What is Antigen?
- Antigens are molecules or molecular structures that are foreign to the body and can trigger an immune response. They can be anything that doesn’t belong to the body and are considered foreign. While all antigens may not induce an immune response, those that do are called immunogens.
- The ability of antigens to elicit an immune response depends on specific regions called antigenic determinants. These determinants bind to receptor molecules on immune cells that have a complementary structure, thereby initiating a response. Antigens can take various forms, such as pollen, viruses, chemicals, or bacteria, and are denoted by the term ‘Ag’.
- The concept of antigens emerged from the fact that our body can distinguish between its own components and foreign particles. When antigens enter the body, the immune system triggers the production of antibodies that act against the antigens. Most antigens in humans are proteins, peptides, or polysaccharides, although lipids and nucleic acids can also act as antigens when combined with proteins or polysaccharides.
- In the field of immunology, an antigen refers to a molecule, moiety, foreign particulate matter, or an allergen that can bind to a specific antibody or T-cell receptor. Antigens can be found on normal cells, cancer cells, parasites, viruses, fungi, and bacteria. They are recognized by antigen receptors, including antibodies and T-cell receptors, which are produced by cells of the immune system. Each immune cell has specificity for a single antigen. Upon exposure to an antigen, only the lymphocytes that recognize it are activated and expanded through a process called clonal selection.
- Antibodies are usually specific to a particular antigen, meaning they can only react to and bind one specific antigen. However, in some cases, antibodies may cross-react and bind to multiple antigens. This interaction between an antigen and an antibody is known as the antigen-antibody reaction.
- Antigens can originate from within the body (self-antigens) or from the external environment (non-self). The immune system identifies and attacks non-self antigens, while typically avoiding a response to self-antigens due to negative selection of T cells and B cells. When antibodies react to self-antigens and damage the body’s own cells, it leads to autoimmune diseases.
- Vaccines are examples of antigens in an immunogenic form intentionally introduced into the body. They aim to induce the adaptive immune system’s memory function against specific antigens from pathogens. By administering vaccines, such as the seasonal influenza vaccine, the body can mount an effective immune response when exposed to the corresponding antigens, helping prevent illness.
Antigen Definition
An antigen is a foreign molecule or substance that triggers an immune response in the body, leading to the production of antibodies.
or
An antigen is a substance that can stimulate an immune response in the body. It can be a molecule or a part of a molecule, such as a protein, carbohydrate, or lipid, that is recognized by the immune system as foreign.
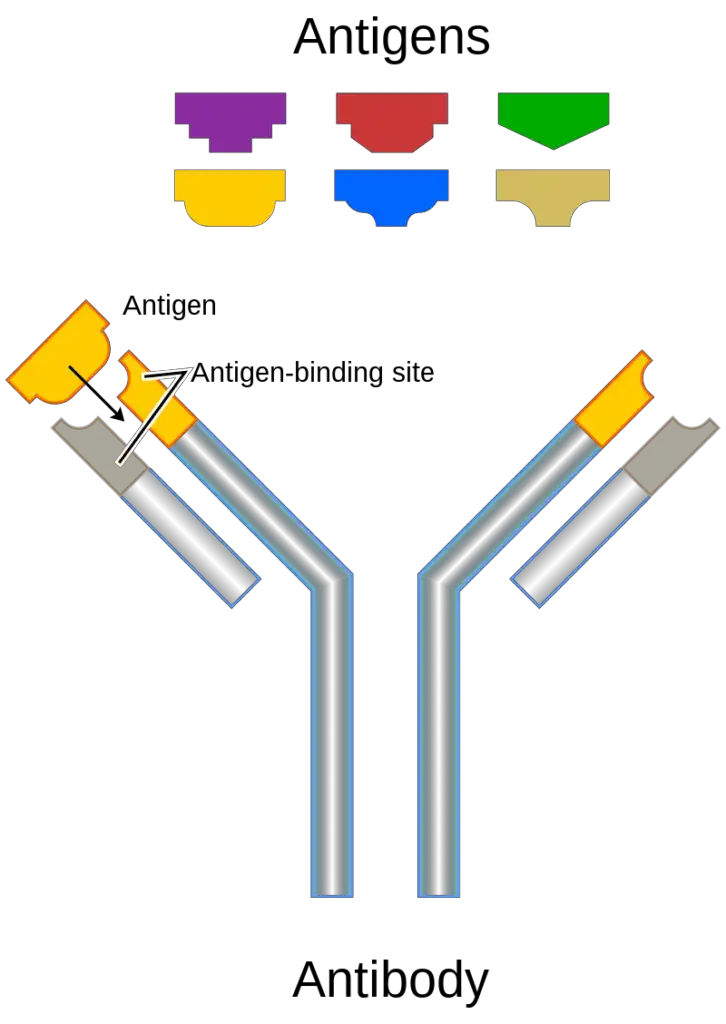
Properties of Antigens
Antigens have distinct properties that determine their immunogenicity and are therefore essential for comprehending the immune response against them. Due to the fact that these properties determine immunogenicity, they are considered necessary for an effective antigen. The following are characteristics of antigens:
1. Foreign Nature
- Antigens are characterized by their foreign nature to the body. All antigens that elicit an immune response in the host are considered foreign and distinct from the normal components of the body. The host body recognizes these antigens as non-self.
- The immunogenicity of an antigen, or its ability to provoke an immune response, increases as its degree of foreignness increases. In the case of biological antigens, the level of foreignness rises with the phylogenetic gap between the species involved. In other words, the more distantly related two species are, the more likely an immune response will be triggered by the antigens.
- However, there are exceptions to this general rule. Some proteins produced within the host’s body, known as autoantigens, can also induce an immune response. In such cases, the immune system mistakenly recognizes self-proteins as foreign and mounts an immune response against them, leading to autoimmune diseases.
- Conversely, not all proteins and molecules from other species necessarily induce an immune response. For an antigen to provoke an immune reaction, it must possess specific antigenic determinants or epitopes that can bind to receptors on immune cells. If a foreign substance lacks these antigenic determinants, it may not elicit an immune response.
- In summary, the foreign nature of antigens refers to their distinction from the body’s own components. The immunogenicity of an antigen increases with its degree of foreignness, although there are exceptions such as autoantigens. The presence of antigenic determinants is crucial for an antigen to induce an immune response, while the absence of these determinants may prevent an immune reaction, even if the substance is from another species.
2. Chemical Nature
- The chemical nature of antigens varies depending on the molecule involved. Proteins are considered the most potent and commonly encountered antigens. They possess complex structures and diverse amino acid sequences, making them highly immunogenic.
- Polysaccharides, which are chains of simple sugars, are also capable of acting as antigens. However, their immunogenicity is generally lower compared to proteins.
- In addition to proteins and polysaccharides, other molecules such as lipids and nucleic acids can also serve as antigens. However, they usually act as antigens when they are complexed with proteins or polysaccharides, forming antigenic conjugates.
- For proteins to function as antigens, they should contain immunogenic regions. These regions typically consist of amino acids such as lysine, glutamine, arginine, glutamic acid, asparagine, and aspartic acid. Moreover, these immunogenic regions should make up at least 30% of the protein’s amino acid composition. Additionally, proteins with a high number of hydrophilic or charged groups tend to be more immunogenic.
- The level of immunogenicity can also be influenced by the heterogeneity of the molecules. Generally, heteropolymers, which consist of different types of subunits, are more immunogenic compared to homopolymers, which consist of repeated subunits of the same type.
- In summary, the chemical nature of antigens encompasses proteins as the most potent antigens, followed by polysaccharides. Lipids and nucleic acids can act as antigens when complexed with proteins or polysaccharides. The immunogenicity of proteins depends on the presence of specific immunogenic regions, amino acid composition, and the abundance of hydrophilic or charged groups. Heterogeneous molecules generally exhibit higher immunogenicity compared to homopolymers.
3. Molecular Size
- The molecular size of antigens plays a significant role in their immunogenicity. It has been established that antigens should have a minimum size greater than 5000 Da (Daltons) to be considered immunogenic. This size threshold is necessary to provide an adequate number of epitopes or antigenic determinants that can interact with immune receptors.
- However, low molecular weight substances, known as haptens, can still demonstrate immunogenicity when coupled with larger carrier molecules. Haptens are considered “partial antigens” because they have at least one antigenic determinant or epitope, but they lack the necessary size and complexity to independently trigger an immune response.
- When haptens bind to carrier molecules, such as proteins or polysaccharides, they form hapten-carrier conjugates. The carrier molecule provides the necessary size and complexity, allowing the hapten to be recognized by the immune system and elicit an immune response. This process is known as hapten-carrier complex formation.
- The immunogenicity of these hapten-carrier conjugates depends on the ability of the carrier molecule to induce an immune response, while the hapten serves as the specific antigenic determinant. The immune system recognizes and responds to the antigenic determinant presented by the hapten-carrier complex.
