What is T cell (T Lymphocyte)?
- T lymphocytes, commonly known as T cells, are a type of white blood cell that play an important part in the adaptive immune system. They are in charge of identifying and combating invading infections, as well as regulating and coordinating immune responses.
- T cells are distinguished by the presence of a T-cell receptor (TCR) on their cell surface. T cells can recognize certain antigens provided by other cells, such as antigen-presenting cells (APCs), thanks to this receptor. T cells, unlike B cells, cannot operate as antigen-presenting cells on their own and must be presented with antigens via major histocompatibility complex (MHC) molecules.
- T cells are formed from bone marrow hematopoietic stem cells. These precursor cells go to the thymus gland, where they mature and differentiate. The thymus is a specialized organ that is involved in T cell development and is in charge of designating T lymphocytes.
- Precursor cells grow into diverse kinds of T lymphocytes within the thymus. T cells continue to specialize and acquire specific roles after exiting the thymus. T cells are divided into subsets, each with its own specialized purpose in the immune system.
- T cells are classified into two types: CD8+ T cells, also known as cytotoxic T cells or “killer” T cells, and CD4+ T cells, also known as helper T cells. Through a process known as immune-mediated cell death, CD8+ T cells are capable of directly killing virus-infected cells as well as malignant cells. To recruit other immune cells, they can emit signaling chemicals known as cytokines.
- CD4+ T cells, on the other hand, function as helper cells, activating other immune cells such as B cells and cytotoxic T cells. They are critical in coordinating immune responses and increasing the immune system’s efficacy. CD4+ T cells can develop into several subtypes based on the cytokines they release, which help to regulate and direct immune responses.
- Tregs, also known as suppressor T cells, are another type of T cell subset. Tregs are essential for immunological tolerance and avoiding autoimmune responses. They keep the immune system from attacking the body’s own cells by inhibiting immunological responses to self-antigens.
- T cells have been linked to a variety of diseases and disorders in addition to their involvement in immunological defense. Certain malignancies, for example, can co-opt regulatory T cells to avoid the immune system and prevent tumor cells from being recognized and destroyed.
- T cells are critical components of the adaptive immune system. They contribute to immune surveillance, pathogen clearance, immunological control, and immune response coordination through their varied subtypes and roles.
Definition of T cell (T Lymphocyte)
T cells, also known as T lymphocytes, are a type of white blood cell that plays a crucial role in the immune system. They are responsible for recognizing and attacking foreign pathogens, infected cells, and cancer cells. T cells have specialized receptors on their surface that allow them to identify specific antigens presented by other cells. They are an integral part of the adaptive immune response and contribute to immune defense, immune regulation, and coordinating immune responses.
Types of T cell (T Lymphocyte)
A. Conventional adaptive T cells
1. Helper T cells (CD4+ T cells)
- Helper T cells, also known as CD4+ T cells, are a subset of lymphocytes that play a crucial role in the immune system. They assist in the maturation and differentiation of other lymphocytes, particularly B cells, into plasma cells and memory B cells.
- CD4+ T cells get their name from the CD4 receptors present on their cell membranes, which are involved in their activation. These cells account for approximately 50-60% of the total T cell population.
- Once activated, helper T cells initiate immune responses by interacting with antigen-presenting cells (APCs) that display antigens using class II major histocompatibility complex (MHC) molecules. This interaction triggers the differentiation of helper T cells and the secretion of cytokines, which regulate the overall immune response.
- Helper T cells can differentiate into different subtypes based on the type of cytokines they produce. Two major subsets of helper T cells are Th1 and Th2 cells, which are distinguished by the toll-like receptors involved in their activation.
- Th1 cells are involved in combating intracellular pathogens by enhancing the phagocytic activity of macrophages. They promote an immune response focused on cell-mediated immunity.
- Th2 cells, on the other hand, are primarily associated with activating B cells. They stimulate B cells to differentiate into plasma cells, which produce antibodies to target extracellular pathogens. Th2 cells also play a role in opsonization, where they coat the surface of pathogens, making them more susceptible to phagocytosis.
