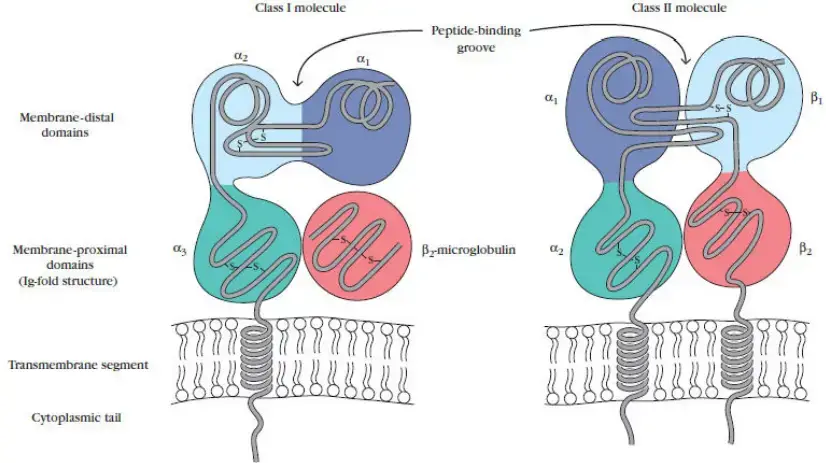
There are two primary classes of MHC molecules: I and II. Both of these MHC molecules are membrane-bound glycoproteins with structural and functional similarities. X-ray crystallography has been utilised to determine the three-dimensional structures of the extracellular domains of both classes of MHC molecules. These membrane glycoproteins serve as highly specialised antigen-presenting molecules with grooves that form exceptionally stable complexes with peptide ligands, displaying them on the cell surface for T-cell recognition via T-cell receptor (TCR) engagement. In contrast, class III MHC molecules are a collection of unrelated proteins that do not have structural or functional similarities with class I and II molecules, although many of them are involved in other elements of the immune response.
Class I and class II molecules have ultimate quaternary structures that are quite similar, despite the fact that their primary through quaternary protein configurations are distinct. MHC molecules assemble within the cell, where they bind with short peptide fragments generated from either the cell’s own proteins or proteins that have been absorbed via phagocytosis or pinocytosis. In addition, they differ in terms of the cells that express them and the antigens they deliver to T cells. Class I molecules are found on all nucleated cells in the body and are specialised for delivering cytosolic antigens, such as viral proteins (MHC class I molecules bind to peptides derived from proteins being synthesised within the cell). These are delivered to CD8+ T lymphocytes, which detect and destroy intracellular antigen-expressing cells. In contrast, class II MHC molecules are produced almost exclusively on antigen-presenting cells (APCs), a subpopulation of leukocytes that specialise in presenting antigens from extracellular spaces that have been ingested by these cells, such as fungus and extracellular bacteria (MHC class II molecules bind to peptides derived from proteins made external to the cell). Once produced on the cell surface, the MHC class II molecule transmits the antigenic peptide to CD4+ T cells, which are then activated and stimulate largely anti-extracellular invading immunity.
1. MHC class I molecules
Structure of MHC class I molecules
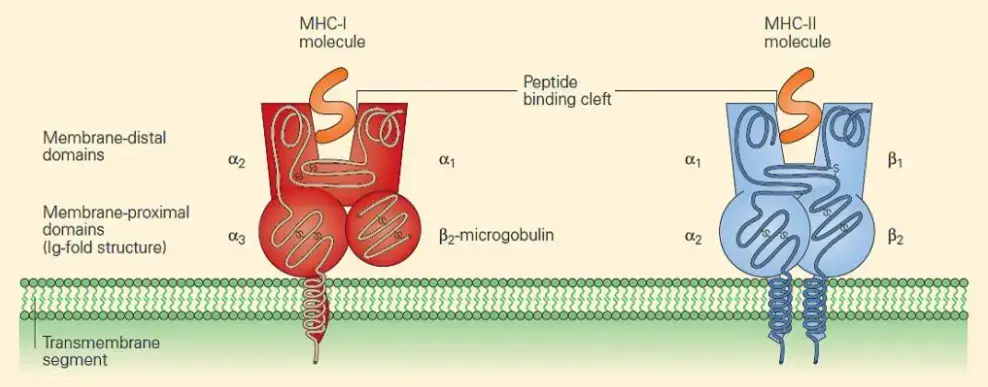
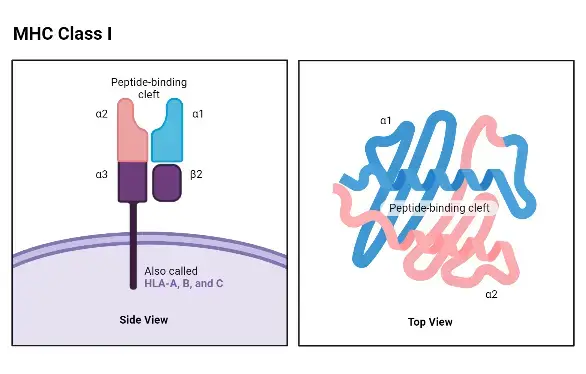
- The structure of MHC class I molecules consists of a 45-kilodalton (kDa) chain and a 12-kDa β2-microglobulin molecule that are noncovalently linked.
- The MHC class I chain is composed of three external domains, namely α1, α2, and α3. Each of these domains consists of approximately 90 amino acids. The α chain also contains a transmembrane domain composed of about 25 hydrophobic amino acids, followed by a short stretch of charged (hydrophilic) amino acids. Additionally, there is a cytoplasmic anchor segment consisting of 30 amino acids.
- The α chain of MHC class I molecules is encoded by polymorphic genes known as HLAA, HLAB, and HLAC in humans, located within the A, B, and C regions of the HLA complex on chromosome 6. In mice, the α chain is encoded by genes H2 K, H2D, and H2 L within the K, D/L region of the H2 complex on chromosome 17. As a result, each cell expresses at least three different class I proteins.
- The β2-microglobulin domain, which is approximately the same size and structure as the α3 domain, is associated with the MHC class I α chain through noncovalent interactions. β2-microglobulin does not possess a transmembrane region and is encoded by a separate gene that is highly conserved.
- It is worth noting that the α3 domain of MHC class I, β2-microglobulin, and the constant-region domains of immunoglobulin molecules exhibit a high degree of sequence homology. As a result, MHC class I has been classified as a member of the immunoglobulin superfamily of proteins.
- In summary, MHC class I molecules consist of an α chain composed of three external domains, a transmembrane domain, and a cytoplasmic anchor segment. They are associated with β2-microglobulin and belong to the immunoglobulin superfamily of proteins.
α-chain of MHC-I
- The α-chain of MHC-I is a transmembrane glycoprotein encoded by a polymorphic gene located in the A, B, and C regions of the human HLA complex.
- The α-chain is anchored to the plasma membrane by a hydrophobic transmembrane segment and a hydrophilic cytoplasmic tail.
- It consists of three domains: α1, α2, and α3. Each domain is composed of approximately 90 amino acids. The transmembrane domain contains around 25 hydrophobic amino acids followed by a short stretch of charged (hydrophilic) amino acids. Additionally, there is a cytoplasmic tail consisting of 30 amino acids.