- In summary, the molecular size of antigens is an important factor in their immunogenicity. Antigens should generally have a size greater than 5000 Da to be considered immunogenic. However, low molecular weight substances, known as haptens, can still demonstrate immunogenicity when coupled with larger carrier molecules. Haptens serve as partial antigens and require carrier molecules to provide the necessary size and complexity for immune recognition and response.
4. Molecular Rigidity and Complexity
- The rigidity and complexity of molecules are important factors in determining their immunogenicity, or their ability to induce an immune response.
- Rigid molecules are generally considered good antigens because they can effectively raise antibodies against specific structures. The rigidity of a molecule allows it to maintain a stable and well-defined conformation, which is crucial for immune recognition. When a rigid molecule interacts with the immune system, it can more effectively stimulate the production of antibodies that target specific regions or epitopes on the molecule’s surface.
- On the other hand, less rigid molecules may have a more flexible and dynamic structure, making it challenging for the immune system to recognize specific epitopes. This can result in a weaker or less targeted immune response.
- In addition to rigidity, the complexity of a molecule also influences its immunogenicity. A molecule with a complex structure has a higher likelihood of containing multiple antigenic determinants or epitopes. These epitopes are regions on the molecule that can specifically bind to immune receptors, triggering an immune response. The presence of multiple epitopes enhances the probability of successful immune recognition and response.
- In contrast, a peptide antigen with a repeating unit of a single amino acid is considered less immunogenic. This is because the repeated structure lacks the complexity required to engage the immune system effectively. A molecule with two or more repeating units of different amino acids, however, offers increased complexity, which can lead to a more robust immune response.
- In summary, the rigidity and complexity of molecules are important factors in determining their immunogenicity. Rigid molecules tend to be more immunogenic as they can raise antibodies against specific structures. The complexity of a molecule, including the presence of multiple epitopes, enhances its immunogenicity. A molecule with a repetitive structure is generally less immunogenic compared to a molecule with a diverse and complex structure.
5. Antigenic Determinants and Cross-reactivity
- Antigenic determinants, also known as epitopes, are specific regions within an antigen molecule that are involved in the interaction with antibodies. These determinants are recognized by the immune system and trigger the production of antibodies that bind to them.
- Antigens that possess two or more antigenic determinants are generally capable of inducing antibody production. This is because multiple determinants provide a greater likelihood of immune recognition, leading to the generation of a diverse range of antibodies that can bind to different epitopes on the antigen. In contrast, smaller antigens with only a single antigenic determinant may have a lower immunogenicity, as they lack the structural complexity required to stimulate a robust antibody response.
- Cross-reactivity is another important aspect of antigen-antibody interactions. Cross-reactivity occurs when antibodies generated in response to one antigen can also recognize and bind to a different antigen. This phenomenon arises when the antigens share common or similar antigenic determinants. As a result, antibodies produced against one antigen may exhibit a degree of binding affinity for another antigen with related determinants.
- Cross-reactivity can be advantageous in certain situations. For example, if a pathogen expresses antigens similar to those of another pathogen previously encountered by the immune system, the preexisting antibodies may be able to recognize and neutralize the new pathogen to some extent. However, cross-reactivity can also have unintended consequences, leading to autoimmune reactions or allergies, where the immune system mistakenly targets self-antigens or harmless substances.
- In summary, antigenic determinants are specific regions within an antigen molecule that interact with antibodies, triggering an immune response. Antigens with multiple determinants are more likely to induce antibody production. Cross-reactivity occurs when antibodies generated against one antigen can also recognize and bind to a different antigen due to shared or similar determinants. While cross-reactivity can be beneficial in certain contexts, it can also lead to immune-related complications.
Properties of Antigens Summery
Antigens exhibit various properties that significantly influence their immunogenicity, determining the response they evoke from the immune system. These properties include:
- Foreignness: Antigens possess the ability to differentiate between self and non-self molecules. Self molecules are not recognized as immunogenic, while non-self molecules are identified as foreign and trigger an immune response.
- Size: Immunogenic molecules are typically large and complex, with a molecular weight of around 10,000. Weak immunogens have low immunogenicity, while extremely small molecules like amino acids are non-immunogenic on their own. However, when amino acids are bound to a carrier protein, they can become immunogenic.
- Structural Complexity: Antigens require a certain level of chemical complexity and structural diversity to induce an effective immune response. Larger proteins, lipopolysaccharides, and glycolipids with multiple epitopes (antigenic determinants) provoke stronger immune responses compared to small peptides with only one or a few epitopes. For example, amino acids are composed of homopolymers with limited epitopes, making them less immunogenic, whereas heteropolymers containing various amino acids exhibit multiple epitopes, resulting in increased immunogenicity.
- Chemical Complexity: Immunogenic molecules need to be enzymatically cleavable by phagocytes. Polypeptides containing L-amino acids are highly immunogenic since proteolytic enzymes can cleave them effectively. Conversely, polypeptides containing O-amino acids are poor immunogens as the proteolytic enzymes can only cleave L-amino acids. Various molecules, including carbohydrates, steroids, and lipids, can serve as immunogens, while haptens (small molecules incapable of inducing an immune response on their own) and amino acids are entirely non-immunogenic.
- Genetic Constitution of the Host: Different strains of the same animal species can exhibit varying responses to identical antigens due to variations in the genes involved in the immune response. Major Histocompatibility Complex (MHC) alleles, for instance, differ between individuals and influence their immune reactions.
- Dosage, Route, and Timing of Antigen Administration: The magnitude of the immune response largely depends on the quantity of antigen administered. Optimizing the immune response involves adjusting the dosage, route of administration, and timing of antigen exposure. Adjuvants, substances that enhance immune responses by facilitating the delivery of antigens to Antigen-Presenting Cells (APCs), can be used to augment immunogenicity.
Understanding these properties of antigens is crucial for designing effective vaccines, diagnostics, and therapeutic interventions that manipulate immune responses for beneficial outcomes.
Antigen Structure
- The structure of an antigen refers to its molecular characteristics that enable it to bind to the antigen-binding site of an antibody. Antibodies are capable of distinguishing between different antigens based on the specific molecular structures present on the surface of the antigen.
- Antigens can be proteins, polysaccharides, lipids, or nucleic acids, depending on their nature and origin. Proteins and polysaccharides are commonly encountered antigens and can include various components of bacteria, viruses, or other microorganisms, such as coats, capsules, flagella, toxins, fimbriae, secretions, and chemicals. Lipids and nucleic acids from microorganisms are typically antigenic when they are combined with proteins or polysaccharides.
- The structure of antigens can vary depending on their size, immunogenicity, and specific characteristics. All immunogenic antigens possess epitopes or antigenic determinants, which are specific structural components that can be recognized by antibodies. Each antigen can have multiple epitopes, and the number of epitopes determines how many antibodies can bind to a single antigen.
- The interaction between antigens and antibodies is based on specific structural components. The region on an antibody that interacts with antigens is called a paratope, while the corresponding region on the antigen is called an epitope. The interaction between epitopes and paratopes can be likened to a lock and key mechanism, as the structures are specific and fit together.
- Antigens are recognized by antibodies according to this lock and key mechanism, leading to the immune response against the antigen. The ability of the immune system to recognize and act against disease-causing agents and antigens is termed immunity, which can be either innate or acquired through vaccinations.
- It’s important to note that antigens can also be categorized as haptens when they have a lower molecular weight and are not independently immunogenic. Haptens become antigenic when they are attached to a carrier molecule. In such cases, the antibody response is directed specifically towards the hapten portion of the complex.
- In summary, the structure of an antigen refers to its molecular characteristics that allow it to interact with antibodies. Antigens can be proteins, polysaccharides, lipids, or nucleic acids, with proteins and polysaccharides being the most common. Antigens possess specific epitopes or antigenic determinants that are recognized by antibodies. The interaction between antigens and antibodies is based on a lock and key mechanism, and the immune response is triggered when antibodies bind to the specific epitopes on the antigen’s surface.
Characteristics/ Determinants of Antigenicity/ Factor affecting the Antigenicity of Antigen
Several factors have been identified that contribute to the immunogenicity of a drug. Among the most important antigenicity determinants are: 1. Molecular size 2. Extraterritoriality 3. Chemical-structural complexity 4. Stability 5. Other variables
The Nature of the Immunogen Contributes to Immunogenicity
1. Molecular Size
- Protein molecules with a high molecular weight are typically highly allergenic.
- In general, compounds having molecular weights of less than 5000 Da are not immunogenic.
- Using high molecular weight proteins such as bovine gamma globulin (MW 150,000 Da) to elicit an immunological response has been utilised to leverage this characteristic in experimental research.
- Low-molecular-weight substances can be rendered antigenic by adsorption on carrier particles such as bentonite, kaolin, and other inert particles.