- The differentiation and function of helper T cells are tightly regulated and coordinated to ensure an effective immune response against specific pathogens or foreign particles.
- In summary, helper T cells, or CD4+ T cells, are an important subset of T lymphocytes that assist in the maturation of other lymphocytes, particularly B cells. They are activated by interactions with antigen-presenting cells and play a crucial role in regulating and coordinating immune responses to protect the body from foreign invaders.
CD4+ Helper T cell subsets
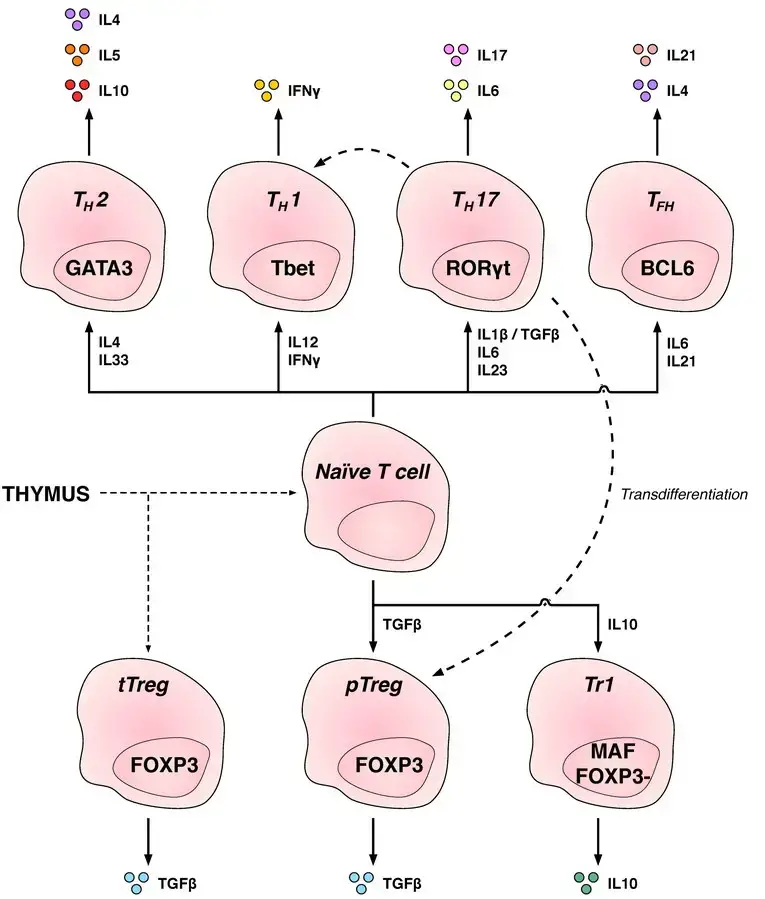
- Th1: Generate an inflammatory response, which is essential for protection against intracellular bacteria, viruses, and cancer. (Related diseases: Multiple Sclerosis and Type 1 diabetes)
- Th2: Immunologically significant against external pathogens, such as worm infections, that are Th2 cells. (Asthma and other allergy illnesses are related)
- Th17: Protection against intestinal pathogens and mucosal barriers. (Related diseases: Multiple Sclerosis, Rheumatoid Arthritis, and Psoriasis)
- Th9: Protection against parasitic helminths and cell-dependent allergic inflammation. Multiple Sclerosis is a related condition.
- Tfh: Aid B cells in producing antibodies.
- (Asthma and other allergy illnesses are related)
- Th22: Pathogenesis of allergic airway disorders is primarily anti-inflammatory, according to Hypothesis 22. (Related diseases: Crohn’s, Rheumatoid, and Tumors)
2. Cytotoxic T cells (CD8+ T cells)
- Cytotoxic T cells, also known as CD8+ T cells, are a subset of T cells that play a crucial role in immune defense against infected cells, tumor cells, and cells involved in transplants. They are named CD8+ T cells because they express the CD8 receptor on their cell surface. CD8+ T cells make up approximately 40% of the total T cell population.