- The α1 and α2 domains of the α-chain interact with each other, forming a peptide-binding groove on the surface of the protein. This groove is responsible for binding antigens, which typically consist of 8 to 10 amino acids.
- The α3 domain and the β2-microglobulin form an immunoglobulin fold, characterized by the arrangement of antiparallel β-strands into β-pleated sheets. This structural similarity to immunoglobulins classifies the α-chain and β2-microglobulin as members of the immunoglobulin superfamily.
- In summary, the α-chain of MHC-I is a transmembrane glycoprotein with three domains: α1, α2, and α3. It forms a peptide-binding groove and interacts with β2-microglobulin to create the MHC-I molecule. Its structure is similar to that of immunoglobulins, and it plays a crucial role in presenting antigens to immune cells.
β2 microglobulin of MHC-I
- β2 microglobulin is a protein that is part of the major histocompatibility complex class I (MHC-I) molecules. It is encoded by a highly conserved gene located on a specific chromosome.
- In terms of size and structure, β2 microglobulin is comparable to the α3 domain of the MHC-I molecule. It is a smaller protein with a similar domain organization.
- β2 microglobulin is non-covalently associated with the α-chain of MHC-I. It lacks a transmembrane region, which means it is not directly embedded in the plasma membrane like the α-chain. Instead, it forms a stable association with the α-chain through non-covalent interactions.
- The main function of β2 microglobulin is to stabilize the structure of MHC-I molecules and facilitate their proper folding. It is essential for the assembly and transport of MHC-I complexes to the cell surface, where they can present antigens to T cells.
- Overall, β2 microglobulin is an important component of MHC-I molecules, playing a crucial role in their structure and function. Its non-covalent association with the α-chain contributes to the stability and proper functioning of MHC-I complexes.
Functions of different domains
The different domains of MHC molecules have specific functions that contribute to their overall structure and function:
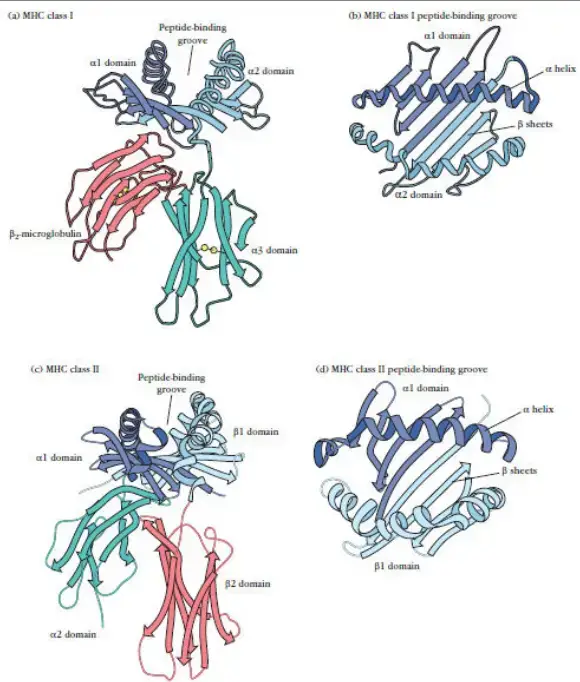
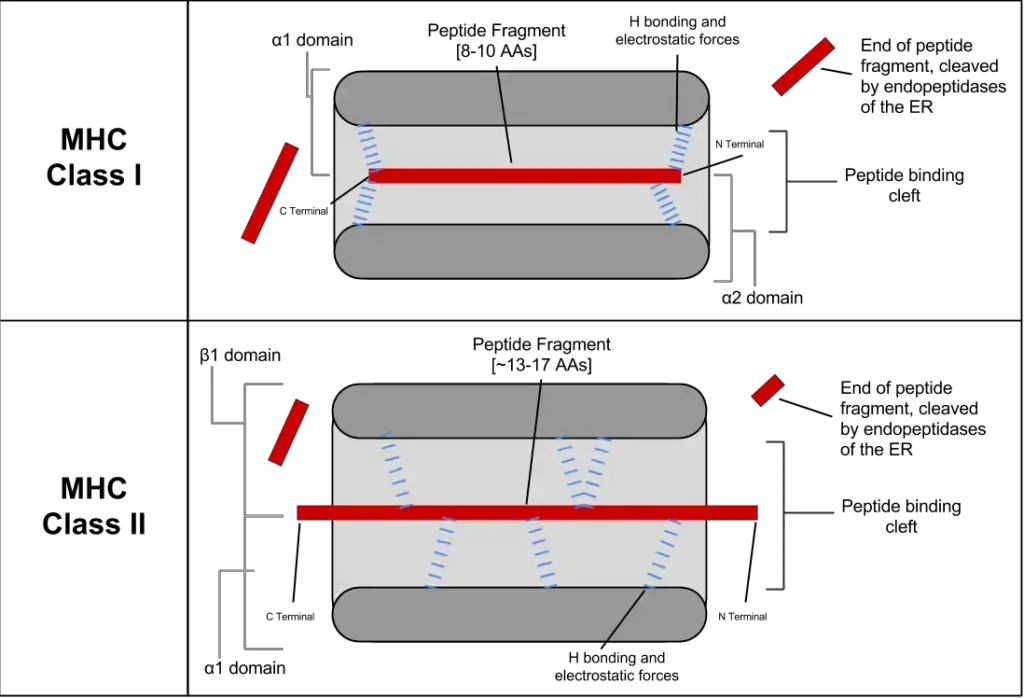
- α1 and α2 domains: These domains interact with each other to form a platform composed of eight antiparallel β strands and two long α-helical regions. This structure creates a deep groove or cleft on the top surface of the class I MHC molecule. The peptide-binding groove, located within this cleft, can accommodate an 8- to 10-amino acid peptide in a flexible and extended conformation. The α1 and α2 domains play a crucial role in binding and presenting peptide antigens to CD8+ T cells.
- α3 domain and β2-microglobulin: The α3 domain of class I MHC molecules is highly conserved and forms part of the overall structure of the molecule. It is structured as a β-pleated sheet composed of antiparallel β-strands. β2-microglobulin, a protein non-covalently associated with the MHC class I α-chain, also contributes to the structure of the MHC complex. The α3 domain and β2-microglobulin together provide stability and proper folding of the MHC class I molecule.
- CD8 interaction: The α3 domain of class I MHC molecules contains a region that interacts significantly with the CD8 protein present on the surface of cytotoxic T cells (TC cells). This interaction plays a role in the activation of TC cells upon recognition of MHC-peptide complexes.
Overall, the different domains of MHC molecules are essential for their peptide-binding capabilities, proper folding, stability, and interaction with other molecules such as CD8. The coordinated function of these domains ensures the effective presentation of antigens to T cells and the subsequent immune response.
Functions of MHC class I
The MHC class I molecules play several important functions in the immune system:
- Binding Peptide Antigens: One of the primary functions of MHC class I molecules is to bind peptide antigens derived from intracellular proteins. These peptides are generated within the cell and can be derived from viral proteins, tumor antigens, or self-proteins. The MHC class I molecule forms a complex with the peptide antigen within the cell.