2. Foreignness
- A molecule must be recognised as nonself, or foreign, in order to be immunogenic.
- The immune system classifies the molecule as self or nonself depending on whether or not it was exposed to the immune system during foetal development.
- Foreignness entails the host’s capacity to accept selfantigens.
- Tolerance to self-antigens develops by contact with them throughout the early stages of immune system development, namely during lymphocyte development.
- The less closely two species are related, the greater the immunogenicity of a molecule from one species when exposed to the other.
- The bovine serum albumin, for instance, is more immunogenic in chickens than in goats.
- A graft from an unrelated human will be rejected within two weeks if immunosuppressive medicines are not utilised, however a chimpanzee graft will be rejected within hours regardless of the use of drugs.
- In contrast, a kidney transplant from an identical twin is readily approved.
3. Chemical-Structural Complexity
- The most potent immunogens are proteins, followed by polysaccharides.
- Although they may serve as haptens, nucleic acids and lipids are ineffective at inducing a strong immunological response.
- Complexity of a protein’s structure contributes to its immunogenicity.
- Single-amino acid or single-sugar chains are not very immunogenic, but when many amino acids or sugars are joined inside the same molecule, the immunogenicity is substantially boosted.
- In cell-mediated immunity, the response of T cells to the peptide component of proteins is dependent on the manner in which the peptide is detected and presented by MHC cells.
- Consequently, the shape of a protein plays a significant impact in its immunogenicity, particularly in eliciting cellular immunity.
- Since lipid-specific antibodies are difficult to manufacture, they do not play a significant role in immunity.
- Nonetheless, these antibodies play an important role in the assessment of particular lipid-based compounds and medications.
- These antibodies are created by first treating lipids with haptens, followed by conjugation with appropriate carrier molecules, such as proteins (e.g., hemocyanin or bovine serum albumin).
4. Stability
- Immunogenic are not things that are highly stable and nondegradable, such as some polymers, metals, or chains of D-amino acids.
- This is due to the fact that internalisation, processing, and presentation by antigen-presenting cells (APCs) are always required for an immune response to be mounted.
- Therefore, extremely stable compounds (such as silicon) have been effective as nonimmunogenic materials for reconstructive procedures, such as breast augmentations.
- In contrast, if a chemical is extremely unstable, it may degrade before an APC can internalise it, hence becoming immunogenic.
- Large, insoluble complexes are also more immunogenic than smaller, soluble ones. This is because macrophages find insoluble complexes easier to phagocytose, breakdown, and present than soluble ones.
5. Lipids As Antigens
- Lipoidal antigens that are appropriately delivered can stimulate B- and T-cell responses. Lipids are employed as haptens and coupled to suitable carrier molecules, such as the proteins keyhole limpet hemocyanin (KLH) or bovine serum albumin, for the stimulation of B-cell responses (BSA).
- By immunising with these lipid-protein conjugates, antibodies that are highly specific for the target lipids can be produced.
- Using this method, antibodies against a wide range of lipid compounds, including steroids, complex fatty-acid derivatives, and fat-soluble vitamins such as vitamin E, have been produced. Numerous clinical assays for the presence and quantities of medically significant lipids are antibody-based, hence these antibodies are of great practical value.
- A determination of the amounts of leukotrienes, a complicated group of lipids, can be helpful in evaluating asthma patients, for instance. Prednisone, an immunosuppressive steroid, is frequently used as part of the endeavour to prevent organ rejection.
6. Susceptibility To Antigen Processing And Presentation
- T cells must engage with antigen that has been processed and presented in conjunction with MHC molecules for the establishment of both humoral and cell-mediated immune responses.
- Large, insoluble macromolecules are often more immunogenic than small, soluble macromolecules, as the larger molecules are more readily phagocytosed and digested.
- Immunogens that cannot be destroyed and delivered to MHC molecules are ineffective. This is exemplified by polymers of D-amino acids, which are stereoisomers of naturally occurring L-amino acids.
- Due to the fact that the degradative enzymes within antigen-presenting cells can only degrade proteins containing L-amino acids, polymers of D-amino acids cannot be digested and are therefore ineffective immunogens.
The Biological System Contributes to Immunogenicity
1. Biological system
- In determining the immunological effectiveness of an antigen, biological system also plays a crucial role.
- Certain chemicals are immunogenic in some people but not others (i.e., responders and nonresponders). Individuals may lack or have altered genes that code for antigen receptors on B cells and T cells, or they may lack the genes required for APC to transmit antigen to helper T (TH) cells.
2. Immunogen Dosage And Route Of Administration
- Antigen immunogenicity is also affected by the antigen’s dosage and the method through which it contacts the immune system.
- Extremely low antigen doses do not activate immunological response, either because too few lymphocytes are touched or because a nonresponsive condition is induced.
- In contrast, an exceedingly high dose does not induce tolerance. It may be necessary to administer antigens repeatedly (booster doses) to improve the immunological response of the host to certain antigens.
- In the case of vaccines, when a minimum degree of immunity must be obtained, this is of special significance. To ensure adequate levels of protective antibodies, booster doses of vaccines such as DPT (Diphtheria, Pertussis, Tetanus), DT (Diphtheria, Tetanus), etc. are administered.
- Antigens are typically delivered parenterally in order to create enough levels of antibodies.
- Antigens can be administered intravenously, subcutaneously, intradermally, intramuscularly, intraperitoneally, and mucosally.
- Typically, subcutaneous injection is superior to intravenous treatment for triggering an immunological response.
3. Adjuvants
Adjuvants are chemicals that, when combined with an antigen and injected with it, enhance the antigen’s immunogenicity. Adjuvants enhance the intensity and persistence of an immunological response. In numerous ways, adjuvants augment the immunogenicity of antigens:
- By establishing a depot at the injection site, adjuvants such as aluminium potassium sulphate (alum) and Freund’s water-in-oil adjuvant prolong the persistence of antigen. Alumprecipitates the antigen and gradually releases it. The water-in-oil emulsion generates antigen-containing droplets that are slowly released over time.
- In addition to emulsifying components, Freund’s complete adjuvant contains heat-killed mycobacteria. The bacterial components stimulate macrophages and boost the immunological response by increasing the synthesis of IL-1 and the concentration of B7 membrane molecules. The increased production of class II MHC enhances APC’s capacity to deliver antigen to TH cells. B7 molecules on the APC attach to CD28, a protein on the cell surface of TH cells, inducing costimulation, an increase of the T-cell immunological response.
- Some adjuvants, such as synthetic polyribonucleotides and bacterial lipopolysaccharides, induce nonspecific lymphocyte proliferation and action.
4. Genotype Of The Recipient Animal
- The genetic makeup (genotype) of an inoculated animal determines both the type and intensity of the immunological response the animal displays.
- Hugh McDevitt demonstrated, for instance, that two distinct inbred mouse strains respond quite differently to a synthetic polypeptide immunogen.
- One strain produced large quantities of serum antibody after exposure to the immunogen, while the other strain produced modest levels.
- When the two strains were crossed, the F1 generation exhibited a moderate immunogenic response.
- The gene governing immunological response was localised to a subregion of the major histocompatibility complex by backcross research (MHC).
- Numerous trials with simply specified immunogens have proven genetic control of immunological responsiveness, which is predominantly confined to MHC genes.
- These findings suggest that MHC gene products, which serve to present processed antigen to T cells, play a crucial role in regulating the degree to which an animal responds to an immunogen.
- The response of an animal to an antigen is also affected by genes encoding B-cell and T-cell receptors as well as genes encoding proteins involved in immune regulation systems.
- The immunogenicity of a specific macromolecule varies among animals based on the genetic diversity of these genes.
Specificity of Antigen
1. Antigenic Specificity
The antigen’s antigenic specificity is determined by antigenic determinants or epitopes.
Epitopes
- A portion of an immunogen that binds to antigen-specific membrane receptors on lymphocytes or released antibodies is referred to as an epitope.
- The interaction between immune system cells and antigens occurs on multiple levels, and the complexity of an antigen is reflected in its epitope.
- B-cell epitopes and T-cell epitopes are the two types of epitopes.
a. B-cell epitopes
- B-cell epitopes are antigenic determinants recognised by B lymphocytes. Only if the antigen molecule is in its original state can B-cell epitope connect with its receptor.
- It would appear that the corresponding surfaces of the antibody and antigen molecules are generally flat.
- Typically, smaller molecules fit snugly within a specific depression or groove in the antigen-binding site of an antibody molecule.
- The length of the B-cell epitope is approximately six or seven sugar residues or amino acids. B-cell epitopes are typically hydrophilic and frequently situated at protein structural bends.
- They are also frequently located in areas of proteins that are more mobile; this may allow an epitope to shift slightly to fit into a nearly-perfect location.
b. T-cell epitopes
- T cells detect amino acids in proteins but do not recognise polysaccharide or nucleic acid antigens.