- The activation of cytotoxic T cells is triggered by the recognition of antigens presented by class I major histocompatibility complex (MHC) molecules on antigen-presenting cells. Once activated, CD8+ T cells undergo clonal expansion and differentiate into cytotoxic or killer T cells.
- Cytotoxic T cells are specialized in targeting and eliminating virus-infected cells, tumor cells, and foreign cells. They accomplish this by recognizing short peptides presented on the surface of target cells in conjunction with class I MHC molecules. The binding of cytotoxic T cells to these peptide-MHC complexes initiates their cytotoxic response.
- Upon activation, cytotoxic T cells release cytotoxic molecules, such as perforin and granzymes, which induce cell death in the target cells. This process is known as immune-mediated cell death or apoptosis. Cytotoxic T cells also secrete cytokines like interleukin-2 (IL-2) and interferon-gamma (IFN-γ), which help regulate the effector functions of other immune cells and enhance the immune response against pathogens.
- The ability of cytotoxic T cells to specifically recognize and eliminate infected or abnormal cells is crucial for immune defense and maintaining overall immune homeostasis.
- In summary, cytotoxic T cells, or CD8+ T cells, are a subset of T cells that are activated by class I MHC molecules. They differentiate into cytotoxic or killer T cells and are responsible for targeting and destroying virus-infected cells, tumor cells, and cells involved in transplants. Through the release of cytotoxic molecules and cytokines, cytotoxic T cells play a vital role in immune-mediated cell death and regulating immune responses.
3. Memory T cells
- Memory T cells are a specialized class of T cells that develop from naïve T cells following exposure to a complete antigen presented by major histocompatibility complex (MHC) molecules. These cells play a crucial role in immunological memory, enabling the immune system to mount a rapid and robust response upon re-exposure to a previously encountered antigen.
- Memory T cells are long-lived and possess the ability to quickly expand into a large number of effector T cells upon encountering the specific antigen again. This rapid response is due to the clonal expansion and differentiation that occurred during the initial antigen exposure.
- Memory T cells can express either CD4+ or CD8+ receptors, although CD45RO is a common marker expressed by memory T cells. CD45RO allows for their distinction from naïve T cells, which express CD45RA.
- Memory T cells can be further classified into subtypes based on their characteristics and localization within the body. These subtypes include central memory T cells, effector memory T cells, tissue-resident memory T cells, and virtual memory T cells.
- Central memory T cells express CD44 and are typically found in lymph nodes and peripheral circulation. They possess high proliferative potential and are capable of generating effector T cells upon antigen re-stimulation.
- Effector memory T cells express CD45RO and are found in peripheral circulation and tissues. These cells are terminally differentiated and serve as a ready-to-respond population of effector T cells upon encountering the specific antigen.
- Tissue-resident memory T cells (TRMs) remain localized in specific tissues, such as the skin, gut, or lungs, providing rapid immune protection at the site of previous antigen exposure. These cells are crucial for long-term immunity in peripheral tissues.
- Virtual memory T cells are distinct from other memory T cells as they do not originate from clonal expansion. They are present in relatively smaller numbers and mainly circulate in the periphery. Virtual memory T cells possess memory-like characteristics despite not being derived from previous antigen encounters.
- The presence of memory T cells allows for a more efficient and effective immune response upon re-exposure to a pathogen or antigen. Their ability to mount a rapid and specific immune reaction is vital in controlling infections and preventing reinfection.
- In summary, memory T cells are a specialized subset of T cells that develop from naïve T cells following exposure to antigens. They exhibit long-term persistence, rapid expansion, and differentiation into effector T cells upon re-encountering specific antigens. The different subtypes of memory T cells contribute to immune memory and provide enhanced protection against pathogens.