- Presentation to CD8+ T Lymphocytes: The main role of MHC class I molecules is to present the peptide antigens to CD8+ T lymphocytes, also known as cytotoxic T cells. CD8+ T cells are crucial for cell-mediated immune responses and are involved in the elimination of infected or abnormal cells. The binding of the peptide antigen to the MHC class I molecule enables CD8+ T cells to recognize and respond to cells that are displaying foreign or abnormal peptides.
- Specificity for CD8 T Lymphocytes: MHC class I molecules are specifically recognized by CD8+ T lymphocytes. CD8 is a co-receptor expressed on the surface of these T cells, and its interaction with MHC class I molecules enhances the activation of CD8+ T cells and their cytotoxic functions.
- Binding and Presentation of Endogenous Antigens: MHC class I molecules primarily bind and present antigens derived from endogenous sources, meaning antigens that originate from within the cell itself. These antigens can be viral proteins synthesized by infected cells, tumor-specific antigens, or self-proteins that have been altered or modified.
- Ubiquitous Expression: MHC class I molecules are expressed on the surface of virtually all nucleated cells in the body. This widespread expression ensures that potential antigenic peptides generated within cells can be presented to CD8+ T cells, allowing for immune surveillance and rapid response against infected or abnormal cells throughout the body.
2. MHC class II molecules
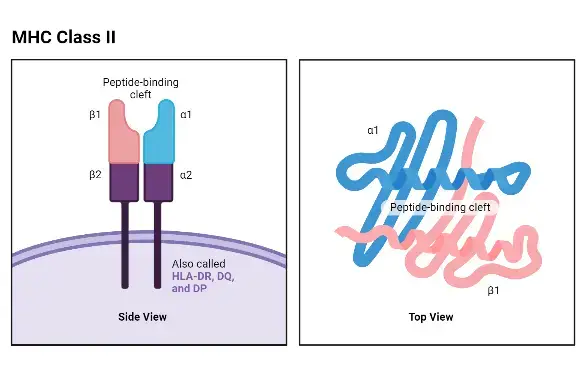
Structure of MHC class II molecules
The structure of MHC class II molecules can be described as follows:
- Composition: MHC class II molecules consist of two distinct polypeptide chains, an α chain and a β chain, which are noncovalently bonded together. The α chain has a molecular weight of about 33 kDa, while the β chain is approximately 28 kDa.
- Membrane-Bound Glycoproteins: Similar to class I α chains, class II MHC molecules are membrane-bound glycoproteins. They possess exterior domains, a transmembrane segment, and a cytoplasmic anchor segment.
- Domain Arrangement: Each chain of a class II molecule consists of two exterior domains. One chain contains α1 and α2 domains, while the other chain contains β1 and β2 domains. These exterior domains contribute to the overall structure and function of the molecule.
- Sequence Similarity and Immunoglobulin Fold: The membrane-proximal α2 and β2 domains of class II MHC molecules exhibit sequence similarity to the membrane-proximal α3/β2-microglobulin domains of class I MHC molecules. They share the immunoglobulin-fold structure, which is characteristic of proteins in the immunoglobulin superfamily.
- Peptide-Binding Groove: The membrane-distal regions of class II molecules, specifically the α1 and β1 domains, provide the peptide-binding groove for processed antigens. This groove allows class II molecules to bind and present exogenous antigens to CD4+ T lymphocytes, also known as helper T cells.
- Multiple Gene Forms: In humans, there are three major forms of MHC class II α and β chain genes, namely HLA-DQ, HLA-DP, and HLA-DR. Mice, on the other hand, express two pairs of class II molecules, H2A (IA) and H2E (IE). This genetic diversity allows for the expression of multiple distinct class II molecules in individuals, increasing the repertoire of antigens that can be presented to helper T cells.
The structure of MHC class II molecules is specifically adapted for the presentation of exogenous antigens to CD4+ T cells, facilitating immune responses and the activation of helper T cell-mediated immune functions.
α-chain and β-chain of MHC-II
The α-chain and β-chain of MHC-II molecules have the following characteristics:
- Membrane-Bound Glycoproteins: Both the α-chain and β-chain of MHC-II molecules are membrane-bound glycoproteins. They are anchored to the cell membrane and extend into the extracellular space.
- Extracellular Domains: Each chain of MHC-II consists of extracellular domains. The α-chain has two extracellular domains known as α1 and α2, while the β-chain has two extracellular domains known as β1 and β2. These domains contribute to the overall structure and function of the MHC-II molecule.
- Transmembrane Segment: The α-chain and β-chain each possess a transmembrane segment. This segment spans the cell membrane and helps anchor the MHC-II molecule in place.
- Cytoplasmic Tail: Both the α-chain and β-chain have a cytoplasmic tail. This tail is located on the intracellular side of the cell membrane and is involved in intracellular signaling and interactions.
- Peptide-Binding Cleft: The α-chain and β-chain of MHC-II molecules come together to form an open-ended groove called the peptide-binding cleft. This cleft is located at the proximal end of the MHC-II molecule, facing outward. It is within this cleft that antigenic peptides bind. The peptide-binding cleft of MHC-II molecules can accommodate peptides that are typically 13 to 18 amino acids in length.
The α-chain and β-chain of MHC-II molecules work together to form a functional MHC-II complex capable of binding and presenting exogenous antigens to CD4+ T lymphocytes. The peptide-binding cleft, formed at the interface of the α-chain and β-chain, plays a critical role in capturing and presenting antigenic peptides for recognition by helper T cells.
Functions of different domains
The different domains of MHC class II molecules have specific functions:
- Peptide-Binding Groove: The extracellular domains of MHC class II molecules, specifically the α1 and β1 domains, form the peptide-binding groove. This groove consists of a floor composed of eight antiparallel β strands and sides made up of antiparallel α helices. The peptide-binding groove is where exogenous antigens, typically containing 13 to 18 amino acids, can bind.
- CD4 Binding: CD4, a co-receptor protein found on the surface of helper T cells, interacts with the β2 domain of MHC class II molecules. This interaction facilitates the binding of the MHC class II-peptide complex to the CD4 molecule, leading to the activation of the helper T cell.
- Polymorphic Residues: Polymorphic residues, which are variations in amino acid sequences, are present in and around the peptide-binding cleft of the α1 and β1 domains of MHC class II molecules. These polymorphic residues contribute to the diversity of antigenic peptides that can be presented by different individuals.
- Invariant Chain (Ii): After synthesis, MHC class II molecules associate with a nonpolymorphic polypeptide called the invariant chain (Ii). The invariant chain helps in the proper folding and intracellular trafficking of the MHC class II molecule. It also blocks the peptide-binding groove, preventing it from binding endogenous peptides in the endoplasmic reticulum. The invariant chain is later cleaved, leaving a small fragment known as the CLIP (class II-associated invariant chain peptide), which is then exchanged for an antigenic peptide before the MHC class II molecule reaches the cell surface.
These functions of different domains in MHC class II molecules are crucial for their role in presenting exogenous antigens to CD4+ T lymphocytes and initiating immune responses.