- This is why polysaccharides are regarded T-independent antigens whereas proteins are called T-dependent antigens.
- Antigenic determinants identified by T cells are established by the main sequence of amino acids in proteins.
- T cells do not identify free peptides, but they do recognise the complex of MHC molecules and peptide.
- In order for a T-cell response to occur, the T-cell must recognise both the antigenic determinant and the MHC; consequently, it is MHC restricted.
- In general, the length of T-cell epitopes or antigenic determinants is between 8 and 15 amino acids.
- Antigenic determinants are restricted to antigen regions that can bind to MHC molecules.
- Given that MHC molecules are vulnerable to genetic diversity, T-cell responses to the same stimuli can vary between individuals. Each MHC molecule can bind numerous peptides, but not all.
- In order for a peptide to be immunogenic in a certain individual, that individual must possess MHC molecules capable of binding to it.
Processing of an antigen by APCs
Antigen processing by APCs is extremely important for T cell recognition. Two distinct methods of processing can prepare a protein antigen for presentation as an antigen. These consist of:
- Processing externally generated antigens: In this process, phagocytic cells, such as macrophages, destroy and lyse phagocytosed microorganisms. The bacterium fragments are subsequently given within the framework of class II MHC molecules.
- Processing of endogenously produced antigens: In this step, viral proteins created within a cell are processed and displayed in the context of class I MHC molecules.
2. Species Specificity
Certain species-specific antigens are present in the tissues of every member of a species. However, there is some cross-reactivity across antigens from closely related species. The specificity of the species indicates phylogenetic relationship. The phylogenetic relationship is useful for:
- tracing species’ evolutionary relationships.
- In forensic medicine, the identification of species from blood and seminal fluid stains.
3. Isospecificity
Isospecificity is determined by the presence of isoantigens or histocompatibility antigens.
Isoantigens
- Isoantigens are antigens found in a subset of a species’ members.
- A species can be classified based on the existence of distinct isoantigens in its individuals.
- These are decided genetically. Human erythrocyte antigens, which are used to classify individuals into various blood groups, are the best examples of isoantigens in humans.
- The blood types play a crucial role in:
- Blood transfusion and transfusion of blood products.
- Isoimmunization during pregnancy.
- Providing crucial evidence in paternity disputes, the results of more modern DNA fingerprinting tests enhance these results.
Histocompatibility
- Antigens Histocompatibility antigens are the species-specific biological determinants for each individual.
- These antigens are connected with tissue cell plasma membranes.
- Human leukocyte antigen (HLA) is the primary histocompatibility antigen that determines the rejection of a homograft.
- Before transplanting tissue or organs from one individual to another, HLA typing is therefore critically necessary.
4. Autospecificity
- In general, self-antigens are not antigenic. Sequestered antigens (such as protein from the eye lens and sperm) are exceptions, as they are not identified as selfantigens.
- This is because the immune system never encounters ocular tissue or sperm during the establishment of self-antigen tolerance.
- Therefore, if these tissues are unintentionally or experimentally discharged into the blood or tissues, they become immunogenic.
5. Organ Specificity
- Antigens that are specific to a particular organ or tissue are known as organ-specific antigens. The antigen specificity of these antigens discovered in the brain, kidney, and lens tissues of diverse animal species is identical.
- Antigens unique to organs, such as brain-specific antigens, are shared by the human and sheep brains.
- When administered, antirabies vaccinations made from sheep brain may provoke an immunological response in some humans, resulting in harm to the recipient’s neural structures.
- Some individuals may get neuroparalytic problems as a result.
6. Heterophile Specificity
- The presence of heterophile antigens determines the specificity of heterophiles. Heterophile antigens are identical or closely related antigens that are sometimes found in the tissues of distinct biological species, groups, or kingdoms.
- Antibodies against heterophile antigens produced by one species react with antigens produced by other species.
- This characteristic is utilised for the diagnosis of numerous infectious disorders. Examples of serological assays that involve heterophile antigens include the Weil–Felix reaction, the Paul-Bunnell test, and cold agglutination tests.
Types of Antigens
Types of Antigens On the basis of Origin
Different types of antigens exist based on their origin:
1. Exogenous Antigens
- Exogenous antigens are antigens that originate outside the body of the host and are considered foreign to the host’s immune system. These antigens can enter the body through various routes such as inhalation, ingestion, or injection. Once inside the body, exogenous antigens can circulate through bodily fluids, including blood and lymphatic vessels.
- The uptake of exogenous antigens is primarily mediated by specialized cells called Antigen Processing Cells (APCs), which include macrophages, dendritic cells, and B cells. These APCs play a crucial role in capturing, processing, and presenting exogenous antigens to the immune system for recognition and response. Upon encountering an exogenous antigen, APCs engulf the antigen through a process called phagocytosis.
- After phagocytosis, the exogenous antigens are broken down into smaller fragments within the APCs. These fragments, also known as antigenic peptides, are then presented on the surface of the APCs using a specialized protein called Major Histocompatibility Complex (MHC) molecules. This process is known as antigen presentation.
- The presentation of exogenous antigens by APCs is vital for the activation of other immune cells, such as T cells. T cells recognize the antigenic peptides displayed on the MHC molecules and initiate immune responses accordingly. This recognition triggers the proliferation and differentiation of T cells, leading to the production of effector cells that can eliminate the antigen.
- It is worth noting that some antigens, like intracellular viruses, can initially enter the body as exogenous antigens but later become endogenous. This occurs when the virus infects host cells and replicates within them, resulting in the generation of endogenous antigens. Endogenous antigens are antigens that originate from within the body’s own cells.
- In summary, exogenous antigens are antigens that originate outside the host’s body and enter through various routes. They are taken up by Antigen Processing Cells (APCs) through phagocytosis and presented to the immune system for recognition. This process plays a crucial role in initiating immune responses against foreign substances and pathogens. Some antigens may transition from being exogenous to endogenous as they infect host cells and replicate within them.
2. Endogenous Antigens
- Endogenous antigens are antigens that originate within the body of the host. They can arise during normal cellular metabolism or as a result of intracellular viral or bacterial infections. Unlike exogenous antigens that enter the body from external sources, endogenous antigens are derived from the cells of the body itself or from the products of cellular metabolism.
- Endogenous antigens are typically processed within specialized immune cells called macrophages. These cells break down the endogenous antigens into smaller fragments and present them on their cell surface using Major Histocompatibility Complex (MHC) molecules. These MHC molecules display the antigenic peptides, allowing them to be recognized by cytotoxic T-cells.
- Cytotoxic T-cells, also known as CD8+ T cells, play a crucial role in the immune response against endogenous antigens. These T-cells have receptors that can specifically recognize the antigenic peptides presented on MHC molecules. Once activated, cytotoxic T-cells can directly target and eliminate cells displaying the specific antigenic peptides. This is particularly important for combating viral or bacterial infections that have infiltrated host cells.
- Endogenous antigens encompass different types based on their origin and relationship to the host. Xenogenic or heterologous endogenous antigens are derived from other species, such as certain transplanted tissues or organs. Autologous endogenous antigens are self-antigens produced by the body’s own cells. Idiotype or allogenic endogenous antigens are antigens produced by other individuals of the same species.
- While the immune system has mechanisms to distinguish self-antigens from foreign antigens, there are instances where the immune system may mistakenly recognize self-antigens as immunogenic. This can lead to autoimmune diseases, where the immune system mounts an immune response against its own cells and tissues. In autoimmune diseases, endogenous antigens are incorrectly recognized as foreign, triggering an immune response that can result in tissue damage and inflammation.
- In summary, endogenous antigens are antigens that originate within the body, either during normal cellular metabolism or as a result of intracellular infections. They are processed and presented by macrophages and recognized by cytotoxic T-cells. Endogenous antigens include self-antigens, antigens from other species, and antigens from other individuals of the same species. While the immune system generally distinguishes self-antigens from foreign antigens, autoimmune diseases can occur when self-antigens are mistakenly recognized as immunogenic, leading to an immune response against the body’s own cells.
3. Autoantigens
- Autoantigens are proteins or protein complexes that are naturally present in the host’s own cells. However, in the context of autoimmune diseases, these autoantigens are mistakenly recognized as foreign or harmful by the immune system, leading to an immune response against the body’s own tissues. This immune response directed towards self-antigens can cause tissue damage and inflammation, characteristic of autoimmune diseases.
- The presence of autoantigens alone does not lead to autoimmune diseases. Normally, the immune system has mechanisms in place to recognize and tolerate self-antigens, preventing an immune response against them. However, in autoimmune diseases, the immune system loses its tolerance to specific autoantigens, resulting in the immune system attacking the body’s own cells and tissues.