Memory T cell subtypes
- Central memory T cells (TCM cells): Central memory T cells (TCM cells) are characterised by the expression of CD45RO, C-C chemokine receptor type 7 (CCR7), and L-selectin (CD62L). CD44 expression is intermediate to high in central memory T cells. This subset of memory cells is typically observed in lymph nodes and peripheral circulation. (Note that CD44 expression is typically utilised to differentiate between murine naïve and memory T cells)
- Effector memory T cells (TEM cells and TEMRA cells): They also exhibit moderate to high CD44 expression. Lacking lymph node-homing receptors, these memory T cells are located in the peripheral circulation and tissues. TEMRA is the abbreviation for terminally developed effector memory cells that re-express CD45RA, a typical marker for naïve T cells.
- Tissue-resident memory T cells (TRM): Tissue-resident memory T cells (TRM) do not recirculate and occupy tissues (skin, lung, etc.). The intern e7, commonly known as CD103, is a cell surface marker that has been linked to TRM.
- Virtual memory T cells (TVM): Virtual memory T cells (TVM) vary from other memory subsets in that they do not arise from a clonal expansion event. Individual virtual memory T cell clones reside at relatively low frequencies, despite the fact that this population as a whole is plentiful in the peripheral circulation. The origin of this T cell population, according to one idea, is homeostatic proliferation. Although CD8 virtual memory T cells were initially identified, it is now understood that CD4 virtual memory T cells also exist.
4. Regulatory CD4+ T cells
- Regulatory CD4+ T cells, also known as regulatory T cells or Tregs, play a crucial role in maintaining immune responses and immune tolerance within the body.
- These cells are primarily responsible for suppressing T-cell mediated immune responses, helping to regulate and balance the immune system. They act to prevent excessive immune activation and inflammation, ensuring that immune responses are appropriately controlled.
- Regulatory T cells have the ability to suppress autoreactive T cells that have escaped the negative selection process during T cell development, thereby preventing autoimmune reactions against self-antigens.
- There are two main classes of regulatory T cells: CD4+ FOXP3+ T cells and CD4+ FOXP3- T cells. FOXP3 is a transcription factor that is essential for the development and function of regulatory T cells. It serves as a marker to identify and characterize these cells.
- Regulatory T cells can be generated through two different mechanisms. Thymic Treg cells are formed during the normal process of T cell development in the thymus. These cells develop from precursors that express FOXP3 and acquire their suppressive function within the thymus.
- Peripherally derived Treg cells, on the other hand, are induced outside of the thymus in response to specific environmental cues. These cells are generated from conventional CD4+ T cells and acquire regulatory function in peripheral tissues.
- Both thymic Treg cells and peripherally derived Treg cells are essential for maintaining immune tolerance and preventing excessive immune responses. They act by suppressing the activation and function of other immune cells, such as effector T cells and antigen-presenting cells, through various mechanisms.
- Disruptions or mutations in the FOXP3 gene can lead to impaired development or function of regulatory T cells, resulting in dysregulated immune responses and the potential development of autoimmune diseases.
- In summary, regulatory CD4+ T cells, or regulatory T cells, play a crucial role in maintaining immune responses and immune tolerance. They suppress T-cell mediated immunity, prevent autoimmune reactions, and help regulate the balance of the immune system. These cells require the expression of the FOXP3 transcription factor for their development and function. Understanding the role and mechanisms of regulatory T cells is important in the context of immune regulation and autoimmune diseases.
B. Innate-like T cells
- Unconventional or innate-like T cells are subsets of T cells that behave differently during immunity.
- In contrast to their traditional counterparts (CD4 T helper cells and CD8 cytotoxic T cells), which are dependent on the identification of peptide antigens in the context of the MHC molecule, they induce fast immune responses regardless of MHC expression.
- NKT cells, MAIT cells, and gammadelta T cells comprise the three largest groups of unconventional T cells. Now, their functional roles in the context of infections and cancer are well-established.
- In addition, these T cell subsets are being translated into a variety of treatments for cancers, such as leukaemia.
Natural killer T cell
- NKT cells connect the adaptive immune system to the innate immune system.
- NKT cells identify glycolipid antigens presented by CD1d, in contrast to typical T cells, which recognise protein peptide antigens presented by major histocompatibility complex (MHC) molecules.