Functions of MHC class II
MHC class II molecules have important functions in the immune system:
- Antigen Presentation: The primary role of MHC class II molecules is to bind peptide antigens derived from exogenous sources, such as pathogens or foreign substances, and present them to CD4+ T lymphocytes. This antigen presentation is essential for the activation of helper T cells, which play a central role in coordinating immune responses.
- Antigen-Presenting Cells (APCs): MHC class II molecules are predominantly found on the surface of specialized antigen-presenting cells (APCs). These APCs include dendritic cells, macrophages, and B cells. By expressing MHC class II molecules, APCs can capture, process, and present antigens to CD4+ T cells, initiating an immune response.
- CD4+ T Cell Recognition: MHC class II molecules are specifically recognized by CD4+ T cells. The interaction between the MHC class II-peptide complex and the T cell receptor (TCR) on CD4+ T cells triggers a series of signaling events that activate the T cell, leading to the initiation of an immune response.
- Activation of B Cells: MHC class II molecules play a crucial role in the activation of B cells. Helper T cells recognize antigens presented by MHC class II molecules on B cells, leading to the activation and differentiation of B cells into antibody-secreting plasma cells. This collaboration between MHC class II-restricted CD4+ T cells and B cells is essential for the generation of effective antibody responses.
- Graft-versus-Host Response and Mixed Lymphocyte Reaction (MLR): In humans, the genes encoding MHC class II molecules are highly polymorphic and are closely linked to the genes involved in immune responses. This close association makes MHC class II molecules crucial for certain immunological reactions, including graft-versus-host response (GVHR) and mixed lymphocyte reaction (MLR). GVHR occurs when immune cells from the transplanted tissue or organ recognize the recipient’s MHC class II molecules as foreign, leading to an immune attack. MLR refers to the interaction between immune cells from different individuals, which is mediated by MHC class II molecules and can influence immune responses.
| Feature | Class I MHC | Class II MHC |
|---|---|---|
| Constituting polypeptide chains | α chain (45KDa in humans)β2 chain (12 KDa in humans) | α chain (30-34 KDa in humans)β chain (26-29 KDa in humans) |
| Antigen binding domain | α1and α2 domains | α1 and β1 domains |
| Binds protein antigens of | 8-10 amino acids residues | 13-18 amino acids residues |
| Peptide bending cleft | Floor formed by β sheets and sides by αhelices, blocked at both the ends | Floor formed by β sheets and sides by αhelices, opened at both the ends |
| Antigenic peptide motifsinvolved in binding | Anchor residues located at amino andcarbon terminal ends | Anchor residues located almost uniformlyalong the peptide |
| Presents antigenic peptide to | CD8+ T cells | CD4+ T cells |
3. MHC class-III Molecules
MHC class III molecules refer to a group of proteins that are encoded by genes located within the major histocompatibility complex (MHC) region. Unlike MHC class I and II molecules, class III molecules do not have a direct role in antigen presentation. Instead, they are involved in other immune-related functions, particularly in the complement system and inflammation.
- Complement System: Class III MHC molecules play a significant role in the complement system, which is a complex cascade of proteins involved in immune defense and inflammation. Several serum proteases that participate in the complement system are categorized as class III MHC molecules. These include components such as C2, C4A, C4B, and factor B. These proteins play essential roles in activating and regulating the complement cascade, which helps to clear pathogens, enhance immune responses, and facilitate the removal of immune complexes.
- Tumor Necrosis Factors: Some members of the tumor necrosis factor (TNF) family are also considered class III MHC molecules. TNF-α and TNF-β, also known as lymphotoxin-alpha (LT-α), are inflammatory cytokines that are involved in various immune processes, such as regulating immune cell activation, promoting inflammation, and inducing cell death in certain contexts.
- Heat Shock Proteins: Certain heat shock proteins (HSPs) are classified as class III MHC molecules. Heat shock proteins are produced in response to cellular stress, and they play a role in protecting cells from damage and assisting in the proper folding of proteins. Some HSPs have immunomodulatory functions and can interact with immune cells to regulate immune responses.
Functions of MHC class-III
MHC class III molecules have important functions in complement activation and inflammation. Here are the key roles they play:
- Complement Activation: Class III MHC molecules participate in the activation of the complement system. The complement system is a complex network of proteins that helps eliminate pathogens, enhance immune responses, and regulate inflammatory processes. Components such as C2, C4A, C4B, and factor B, which are classified as class III MHC molecules, are involved in different stages of the complement cascade. These molecules promote the binding, activation, and assembly of complement proteins, leading to the formation of membrane attack complexes that can destroy pathogens or mark them for removal by immune cells.
- Inflammation Induced by Cytokines and Tumor Necrosis Factors: MHC class III molecules are implicated in the inflammatory response induced by various cytokines and tumor necrosis factors. Cytokines are small signaling molecules secreted by immune cells that regulate immune responses and inflammation. Tumor necrosis factors, including TNF-α and TNF-β (lymphotoxin-alpha), are inflammatory cytokines that play crucial roles in mediating immune responses and promoting inflammation. Class III MHC molecules are involved in the signaling pathways activated by these cytokines, contributing to the initiation and regulation of inflammatory processes.
- Other Functions: In addition to complement activation and cytokine-induced inflammation, class III MHC molecules may have additional roles in immune regulation and response. Some heat shock proteins, which are classified as class III MHC molecules, can modulate immune reactions and interact with immune cells. These heat shock proteins are produced in response to cellular stress and assist in protein folding and cellular homeostasis. They can also act as danger signals, alerting the immune system to potential threats and influencing immune cell functions.
Formation of the Membrane Attack Complex (MAC) Animation
Antigen Presentation and Processing
Antigen presentation and processing play a critical role in the immune response by allowing T cells to recognize foreign antigens. Here is an overview of the key steps involved:
- Antigen Degradation: Protein antigens, which can be derived from intracellular or extracellular sources, need to be degraded into smaller fragments for efficient presentation. This degradation process is known as antigen processing. Intracellular antigens are processed through the cytosolic pathway (Class I MHC molecules), while extracellular antigens are processed through the endocytic pathway (Class II MHC molecules).
- MHC-Peptide Complex Formation: During antigen processing, the protein antigens are broken down into smaller peptide fragments. These peptide fragments then bind to specific regions of MHC molecules, forming MHC-peptide complexes. In the cytosolic pathway (Class I MHC molecules), the peptides bind to the peptide-binding groove of Class I MHC molecules. In the endocytic pathway (Class II MHC molecules), the peptides displace a small fragment called CLIP (Class II-associated invariant chain polypeptide) and bind to the Class II MHC molecules.
- MHC-Mediated Transport: After the formation of the MHC-peptide complexes, the MHC molecules, along with the bound antigenic peptides, are transported from the intracellular compartments to the cell membrane. This transport process involves various cellular mechanisms, including vesicular trafficking and fusion events.
- Antigen Presentation to T Cells: Once the MHC-peptide complexes reach the cell membrane, they are exposed to the external environment. T cells, specifically T cell receptors (TCRs) on the surface of CD4+ or CD8+ T cells, can recognize the antigenic peptides presented by MHC molecules. The TCRs bind to the MHC-peptide complex with high specificity, initiating a signaling cascade that leads to T cell activation.