- The loss of immunological tolerance to autoantigens can occur due to a combination of genetic and environmental factors. Genetic predisposition plays a role in determining an individual’s susceptibility to developing autoimmune diseases. Certain genes involved in immune regulation and self-tolerance can contribute to an increased risk of autoimmune disorders.
- Environmental factors, such as infections, exposure to certain chemicals or drugs, hormonal imbalances, and other external triggers, can also influence the development of autoimmune diseases. These factors can disrupt the normal immune tolerance mechanisms, leading to the immune system mistakenly recognizing autoantigens as foreign and initiating an immune response against them.
- Autoimmune diseases can affect various organs and tissues in the body, depending on the specific autoantigens involved. Examples of autoimmune diseases include rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, type 1 diabetes, and Hashimoto’s thyroiditis, among others.
- Managing autoimmune diseases typically involves controlling the immune response and reducing inflammation to alleviate symptoms and slow down disease progression. This is often achieved through immunosuppressive medications that dampen the immune system’s activity. Understanding the specific autoantigens involved in an autoimmune disease can aid in developing targeted therapies that aim to restore immune tolerance or modulate the immune response.
- In conclusion, autoantigens are proteins or protein complexes present in the body’s own cells. In autoimmune diseases, these autoantigens are mistakenly recognized as foreign by the immune system, leading to an immune response against the body’s own tissues. The loss of immunological tolerance to autoantigens is influenced by both genetic and environmental factors. Autoimmune diseases can result in chronic inflammation and tissue damage. Managing these conditions involves modulating the immune response and reducing inflammation to alleviate symptoms and prevent further damage.
4. Tumour Antigens
- Tumor antigens, also known as neoantigens, are specific antigens that are expressed on the surface of tumor cells. They arise as a result of genetic mutations that occur during the development of cancer. These mutations lead to the production of abnormal proteins or protein fragments that are not present in normal, healthy cells.
- Neoantigens are presented to the immune system by the Major Histocompatibility Complex (MHC) molecules on the surface of tumor cells. MHC class I molecules present antigens to cytotoxic T cells, while MHC class II molecules present antigens to helper T cells. This antigen presentation is crucial for the immune system to recognize and mount an immune response against the tumor cells.
- However, tumor cells have developed various mechanisms to evade immune recognition and defense. They can downregulate the expression of MHC molecules or interfere with antigen processing and presentation pathways. This immune evasion allows tumor cells to escape detection and destruction by the immune system.
- Despite the immune evasion strategies employed by tumor cells, the immune system can still recognize and respond to tumor antigens in certain cases. Neoantigens derived from tumor-specific mutations have unique sequences that distinguish them from normal antigens, making them potential targets for immune responses.
- In recent years, advances in genomic sequencing technologies have allowed for the identification of neoantigens in individual tumors. This has led to the development of personalized cancer immunotherapies that aim to specifically target and activate the immune system against the neoantigens present in a patient’s tumor. These approaches, such as tumor-infiltrating lymphocyte therapy and personalized cancer vaccines, hold promise for enhancing the immune response against tumors and improving patient outcomes.
- In summary, tumor antigens or neoantigens are antigens that are expressed on the surface of tumor cells as a result of genetic mutations. These antigens can potentially elicit an immune response, but tumor cells have developed mechanisms to evade immune recognition and defense. Nonetheless, advancements in understanding and targeting neoantigens are paving the way for personalized cancer immunotherapies that aim to harness the immune system to selectively attack tumor cells.
5. Native Antigens
- Native antigens refer to antigens that are presented in their natural, unprocessed form without being processed by antigen-presenting cells (APCs) such as macrophages or dendritic cells. These antigens can directly interact with B cells, leading to the activation of the humoral immune response.
- Unlike T cells, which require antigen processing and presentation by APCs, B cells possess B cell receptors (BCRs) on their surface that can recognize and bind to native antigens. The BCRs are membrane-bound immunoglobulins, also known as antibodies, that are specific to particular epitopes or antigenic determinants on the surface of the native antigen.
- When a B cell encounters a native antigen that matches its BCR, the antigen binds to the BCR, triggering a series of signaling events within the B cell. This activation leads to the proliferation and differentiation of the B cell into plasma cells, which are specialized cells that produce large amounts of antibodies specific to the native antigen. These antibodies can then circulate in the bloodstream and other body fluids, targeting the native antigen for neutralization or elimination.
- Native antigens can be derived from various sources, including pathogens, foreign particles, or substances introduced into the body. Examples of native antigens include intact viruses, bacteria, toxins, or other molecules that are recognized as foreign by the immune system.
- It’s important to note that while B cells can directly interact with and respond to native antigens, the activation of T cells is primarily dependent on antigen processing and presentation by APCs. T cells recognize antigenic peptides that are derived from the processing of antigens by APCs and presented in the context of major histocompatibility complex (MHC) molecules.
- In summary, native antigens are antigens that can directly interact with B cells without requiring prior processing by APCs. B cell activation leads to the production of antibodies specific to the native antigen, contributing to the humoral immune response. T cells, on the other hand, generally require antigen processing and presentation by APCs to recognize and respond to antigens.
Types of Antigens On the Basis of Immune Response
Based on the immunological reaction, antigens are categorised as:
1. Complete antigens/ Immunogen
- Complete antigens, also known as immunogens, are substances that possess the ability to elicit a specific immune response in an organism. Unlike haptens, which require carrier molecules to induce an immune response, complete antigens have the capacity to stimulate the immune system on their own.
- Immunogens are typically composed of proteins, peptides, or polysaccharides, and they often have a molecular weight greater than 10,000 Daltons. This larger size allows them to be more easily recognized by the immune system and promotes their immunogenicity.
- When a complete antigen enters the body, it interacts with the cells of the immune system, such as B cells and T cells. B cells, through their membrane-bound antibodies (BCRs), can directly bind to the antigen if it contains epitopes that match their specific receptors. This binding initiates a cascade of events that lead to B cell activation, proliferation, and the production of antibodies. These antibodies, in turn, can target the antigen for neutralization or destruction.
- T cells, on the other hand, recognize antigenic peptides derived from the processing of complete antigens. Antigen-presenting cells, such as macrophages or dendritic cells, capture the antigen, break it down into smaller peptides, and present these peptides on their cell surface using major histocompatibility complex (MHC) molecules. T cells with specific receptors, called T cell receptors (TCRs), can recognize these antigenic peptides when presented by the MHC molecules, leading to T cell activation and the initiation of cellular immune responses.
- The immunogenicity of a complete antigen depends not only on its composition but also on other factors such as its structure, complexity, and foreignness to the host. The presence of specific antigenic determinants or epitopes on the antigen is crucial for interaction with immune receptors and the subsequent induction of an immune response.
- In summary, complete antigens or immunogens are capable of independently inducing an immune response without the need for carrier molecules. These antigens, often proteins, peptides, or polysaccharides, have a high molecular weight and possess specific antigenic determinants that interact with B cell receptors or are presented by antigen-presenting cells to T cells. Through these interactions, immunogens stimulate the production of antibodies and activate cellular immune responses, contributing to the body’s defense against foreign substances and pathogens.
2. Incomplete antigens/ Hapten
- Incomplete antigens, also known as haptens, are substances that are incapable of generating an immune response on their own. Unlike complete antigens, haptens require the assistance of a carrier molecule to become immunogenic and elicit an immune response.
- Haptens are typically non-protein substances with a relatively low molecular weight, usually less than 10,000 Daltons. Examples of haptens include small molecules like drugs, certain chemicals, or environmental allergens. Due to their small size and chemical nature, haptens do not possess enough antigenic determinants or epitopes to interact directly with immune receptors and trigger an immune response.
- To become immunogenic, haptens must bind to a larger carrier molecule, typically a protein or a polysaccharide. The carrier molecule acts as a scaffold or carrier for the hapten, enabling it to be recognized by the immune system. The carrier molecule provides the necessary antigenic determinants that can interact with immune receptors and initiate an immune response. The hapten, in this context, functions as a “non-antigenic” component and does not independently induce an immune response.
- When a hapten-carrier complex enters the body, the immune system recognizes the combined structure as a complete antigen. The carrier molecule, with its inherent antigenic determinants, provides the immunogenic properties necessary for an immune response to be triggered. Immune cells, such as B cells, can recognize the complete antigen and initiate the production of antibodies specifically targeting the hapten. These antibodies can bind to the hapten and form immune complexes, which can have various consequences depending on the specific circumstances.
- It is worth noting that while haptens themselves are incomplete antigens, they can still cause immune-mediated reactions when they bind to carrier molecules in the body. In some cases, these reactions can manifest as allergies or hypersensitivity responses.
- In summary, incomplete antigens or haptens are non-protein substances that lack the ability to generate an immune response on their own. They require a carrier molecule, usually a protein or a polysaccharide, to form a complete antigen and become immunogenic. The carrier molecule provides the necessary antigenic determinants, while the hapten functions as a non-antigenic component that binds to the carrier. Together, the hapten-carrier complex can stimulate an immune response, leading to the production of antibodies specifically targeting the hapten.