- Once activated, these cells can perform both helper and cytotoxic T cell tasks, including cytokine production and the release of cytolytic/cell-killing chemicals.
- They can also identify and kill some tumour cells and herpes virus-infected cells.
Mucosal associated invariant T cells
- Mucosal associated invariant T (MAIT) cells have innate effector-like characteristics. MAIT cells are present in the blood, liver, lungs, and mucosa of humans, where they guard against microbial activity and infection.
- The MHC class I-like protein MR1 is responsible for delivering vitamin B metabolites generated by bacteria to MAIT cells.
- MAIT cells are capable of lysing bacterially infected cells upon the presentation of foreign antigen by MR1.
- MAIT cells can potentially be triggered via signalling independent of MR1.
- In addition to exhibiting innate-like activities, this subgroup of T cells possesses a memory-like phenotype and supports the adaptive immune response.
- In addition, it is believed that MAIT cells play a role in autoimmune disorders such as multiple sclerosis, arthritis, and inflammatory bowel disease, although definitive proof has not yet been published.
Gamma delta T cells
- Gamma delta T cells ( γδ T cells) are a small group of T cells that have a γδTCR on the cell surface instead of a αβTCR. A large number of T cells have αβ TCR chains.
- This group of T cells makes up only about 2% of all T cells in humans and mice. They are mostly found in the gut mucosa, where they are part of a group of intraepithelial lymphocytes.
- γδT cells can make up as much as 60% of all T cells in rabbits, sheep, and chickens. Most of what we know about the antigenic molecules that turn on γδ T cells is still pretty new. But γδ T cells are not limited by MHC and seem to be able to recognise whole proteins without the need for MHC molecules on APCs to present peptides.
- Some murine γδT cells can recognise molecules called MHC class IB. Most of the γδ T cells in the peripheral blood of humans are those that use the Vγ9 and Vδ2 gene fragments.
- These cells are unique because they respond quickly and specifically to a group of nonpeptidic phosphorylated isoprenoid precursors called phosphoantigens. Almost all living cells make phosphoantigens.
- Isopentenyl pyrophosphate (IPP) and its isomer, dimethylallyl pyrophosphate, are found in most animal and human cells, including cancer cells (DMPP).
- Along with IPP and DMAPP, many microbes also make the active compound hydroxy-DMAPP (HMB-PP) and the corresponding mononucleotide conjugates. Both types of phosphoantigens are made by plant cells.
- Human Vγ9/Vδ2 T cells can be turned on by drugs that contain synthetic phosphoantigens and aminobisphosphonates, which turn on the body’s own IPP/DMAPP.
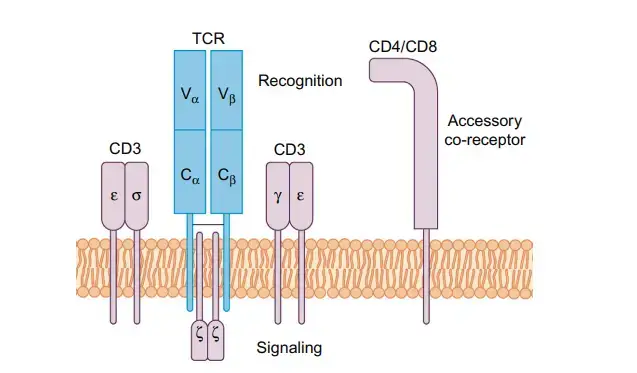
The T Cell Receptor
- The T cell receptor (TCR) is a transmembrane heterodimer made up of two polypeptide chains that are linked by disulfide bonds.
- Each lymphocyte has a TCR that is only good for one thing. Antigens can cause T lymphocytes to divide and make copies of themselves with the same antigenic specificity.
- Alpha (α) and beta (β) chains are found on the outside of almost all T lymphocytes.
- Only 5% of a healthy adult’s normal T cell population is made up of cells that have gamma (γ) and delta (δ) chains.