By presenting antigens in the form of MHC-peptide complexes, antigen presentation and processing enable T cells to detect and respond to foreign invaders, triggering immune responses tailored to the specific antigens encountered. This process is crucial for the recognition of pathogens and the initiation of appropriate immune reactions to protect the body from infections and diseases.
Two classical routes are used to digest and present peptides:
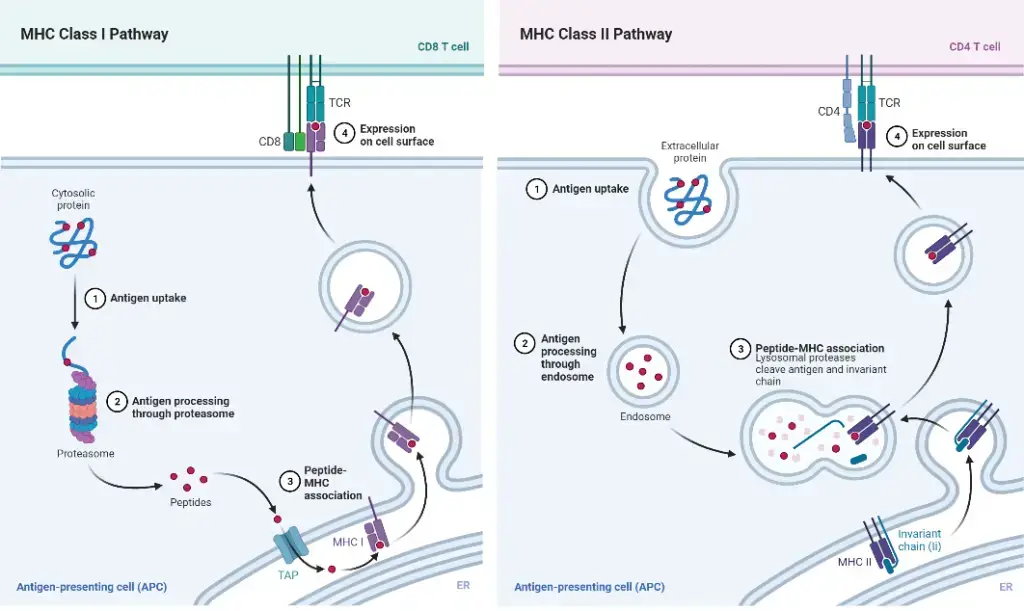
Antigen Presentation Pathway: Class II MHC molecules – Endocytic Pathway
The antigen presentation pathway involving Class II MHC molecules, known as the endocytic pathway, plays a crucial role in presenting exogenous or extracellular pathogens or antigens to T cells. Here are the key steps involved:
- Exogenous Pathogens: Class II MHC molecules are responsible for presenting antigens derived from extracellular pathogens that can grow and reproduce outside of host cells, such as bacteria, exotoxins, and parasites. These antigens are taken up by antigen-presenting cells through endocytosis or phagocytosis.
- Antigen-Presenting Cells: Only antigen-presenting cells, including B cells, macrophages, and dendritic cells, are involved in antigen processing and presentation via Class II MHC molecules. This pathway occurs after the antigen is engulfed by the antigen-presenting cell.
- Formation of Endolysosomes: Inside the antigen-presenting cell, the antigen is enclosed in a vesicle called an endosome. The endosome fuses with a lysosome in the cytoplasm, forming an endolysosome. Within the endolysosome, the foreign protein is degraded by proteolytic enzymes, resulting in the formation of small peptides.
- MHC Class II Synthesis and Association: The Class II MHC molecules are synthesized and formed in the endoplasmic reticulum. The alpha (α) and beta (β) chains of the molecule are associated with a protein called the invariant chain. This association helps prevent the binding of self-antigens to the Class II MHC molecule. The invariant chain-MHC complex is then transported from the endoplasmic reticulum to the Golgi apparatus and subsequently to another vesicle.
- CLIP Formation and Exchange: Inside the vesicle, the invariant chain is digested, leaving a small fragment called Class II-associated invariant chain polypeptide (CLIP) attached to the MHC class II molecule.
- Peptide Binding: The vesicle containing the MHC class II molecule fuses with another vesicle containing the fragmented peptides. The fragmented peptides displace CLIP and bind to the MHC class II molecule. This forms a newly formed MHC class II-peptide complex.
- Transport to Cell Surface: The MHC class II-peptide complex is transported to the surface of the antigen-presenting cell.
- T Cell Recognition: At the cell surface, the antigen is presented to T cells. The T cell receptor on T cells recognizes the peptide bound to the MHC class II molecule. Additionally, the co-receptor CD4 binds to the β2 domain of the Class II MHC molecule, facilitating antigen presentation to the T cell.
In summary, the endocytic pathway involving Class II MHC molecules enables the presentation of antigens derived from extracellular pathogens or antigens to T cells, leading to the activation of the immune response.
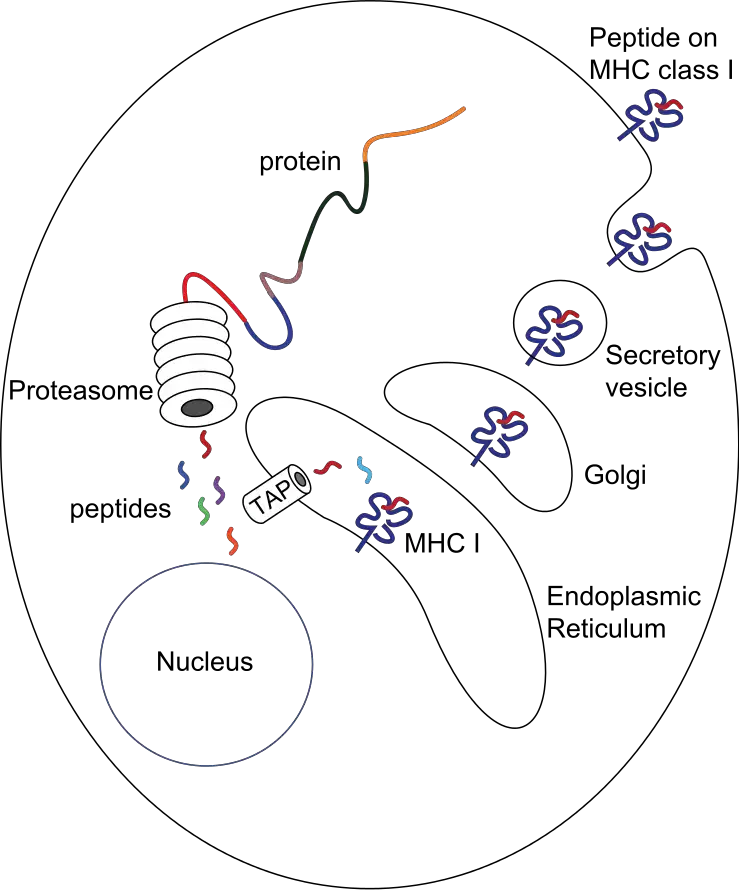
Antigen Presentation Pathway: Class I MHC molecules – Cytosolic pathway
The antigen presentation pathway involving Class I MHC molecules, also known as the cytosolic pathway, plays a crucial role in presenting intracellular or endogenous pathogens or antigens to T cells. Here are the key steps involved:
- Intracellular Pathogens: Class I MHC molecules are involved in presenting antigens derived from intracellular pathogens, such as viruses, which reside and replicate inside host cells.