Antigen Presenting Cells (APCs)
Antigen-presenting cells are cells that can absorb antigen and provide pieces to T cells (APCs). In the body, there are three types of antigen-presenting cells: macrophages, dendritic cells, and B cells.
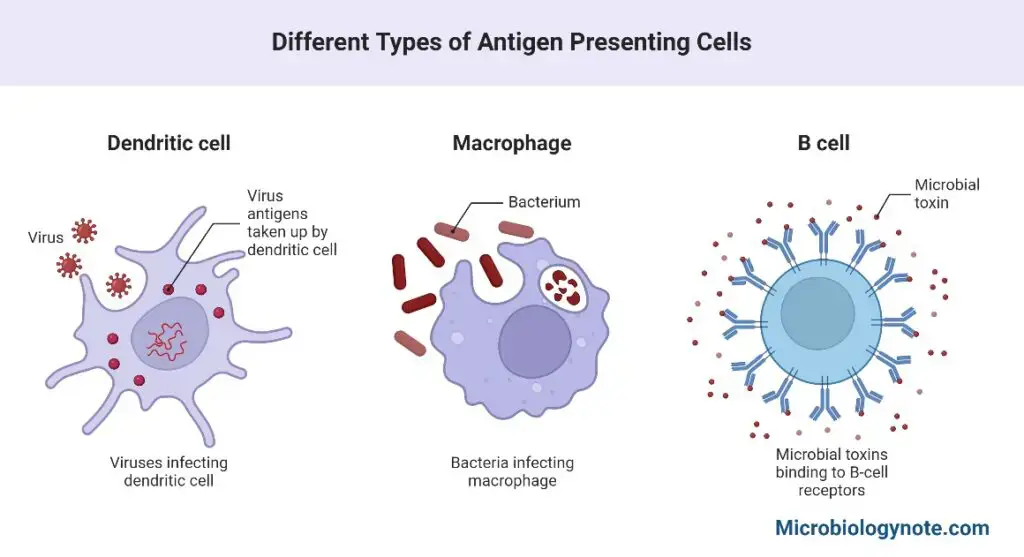
1. Macrophages
- Typically, macrophages are present in a resting state. When induced to become activated macrophages, their phagocytic capacities are significantly enhanced.
- Almost all lymphoid tissues contain macrophages alongside lymphocytes, such as monocytes as blood macrophages and histocytes as tissue macrophages.
2. Dendritic Cells
- These cells are distinguished by their lengthy cytoplasmic processes.
- Their principal job is to serve as very efficient antigen-capture and antigen-presentation cells.
- These cells are incapable of phagocytosis. The lymph nodes, spleen, thymus, and skin contain these cells.
- These are the various types of dendritic cells:
- Langerhan’s dendritic cells: The epidermis of skin contains dendritic cells of Langerhans that capture organisms coming into touch with the body surface.
- Dendritic cells in spleen: The spleen contains dendritic cells that capture the antigen in the blood.
- Follicular dendritic cells: In lymph nodes, follicular dendritic cells capture the antigen in the lymph.
- Therefore, macrophages and dendritic cells play a crucial role in the capture and presentation of antigens to T and B cells in order to trigger an immune response.
- Steinman received the 2011 Nobel Prize for his discovery of the dendritic cell and its function in adaptive immunity.
3. B-cells
- B-cells express intramembrane immunoglobulin (Ig) molecules that serve as V cell antigen receptors on their surface.
- Due to the fact that all the receptors on a V cell are identical, each V cell can only connect to a single antigen.
- This makes them significantly more effective antigen-presenting cells than macrophages, which must consume any foreign material they encounter.
- Descendants of V-cells (plasma cells) generate antibodies.
Antigen-antibody Complex Formation
- Antigen-antibody complex formation, also known as immunogenic complex formation, occurs when multiple antigens bind to antibodies, resulting in the formation of a complex molecule. The binding of antibodies and antigens is highly specific and is mediated by the interaction between epitopes on the antigen and paratopes on the antibody.
- Antibodies are able to recognize and fight against a wide range of pathogens due to their ability to distinguish between different antigens. The specificity of the interaction between antigens and antibodies is determined by the amino acid sequence in the epitope of the antigen and the paratope of the antibody, which forms a complementary fit.
- The formation of antigen-antibody complexes triggers various immune responses. These include complement deposition, which activates the complement system leading to inflammation, opsonization, which enhances phagocytosis of the complex by immune cells, and phagocytosis itself, where immune cells engulf and eliminate the complex.
- The shape and size of the immune complex depend on the ratio of antigen to antibody. The size of the complex, in turn, determines its effects within the immune system. Large immune complexes can activate immune cells and initiate immune responses, while smaller complexes may be eliminated more efficiently.
- Antigen-antibody complexes have been instrumental in understanding the interaction between antibodies and antigens, providing insights into the basis of molecular recognition in immune responses. They have also been used as important tools in various research techniques and diagnostic assays.
- In addition to their role in immune functions, immune complexes can also play a role in regulating antibody production. Binding of antigens to cell receptors can activate signaling cascades that lead to the activation of antibody production, contributing to the immune response.
- However, the deposition of immune complexes can also lead to autoimmune diseases. In certain conditions, immune complexes can accumulate and deposit in tissues, causing inflammation and tissue damage. Examples of such diseases include arthritis and scleroderma, where the presence of immune complexes triggers pathological immune responses.
- In summary, antigen-antibody complex formation is a crucial aspect of the immune response. It allows for the recognition and elimination of pathogens, regulates antibody production, and serves as a tool for understanding the mechanisms of immune recognition. While immune complexes have important functions, their deposition can also contribute to the development of autoimmune diseases.
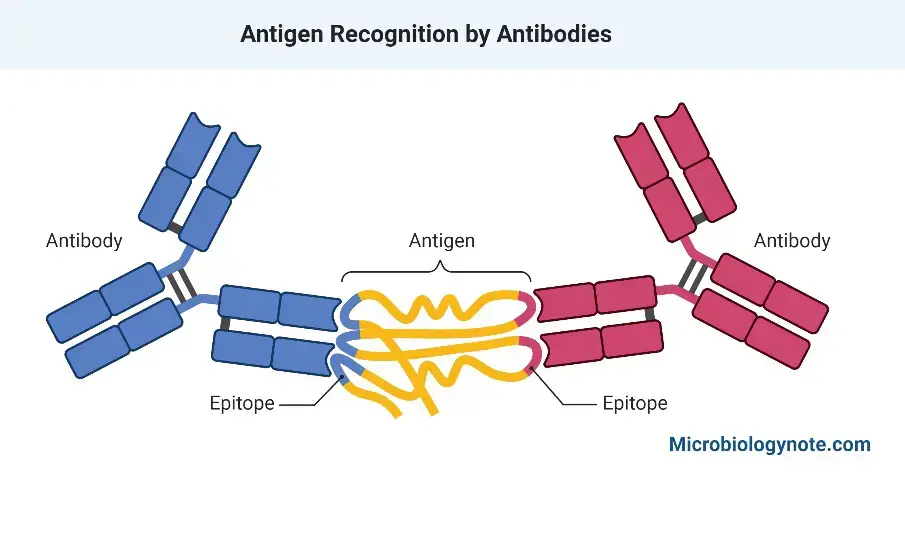
Antigen Processing and Presentation
Antigen processing and presentation refers to the decomposition of antigens into smaller peptide fragments by antigen-presenting cells (APCs), which are then displayed on the cell surface by antigen-presenting molecules such as MHC class I and II for recognition by lymphocytes.
Three distinct antigen processing and presentation pathways are possible;
1. Endogenous Pathway or Classical MHC class I Presentation
- The endogenous pathway, also known as the classical MHC class I presentation pathway, plays a crucial role in the immune system’s response to intracellular pathogens and the recognition of infected cells by cytotoxic CD8+ T cells.
- In this pathway, the processing and presentation of antigens involve mechanisms similar to the normal turnover of intracellular proteins. Antigen-presenting cells (APCs), such as dendritic cells, macrophages, and some B cells, are responsible for executing these processes.
- The first step in the endogenous pathway is the degradation of the protein antigen into short peptide fragments. This degradation occurs within the cytosol and is facilitated by a specialized cytosolic proteolytic system called the proteasome. It breaks down the proteins into small peptides, typically 7 to 10 amino acids in length.
- Within the immune system, a modified form of the proteasome known as the immunoproteasome is involved in antigen processing. The immunoproteasome is induced by exposure to interferon-γ or TNF-α, and its components are optimized for generating peptides that can be presented by MHC class I molecules.
- After proteasomal degradation, the resulting peptide fragments are further trimmed by aminopeptidases in the endoplasmic reticulum (ER) lumen. This trimming step helps ensure that the peptides reach the appropriate length for binding to MHC class I molecules.
- The peptides generated in the cytosol and ER lumen are then transported into the ER lumen by a specialized transport protein called the Transporter associated with Antigen Processing (TAP). TAP ensures the delivery of peptides from the cytosol to the ER, where MHC class I molecules are synthesized.
- Once inside the ER, the peptides undergo further processing and loading onto MHC class I molecules. This process is facilitated by several proteins, including tapasin and the calnexin-calreticulin system. Tapasin helps to stabilize the interaction between MHC class I molecules and the peptides, ensuring proper peptide loading.
- Depending on the affinity of the loaded peptides for MHC class I molecules, different outcomes occur. Peptide-MHC class I complexes with high affinity are transported from the ER through the Golgi apparatus and finally reach the cell membrane. These complexes are then displayed on the cell surface, where they can be recognized by antigen-specific CD8+ T cells. This recognition triggers an immune response against infected or abnormal cells.
- Peptide-MHC class I complexes with lower affinity are not transported to the cell membrane but are instead recycled by a mechanism involving UDP-glucose glycoprotein transferase-1. This recycling process prevents the presentation of non-specific or low-affinity peptides to the immune system.
- In summary, the endogenous pathway, or classical MHC class I presentation pathway, involves the proteasomal degradation of intracellular proteins, trimming of resulting peptides in the ER, transport of peptides to the ER lumen by TAP, and subsequent loading of peptides onto MHC class I molecules with the help of tapasin and the calnexin-calreticulin system. High-affinity peptide-MHC class I complexes are transported to the cell membrane to initiate antigen-specific CD8+ T cell responses, while low-affinity complexes are recycled to prevent non-specific immune activation.
2. Exogenous Pathway/ Classical MHC class II Presentation
- The exogenous pathway, also known as the classical MHC class II presentation pathway, is responsible for the processing and presentation of antigens derived from extracellular sources. It involves the internalization and degradation of antigens by antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells.
- In the exogenous pathway, the antigen is initially internalized by the APC through a process called phagocytosis or receptor-mediated endocytosis. The antigen binds to specific surface receptors on the APC, facilitating its uptake into the cell.
- Once inside the cell, the antigen progresses through different acidic compartments, including endosomes and lysosomes. These compartments contain hydrolytic enzymes and have low pH, which aid in the degradation of proteins into smaller peptide fragments.
- Within the APC, there is a unique endosome known as the MHC class II-containing compartment (MIIC). It is in this compartment that the final degradation of proteins and loading of peptide fragments onto MHC class II molecules occur.
- To prevent the interaction of antigenic peptides with MHC class I molecules, which are also expressed by APCs, a distinct mechanism is in place. When MHC class II molecules are synthesized, they associate with a protein called the invariant chain. The invariant chain interacts with the peptide-binding groove of the MHC class II molecule, blocking the binding of endogenously derived peptides.
- As the invariant chain travels through different compartments, it is gradually degraded, resulting in the formation of a short fragment known as CLIP (class II-associated invariant chain peptide). CLIP occupies the peptide-binding groove of the MHC class II molecule until it is replaced by an antigenic peptide.
- The exchange of CLIP with an antigenic peptide is catalyzed by a specialized class II MHC molecule. This peptide binding is crucial for maintaining the structure and stability of the MHC class II molecule.
- Once the antigenic peptide is bound, the MHC class II-peptide complex is transported to the plasma membrane of the APC. In the neutral pH environment of the plasma membrane, the complex undergoes conformational changes, adopting a compact and stable form.
- At the cell surface, the MHC class II-peptide complex is displayed to CD4+ T cells, which can recognize the complex and initiate immune responses specific to the presented antigen.
- In summary, the exogenous pathway of antigen processing and presentation involves the internalization of antigens by APCs, degradation of proteins into peptide fragments within acidic compartments, loading of peptides onto MHC class II molecules in the MIIC, and transportation of the MHC class II-peptide complex to the cell surface. This pathway allows for the presentation of extracellular antigens to CD4+ T cells, enabling immune recognition and activation.
3. Cross-presentation
- Cross-presentation is a unique process observed primarily in dendritic cells, where antigens acquired through endocytosis or the exogenous pathway are diverted to the class I major histocompatibility complex (MHC) loading pathway for peptide presentation. This phenomenon allows dendritic cells to present exogenous antigens on MHC class I molecules, which are typically responsible for presenting endogenous antigens.
- The exact mechanisms by which cross-presentation occurs are not fully understood, but two possible mechanisms have been proposed. The first mechanism suggests that cross-presenting cells possess specialized antigen-processing machinery that allows them to load exogenously derived peptides onto class I MHC molecules. This machinery may involve unique proteases and peptide transporters that facilitate the generation and transport of peptide fragments into the endoplasmic reticulum (ER), where class I MHC molecules are loaded with peptides.
- The second mechanism proposes that certain cells possess specialized endocytosis machinery that directs internalized antigens to a specific organelle where peptide loading onto class I MHC molecules takes place. This organelle could be an early endosome or a specialized subcompartment within the endosomal system. The internalized antigens are processed within this organelle, and the resulting peptides are subsequently loaded onto class I MHC molecules for presentation.
- Cross-presentation of antigens has significant advantages for the immune response. By capturing and processing antigens from the extracellular environment, APCs, especially dendritic cells, can activate cytotoxic T-cell lymphocytes (CTLs) through MHC class I presentation. This allows the CTLs to recognize and eliminate virus-infected cells or cells presenting antigens derived from other intracellular pathogens. In essence, cross-presentation enables the immune system to target and eliminate infected or abnormal cells, preventing the further spread of the pathogen.
- Overall, cross-presentation is a crucial mechanism by which dendritic cells bridge the gap between the innate and adaptive immune responses. It allows for the activation of cytotoxic T cells against extracellular antigens, enhancing the immune response against pathogens and contributing to immune surveillance and defense.
Antigen Examples
- Microbial antigens: These are components of pathogens, such as bacteria, viruses, fungi, and parasites, that trigger an immune response. Examples include bacterial cell wall components (e.g., lipopolysaccharides, peptidoglycan), viral proteins (e.g., viral capsid proteins), and fungal cell wall components (e.g., β-glucans).
- Allergens: Allergens are antigens that induce allergic reactions in susceptible individuals. Common allergens include pollen, dust mites, pet dander, certain foods (e.g., peanuts, shellfish), and insect venom.
- Autoantigens: Autoantigens are self-antigens, which are the body’s own proteins or cellular components. In autoimmune diseases, the immune system mistakenly recognizes these self-antigens as foreign, leading to an immune response against them. Examples include antigens associated with autoimmune diseases like rheumatoid arthritis (e.g., rheumatoid factor), systemic lupus erythematosus (e.g., anti-nuclear antibodies), and multiple sclerosis (e.g., myelin antigens).
- Tumor antigens: Tumor antigens, also known as neoantigens, are derived from mutations or abnormal expression of proteins in tumor cells. These antigens are recognized by the immune system, leading to an immune response against the tumor. Tumor antigens can be specific to certain types of cancer or shared among different types.
- Blood group antigens: Blood group antigens are present on the surface of red blood cells and determine the different blood groups (e.g., A, B, AB, O). Examples include the ABO antigens (A and B antigens) and the Rh antigen (positive or negative).
- Transplant antigens: Transplant antigens, also known as histocompatibility antigens, are proteins found on the surface of cells that determine the compatibility between donor and recipient in organ or tissue transplantation. Major histocompatibility complex (MHC) antigens, such as HLA antigens in humans, play a crucial role in transplant rejection or acceptance.
These are just a few examples of antigens. Antigens can be diverse and can include proteins, carbohydrates, lipids, and other molecules that can elicit an immune response when recognized by the immune system.
Antigen Detection
Antigen detection methods are important tools used in the field of immunology to identify and characterize antigens in various biological samples. Here are some key points regarding antigen detection methods:
- B-cell Receptor (BCR) and T-cell Receptor (TCR): Antigens can be detected by the body through BCRs in humoral immunity or TCRs in cellular immunity. BCRs recognize extracellular or free antigens, while TCRs detect intracellular antigens.
- Pathogen-Associated Molecular Patterns (PAMPs): Antigens can be components of invading pathogens, known as PAMPs. These include cell wall components, fimbriae, flagella, bacterial toxins, viral coats, and other surface molecules. PAMPs are recognized by host toll-like receptors (TLRs) as a warning signal of an invading organism.
- Antibody Formation: Antigenic molecules or cells stimulate specific immune responses when they bind to BCRs. This leads to the production of antibodies that are specific to the antigen, aiming to eliminate the invading pathogen.
- T-Dependent Antigens: Some antigens, called T-dependent antigens, require the help of T-helper cells to stimulate antibody production. Epitopes, which are specific regions on antigens, are recognized by antibodies and play a crucial role in antigen-antibody interactions.