- Formation of MHC-Self Peptide Complex: Under normal conditions, Class I MHC molecules form complexes with self-peptides or self-antigens. However, during viral infection, MHC Class I molecules present peptides derived from the virus to be recognized by T cells.
- Cell Components: Various cell components, including the nucleus, endoplasmic reticulum (ER), and Golgi apparatus, play important roles in antigen processing and presentation.
- Viral Protein Synthesis: When a virus infects a normal cell, its DNA enters the cell and utilizes the host cell machinery to produce viral proteins. These viral proteins are synthesized in the cytosol.
- Proteasome Degradation: The cytosol contains a cylindrical protein complex called the proteasome, which degrades unwanted or damaged proteins into smaller peptides. During viral infection, viral proteins interact with proteasomes in the cytosol, leading to their degradation into smaller peptides (8-15 amino acids long).
- Peptide Transport: The fragmented peptides generated in the cytosol are transported into the endoplasmic reticulum. This transport is facilitated by a peptide delivery system called the transporter associated with antigen processing (TAP), consisting of two subunits, TAP1 and TAP2.
- MHC Class I Synthesis: Inside the endoplasmic reticulum, the alpha (α) and beta-2 microglobulin (β2m) chains of MHC Class I molecules are synthesized. With the help of chaperone proteins, the MHC Class I molecule is formed and moves toward TAP.
- Peptide Binding: The peptides bind to the peptide-binding site of the Class I MHC molecule inside the endoplasmic reticulum, forming the MHC Class I-peptide complex.
- Golgi Apparatus and Plasma Membrane: The MHC Class I-peptide complex moves to the surface of the Golgi apparatus and, through secretory vesicles, is transported to the plasma membrane.
- T Cell Recognition: Once the MHC Class I-peptide complex reaches the cell surface, T cell receptors on T cells recognize the antigen peptide complex. Additionally, the co-receptor CD8 on T cells attaches to the α3 domain of the MHC Class I molecule, facilitating antigen presentation to the T cell.
Characteristics of the antigen processing pathways
| Characteristic | MHC-I pathway | MHC-II pathway |
|---|---|---|
| Composition of the stable peptide-MHC complex | Polymorphic chain α and β2 microglobulin, peptide bound to α chain | Polymorphic chains α and β, peptide binds to both |
| Types of antigen-presenting cells (APC) | All nucleated cells | Dendritic cells, mononuclear phagocytes, B lymphocytes, some endothelial cells, epithelium of thymus |
| T lymphocytes able to respond | Cytotoxic T lymphocytes (CD8+) | Helper T lymphocytes (CD4+) |
| Origin of antigenic proteins | cytosolic proteins (mostly synthetized by the cell; may also enter from the extracellular medium via phagosomes) | Proteins present in endosomes or lysosomes (mostly internalized from extracellular medium) |
| Enzymes responsible for peptide generation | Cytosolic proteasome | Proteases from endosomes and lysosomes (for instance, cathepsin) |
| Location of loading the peptide on the MHC molecule | Endoplasmic reticulum | Specialized vesicular compartment |
| Molecules implicated in transporting the peptides and loading them on the MHC molecules | TAP (transporter associated with antigen processing) | DM, invariant chain |
Regulation of MHC Expression
- The study of the regulatory mechanisms that control the differential expression of MHC genes in various cell types is still in its infancy, although a great deal has been learnt.
- It is anticipated that the release of the whole genetic map of the MHC complex will considerably accelerate the identification and examination of coding and regulatory sequences, leading to new research avenues into how the system is controlled.
- Both class I and class II MHC genes have five promoter regions that bind sequence-specific transcription factors.
- For a number of MHC genes, the promoter motifs and transcription factors that bind to these motifs have been identified.
- Positive and negative elements both regulate the MHC’s transcriptional regulation. CIITA, an MHC II transactivator, and RFX, another transcription factor, have both been demonstrated to bind to the promoter region of class II MHC genes.
- These transcription factor deficiencies induce one variant of bare lymphocyte syndrome. Due to the crucial role of class II MHC molecules in T-cell maturation and activation, patients with this illness suffer from severe immunodeficiency due to the absence of class II MHC molecules on their cells.
- Various cytokines control the expression of MHC molecules as well. It has been demonstrated that the interferons (alpha, beta, and gamma) and tumour necrosis factor promote the expression of class I MHC molecules on cells.
- Interferon gamma (IFN-γ), for instance, seems to cause the production of a transcription factor that binds to the promoter sequence bordering the class I MHC genes.
- This transcription factor appears to coordinate the up-regulation of transcription of the genes encoding the class I chain, α chain, β2-microglobulin, the proteasome subunits (LMP), and the transporter subunits by binding to the promoter region (TAP).
- It has been demonstrated that IFN-γ induces expression of the class II transactivator (CIITA), thereby indirectly enhancing expression of class II MHC molecules on a range of cells, including non-antigen-presenting cells (e.g., skin keratinocytes, intestinal epithelial cells, vascular endothelium, placental cells, and pancreatic beta cells).
- Other cytokines only alter MHC expression in specific cell types; for instance, IL-4 promotes class II molecule expression in resting B cells.
- IFN-γ inhibits B cell expression of class II molecules; corticosteroids and prostaglandins also inhibit B cell expression of class II molecules.
- Infection with several viruses, including human cytomegalovirus (CMV), hepatitis B virus (HBV), and adenovirus 12, reduces MHC expression (Ad12).
- In some instances, decreased production of class I MHC molecules on cell surfaces is owing to decreasing quantities of a component required for peptide transport or MHC class I assembly, as opposed to transcription.
- In CMV infection, for instance, a viral protein attaches to β2-microglobulin, inhibiting the assembly and transport of class I MHC molecules to the plasma membrane.
- Infection with Adenovirus 12 significantly reduces the transcription of transporter genes (TAP1 and TAP2). As shown in the following chapter, the TAP gene products play a crucial function in the transport of peptides from the cytoplasm to the rough endoplasmic reticulum.
- Blocking the expression of the TAP gene reduces peptide transport, preventing class I MHC molecules from assembling with β2-microglobulin or being delivered to the cell membrane.
- By reducing the likelihood that virus-infected cells will show MHC–viral peptide complexes and become targets for CTL-mediated destruction, decreased production of class I MHC molecules, regardless of the underlying mechanism, may aid viruses in evading the immune response.
MHC and Immune Responsiveness
- Immunologists have recognised for a long time that some foreign proteins that elicit robust immune responses in some individuals do not do so in others. Those who did not mount a reaction were previously referred to as “non-responders,” while those who did were referred to as “responders.”