- Non-Microbial Antigens: Antigens can also be non-microbial, such as those found on the surface of transplanted organs or tissues, blood cells, pollens, serum proteins, or egg white. These antigens can still trigger an adaptive immune response.
- Blood Group Antigens: Antigens present on the surface of human red blood cells determine blood groups. These antigens, such as ABO blood group antigens, are non-immunogenic under normal conditions but can elicit an immune response if a person receives blood from a donor with a different blood group. Matching blood groups is essential to avoid adverse immune responses during blood transfusions or organ donations.
In antigen detection methods, various techniques are used to identify and characterize antigens, including enzyme-linked immunosorbent assays (ELISA), immunohistochemistry (IHC), flow cytometry, and Western blotting, among others. These methods utilize the specific binding of antibodies to antigens to enable their detection and analysis in different samples.
Key Point
- In the Weil–Felix reaction, strains of Proteus species (such as OX 19, OX 2, and OX K) are used to detect heterophile antibodies produced in response to rickettsial pathogens.
- By demonstrating heterophile antibodies that agglutinate sheep erythrocytes, the Paul–Bunnell test is utilised to diagnose infectious mononucleosis caused by Epstein–Barr virus infection.
- By demonstrating heterophilic antibodies, a cold agglutinin test is performed to diagnose primary atypical pneumonia caused by Mycoplasma pneumoniae.
What are Superantigens?
- Superantigens are a class of molecules that can interact nonspecifically with APCs and T lymphocytes.
- By interacting with MHC class II molecules of the APC and the Vb domain of the T-lymphocyte receptor, superantigens exert distinct effects.
- This interaction activates a greater proportion of T cells (10%) than conventional antigens (1%), resulting in massive cytokine production and immunomodulation.
- Staphylococcal enterotoxins, toxic shock syndrome toxin, exfoliative toxins, and certain viral proteins are examples of superantigens.
Examples of Bacterial Superantigens and their roles
- Staphylococcal enterotoxins are the cause of food poisoning.
- Toxic shock syndrome caused by the Staphylococcal toxin TSST-1
- Scalding skin condition due to Staphylococcal exfoliating toxins
- Streptococcal pyrogenic exotoxins (exotoxins A and B)
What is Adjuvant?
- Adjuvants (from the Latin adjuvare, to aid) are chemicals that enhance the immunogenicity of an antigen when mixed with it and administered.
- When an antigen has poor immunogenicity or when only a little amount of an antigen is available, adjuvants are frequently utilised to stimulate the immune system.
- If BSA is delivered with an adjuvant, for instance, the antibody response of mice after immunisation with BSA can be boosted by a factor of five or more.
- It is not totally understood how adjuvants enhance the immune response, however they appear to exert one or more of the following effects:
- Antigen persistence is prolonged.
- Co-stimulatory signals are enhanced.
- Local inflammation is increased.
- The nonspecific proliferation of lymphocytes is stimulated.
- Aluminum potassium sulphate (alum) increases the antigen’s persistence. When an antigen is combined with alum, the salt causes the antigen to precipitate.
- Injection of this alum precipitate causes a slower release of antigen from the injection site, hence increasing the effective exposure duration to the antigen from a few days without adjuvant to several weeks with adjuvant.
- Additionally, the alum precipitate enlarges the antigen, hence boosting the possibility of phagocytosis. Additionally, water-in-oil adjuvants extend antigen persistence.
- Freund’s incomplete adjuvant consists of antigen in aqueous solution, mineral oil, and an emulsifying ingredient such as mannide monooleate that disperses the oil into small droplets surrounding the antigen; the antigen is thus released very slowly from the injection site.
- This treatment is based on Freund’s complete adjuvant, the first purposefully formulated, extremely efficient adjuvant, which was created by Jules Freund many years ago and contains heat-killed Mycobacteria as an extra component.
- Muramyl dipeptide, a component of the mycobacterial cell wall, stimulates macrophages, rendering Freund’s complete adjuvant considerably more effective than its incomplete counterpart.
- Activated macrophages are more phagocytic than unactivated macrophages and express more class II MHC molecules and B7 family membrane molecules.
- The increased expression of class II MHC enhances the antigen-presenting cell’s capacity to present antigen to T-helper cells. B7 molecules on the antigen-presenting cell attach to CD28, a cell-surface protein on TH cells, inducing co-stimulation, an intensification of the T-cell immunological response.
- In the presence of adjuvant, antigen presentation and the necessary co-stimulatory signal are often enhanced.
- Alum and Freund’s adjuvants also induce a prolonged, local inflammatory response that draws both phagocytes and lymphocytes.
- This infiltration of cells at the site of adjuvant injection frequently culminates in the creation of a granuloma, a thick, macrophage-rich mass of cells. Due to the activation of macrophages within a granuloma, this technique also promotes the activation of TH cells.
- Other adjuvants (such as synthetic polyribonucleotides and bacterial lipopolysaccharides) improve the chance of antigen-induced clonal selection of lymphocytes by stimulating nonspecific lymphocyte proliferation.
FAQ
What is an antigen?
An antigen is a substance that can stimulate an immune response in the body. It can be a molecule or a part of a molecule, such as a protein, carbohydrate, or lipid, that is recognized by the immune system as foreign.
How do antigens trigger an immune response?
Antigens interact with specific immune cells, such as B cells and T cells, through their unique molecular structures called epitopes. This interaction activates the immune system to produce an immune response, including the production of antibodies or the activation of immune cells.
What are the different types of antigens?
Antigens can be classified into various categories, including microbial antigens (e.g., from bacteria, viruses), allergens (e.g., pollen, pet dander), autoantigens (self-antigens triggering autoimmune responses), tumor antigens (neoantigens produced by cancer cells), blood group antigens (determining ABO and Rh blood types), and transplant antigens (histocompatibility antigens for organ transplantation).
How are antigens recognized by the immune system?
The immune system recognizes antigens through specialized receptors on immune cells. B cells have antigen-specific receptors called B cell receptors (BCRs), while T cells have T cell receptors (TCRs). These receptors can bind to specific epitopes on antigens, initiating an immune response.
Can antigens be harmful to the body?
Antigens themselves are not inherently harmful. However, certain antigens, such as those from pathogens or allergens, can trigger an immune response that leads to disease symptoms or allergic reactions. In some cases, the immune response may also target normal body tissues, leading to autoimmune diseases.
Can antibodies bind to any antigen?
Antibodies are highly specific and can bind to specific epitopes on antigens. Each antibody recognizes a particular antigen or a closely related group of antigens. The binding occurs through the complementary interaction between the antigen’s epitope and the antibody’s paratope.
How are antigens involved in vaccinations?
Vaccinations introduce harmless forms of antigens, such as weakened or inactivated pathogens or specific parts of pathogens, into the body. This stimulates the immune system to produce an immune response and develop immunological memory, providing protection against future infections by the same pathogen.
Can antigens change or mutate?
Antigens can undergo changes or mutations, particularly in the case of infectious agents like viruses. These changes can affect the antigenicity of the pathogen, allowing it to evade the immune system and potentially cause recurrent or more severe infections.
How are antigens detected in diagnostic tests?
Various diagnostic tests utilize the detection of antigens to identify the presence of pathogens or specific substances in the body. Techniques such as enzyme-linked immunosorbent assay (ELISA), lateral flow assays, and immunohistochemistry rely on the specific binding of antibodies to antigens for detection and diagnosis.
Can the body develop tolerance to antigens?
The immune system has mechanisms to develop tolerance to self-antigens, preventing an immune response against normal body tissues. However, in certain circumstances, tolerance mechanisms can break down, leading to autoimmune diseases where the immune system mistakenly targets self-antigens.
References
- Saylor, K., Gillam, F., Lohneis, T., & Zhang, C. (2020). Designs of Antigen Structure and Composition for Improved Protein-Based Vaccine Efficacy. Frontiers in Immunology, 11. doi:10.3389/fimmu.2020.00283
- https://www.biologydiscussion.com/antigens/antigens-in-body-definition-types-and-structure/1437
- https://microbiologyinfo.com/antigen-properties-types-and-determinants-of-antigenicity/
- https://www.rcsb.org/structure/3hfm
- https://www.iith.ac.in/K-PAM/o_antigen.html
- https://www2.nau.edu/~fpm/immunology/documents/Ch-04_000.pdf
- https://www.biologyonline.com/dictionary/antigen
- https://theconversation.com/antigen-tests-for-covid-19-are-fast-and-easy-and-could-solve-the-coronavirus-testing-problem-despite-being-somewhat-inaccurate-137977
- https://www.vedantu.com/biology/antigen
- https://microbenotes.com/antigen/
- https://www.news-medical.net/life-sciences/What-is-an-Antigen.aspx