- There were subtle disparities in the level of reaction across respondents, leading to the classification of individuals as low or high responders.
- Immunologists quickly pinpointed the genes responsible for immunological responsiveness to the MHC and demonstrated that mice with distinct MHC haplotypes respond differentially to a given peptide.
- These variations in response levels to a given antigen might be read as changes in the ability of various MHC alleles to present antigen-derived peptides that T cells can detect.
- In an inbred population, there is a larger likelihood that an individual will be a non-responder; that is, a person will lack an MHC allele that can result in specific T cell activation in response to a specific antigen challenge.
- Two ideas, neither of which is mutually incompatible, have been offered to explain non-response: the determinant selection model and the hole in the T cell repertoire model.
a. Determinant Selection Model
- To mount a T cell response against a foreign protein, the host must have at least one MHC allele with a groove that can accommodate a peptide generated from that protein.
- The responsiveness is thus determined by the strength of binding between a certain MHC allele and a given determinant (peptide), which is in turn determined by structural compatibility. In other words, the MHC proteins in an individual “choose” the immunogenic factors and the magnitude of the immune response.
- Since a foreign protein is often digested into three to four immunogenic peptides, it is quite likely that an outbred individual will possess an MHC allele capable of binding to at least one of these peptides and eliciting an immune response.
- The individual is therefore a responder to this particular antigen, and his or her position as a high or low responder coincides with the peptide’s strong or weak binding to MHC, respectively.
- The individual is a non-responder to this antigen if none of his or her MHC molecules can bind to any of the peptides derived from the protein.
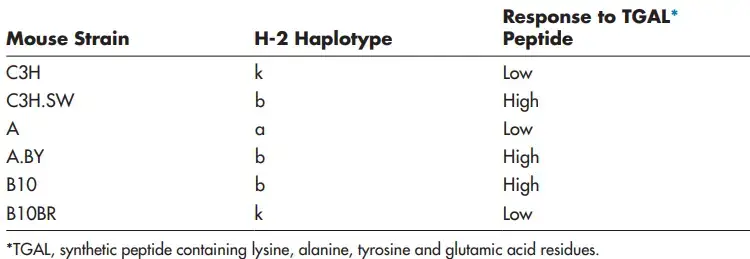
- Experiments demonstrating that a peptide is only immunogenic when coupled to a specific MHC allele have provided support for the determinant selection paradigm. For instance, a specific peptide of an influenza virus protein rapidly induces an immunological response in H-2k haplotype inbred mice.
- Specifically, T lymphocytes identify the determinant when it is given to them on the MHC class I H-2Kk molecule.
- However, this peptide fails to excite T cells in H-2b haplotype mice. When displayed on the MHC class I H-2Db molecule, a peptide from a different portion of the same influenza virus protein induces an antiviral response in H-2b mice.
b. Hole in the T Cell Repertoire Model
- Immune non-reactivity may potentially be caused by tolerance mechanisms.
- In non-responders, it is possible that a particular foreign peptide–MHC combination resembles the structure of a self peptide–MHC combination so closely that T cell clones capable of recognising the foreign peptide–MHC combination were eliminated as autoreactive during the establishment of central tolerance.
- This would result in a lack of T cell specificity or a “hole” in a non-T responder’s cell repertoire relative to a responder’s repertoire.
MHC and Disease Predisposition
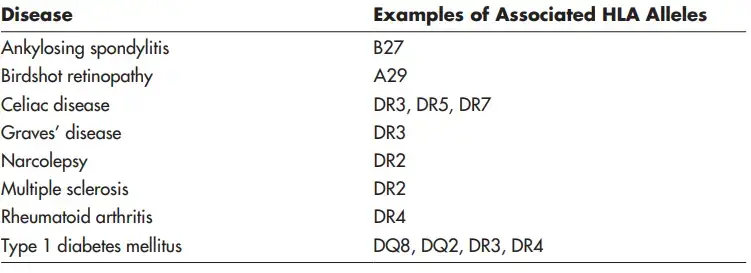
- The MHC haplotype of an individual determines his or her susceptibility to immunogens. If a person is unable to establish an adequate immune response to an immunogen associated with an infection or malignancy, he or she will likely develop a disease.
- A disease in the form of autoimmunity or hypersensitivity (including allergy) may result from an improper immune response.
- Due to the direct association between immunological responsiveness and distinct MHC alleles, specific MHC haplotypes may predispose individuals to particular susceptibilities or diseases. Numerous illnesses associated with specific MHC alleles manifest as autoimmune disease in humans.
- When self-reactive T cell clones escape the tolerance mechanisms that would usually prevent them from entering or activating in the periphery, autoimmune illness occurs.
- The individual may then contain T cells that can recognise self-components and assault tissues that express them. For instance, type 1 (insulin-dependent) diabetes mellitus is believed to be caused by an autoimmune attack on antigens expressed by insulin-producing β cells in the pancreatic islets. The elimination of β islet cells by the immune system results in insulin insufficiency and diabetes.
- The HLA-DQ8 allele is eight times more frequent in human communities with type 1 diabetes than in healthy ones for unknown causes.
- Similarly, 90% of Caucasian patients with ankylosing spondylitis contain the HLA-B27 allele, compared to 9% of healthy Caucasian individuals.
- Note, however, that the mere presence of a predisposing HLA allele is typically insufficient to produce disease; it is believed that additional genetic and environmental variables are required.
- This finishes our examination of the MHC’s anatomy and physiology.
- In the following chapter, headed “Antigen Processing and Presentation,” we detail how antigenic peptides are produced and how MHC molecules bind to and present these peptides to T cells.
2003 marked the conclusion of the Human Genome Project (HGP), which found ∼25,000 genes in human DNA and sequenced 3 billion base pairs in the human genome. The HGP has enabled unparalleled precision in determining the chromosomal locations and DNA sequences of HLA alleles. By comparing the sequences of HLA alleles derived from distinct populations around the world, researchers have explored the relationships between certain HLA alleles and illness risk among individuals of various nationalities. Moreover, other non-HLA genes have been linked to illness occurrence. The term “Genome-Wide Association Studies” refers to research that utilises the extensive data of the HGP to identify the locations of genes associated with certain diseases (GWAS).
Polymorphism and MHC Restriction
- How did MHC genes get so polymorphic? In an earlier, antigenically simple environment, a primordial MHC molecule that showed endogenous and foreign protein fragments to T cells probably existed, but its diversity was extremely limited (or nonexistent).
- Individuals with many duplications of this primordial MHC gene likely survived as the world became more antigenically complex because they possessed more than one gene devoted to displaying protein fragments.
- Possibly concurrently, distinct alleles of each gene, each with a distinct sequence in the peptide-binding groove, also emerged.
- A wider variety of peptide-binding and -presentation molecules would have been produced. Today, the ensuing polymorphism at numerous MHC loci assures that each member of an outbred species is heterozygous at the majority, if not all, MHC loci, and hence has a high probability of harbouring at least one MHC allele capable of binding to any given antigenic peptide.
- MHC polymorphism indicates that a significant number of MHC alleles are distributed throughout the entire population. In the event of a devastating disease attack, a considerable portion of the population will be able to respond to the infection and survive in order to reproduce the species.
- However, the plurality of MHC molecules does not permit anarchy in terms of T-cell presentation. Exists a phenomena known as MHC limitation, which demands that the epitope detected by a given TCR is a combination of a certain peptide and a particular MHC molecule.
Important Aspects of MHC Molecules
- Although a species exhibits a significant degree of polymorphism, an individual possesses no more than six distinct class I MHC products and only slightly more class II MHC products (considering only the major loci).
- Each MHC molecule contains a single binding site. The various peptides that a particular MHC molecule can bind bind to the same location, but only one at a time.
- Because one MHC molecule can bind numerous distinct peptides, this type of binding is known as degenerate.
- MHC polymorphism is solely determined at the germline level. There are no processes for recombination that generate variety.
- MHC molecules are membrane-bound, and T cell recognition needs cell-cell interaction.
- Alleles for MHC genes are co-dominant. Each MHC gene product is expressed on the surface of a nucleated cell.
- A peptide must bind to a specific MHC of that individual in order for an immune response to occur. That is one control level.
- Mature T cells must have a T cell receptor capable of recognising the MHC-associated peptide. This is the second control level.
- Cytokines (particularly interferon-γ) boost the level of MHC expression.
- Tc cells identify peptides from the cytosol when they bind to class I MHC. Th cells identify peptides from inside vesicles that connect with class II MHC.
- MHC polymorphism is essential for the survival of the species.
How Do Peptides Get Into The MHC Groove?
- CTL cells identify cytosolic peptides through their association with class I MHC. The peptides penetrate the endoplasmic reticulum, where they bind to the MHC class I groove.
- Through the Golgi, this complex is then transferred to the cell surface. In the ER and Golgi, MHC class II molecules are synthesised using an invariant (Ii) chain as a placeholder.
- Once the complex enters a vesicle, the Ii chain is cleaved and eliminated. Within the vesicle, peptides bind to class II MHC before being ejected to the cell surface, where they are identified by Th cells.
FAQ
What is the Major Histocompatibility Complex (MHC)?
The Major Histocompatibility Complex (MHC) is a set of genes that code for proteins involved in the immune response. These proteins are responsible for presenting antigens to T cells, which play a crucial role in immune recognition and response.
What are the two main classes of MHC molecules?
There are two main classes of MHC molecules: Class I and Class II. Class I MHC molecules are found on the surface of almost all nucleated cells, while Class II MHC molecules are primarily found on antigen-presenting cells such as macrophages, dendritic cells, and B cells.
What is the function of MHC molecules?
The primary function of MHC molecules is to present antigens to T cells. This antigen presentation allows T cells to recognize and respond to foreign invaders, such as viruses or bacteria, as well as abnormal cells, such as cancer cells.
How do MHC molecules present antigens?
MHC molecules bind to small peptide fragments derived from proteins (antigens) and present them on the cell surface. Class I MHC molecules present intracellular antigens to CD8+ T cells, while Class II MHC molecules present extracellular antigens to CD4+ T cells.
Why is MHC polymorphism important?
MHC molecules are highly polymorphic, meaning they have many different variants or alleles within a population. This polymorphism allows MHC molecules to present a wide range of antigens, increasing the likelihood of immune recognition and response to diverse pathogens.
What is the role of MHC in transplantation?
MHC molecules play a crucial role in transplant rejection. Mismatched MHC molecules between the donor and recipient can trigger an immune response, leading to graft rejection. Matching MHC compatibility is important for successful organ or tissue transplantation.
Can MHC molecules influence susceptibility to diseases?
Yes, certain MHC alleles have been associated with increased or decreased susceptibility to certain diseases. For example, specific MHC alleles are linked to autoimmune diseases, such as rheumatoid arthritis or type 1 diabetes, where the immune system mistakenly attacks healthy tissues.
How are MHC molecules inherited?
MHC molecules are inherited in a codominant manner, meaning that an individual inherits one set of MHC alleles from each parent. This genetic diversity contributes to the wide range of MHC molecules present in a population.
Can MHC molecules change in response to infections or diseases?
Yes, during infections or diseases, the expression of MHC molecules can be upregulated, allowing for increased antigen presentation to T cells. This helps to enhance the immune response and facilitate the clearance of pathogens.
Are MHC molecules only found in humans?
No, MHC molecules are found in many vertebrate species, including humans. However, the specific genes and alleles within the MHC vary between species. Studying MHC molecules in different organisms helps us understand the evolution and diversity of immune responses.
References
- Kuby Immunology Seventh edition.
- Roitt’s Essential Immunology Thirteenth edition
- Wieczorek, M., Abualrous, E. T., Sticht, J., Álvaro-Benito, M., Stolzenberg, S., Noé, F., & Freund, C. (2017). Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Frontiers in Immunology, 8. doi:10.3389/fimmu.2017.00292
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The major histocompatibility complex and its functions. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27156/
- Hohl, T. M. (2015). Cell-Mediated Defense against Infection. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 50–69.e6. doi:10.1016/b978-1-4557-4801-3.00006-0
- Mak, T. W., & Saunders, M. E. (2006). MHC: The Major Histocompatibility Complex. The Immune Response, 247–277. doi:10.1016/b978-012088451-3.50012-0
- The Major Histocompatibility Complex. (2014). Primer to the Immune Response, 143–159. doi:10.1016/b978-0-12-385245-8.00006-6
- https://www.ebi.ac.uk/interpro/entry/InterPro/IPR001039/
- https://www2.nau.edu/~fpm/immunology/documents/Ch-08.pdf
- https://www.ndvsu.org/images/StudyMaterials/Micro/MHC-Antigens.pdf
- https://pdb101.rcsb.org/motm/62
- https://www.jrc.ac.in/working_folder/DOWNLOAD-D-11-53-5F0F117D553FB.pdf
- https://www.microbiologybook.org/bowers/mhc.htm
- https://www.azolifesciences.com/article/What-is-the-Function-of-MHC-Molecules.aspx
- https://www.onlinebiologynotes.com/major-histocompatibility-complex-mhc-structure-types-and-functions/
- https://immunobites.com/2018/07/23/what-is-mhc-and-why-does-it-matter/
- https://www.vedantu.com/biology/major-histocompatibility-complex
- https://hmmcollege.ac.in/uploads/Study_Materailfor_CC10.pdf
- https://med.seu.edu.cn/_upload/article/files/4c/37/03879d2643f0a3ee510a3f684c84/4e155996-3728-4951-8d6f-2aa6ef0c4f6f.pdf
- https://microbeonline.com/difference-mhc-class-mhc-class-ii-proteins/
- https://www.biologydiscussion.com/immunology/major-histocompatibility-complex-mhc-molecule-microbiology/66216
- https://www.immunopaedia.org.za/immunology/basics/4-mhc-antigen-presentation/
- https://www.uptodate.com/contents/major-histocompatibility-complex-mhc-structure-and-function
- https://www.news-medical.net/life-sciences/Functions-of-MHC-in-the-Immune-System.aspx
- https://microbenotes.com/mhc-molecules/
- https://en.wikipedia.org/wiki/Major_histocompatibility_complex