- Each chain (α, β, γ, or δ) is a different protein with an approx. 45 kDa molecular weight. As its receptor, a T cell can have either a heterodimer αβ or a γδ a heterodimer, but never both.
- The TCR is always expressed with the related CD3 complex, which is made up of multiple units that can be expressed on their own and is needed for signal transduction once antigen is presented.
T Cell (T Lymphocyte) Development
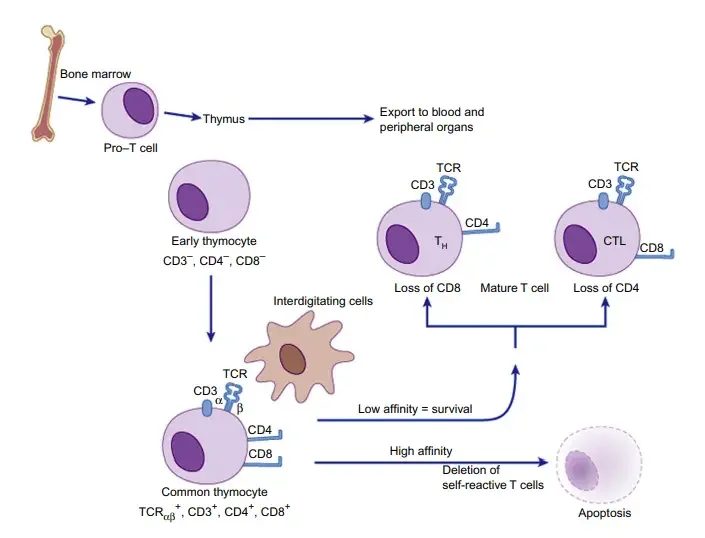
- T cells, also known as T lymphocytes, undergo a complex process of development and maturation. It begins with the differentiation of common lymphoid progenitor cells from hematopoietic stem cells in the bone marrow. These progenitor cells give rise to various lymphoid cells, including T cells.
- The precursors of T cells migrate from the bone marrow to the thymus, where their development and selection take place. This migration is essential for the maturation of T cells. In the thymus, T cells undergo a series of stages and are subjected to a process called thymic selection.
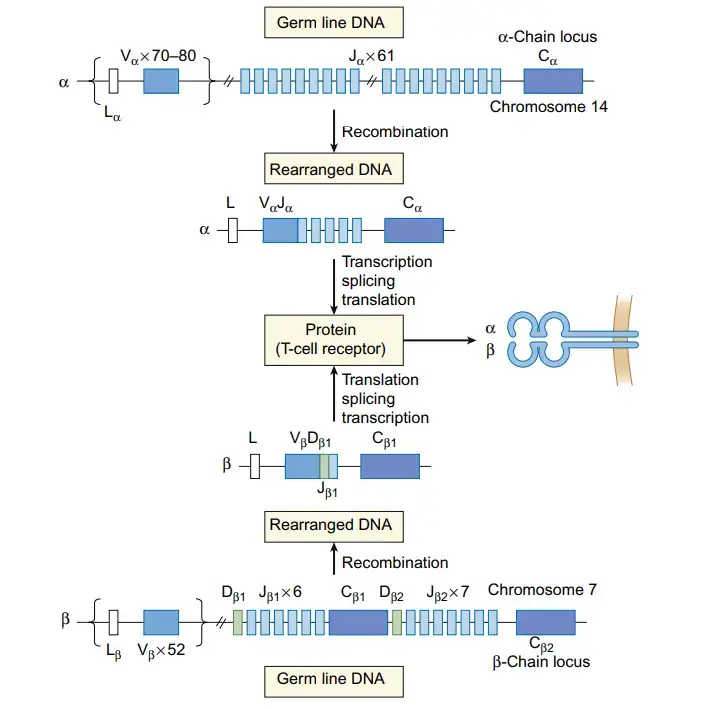
- Gene rearrangements occur during T cell development and are crucial for the generation of T cell receptor (TCR)-specific T cells. V(D)J recombination is the process by which the TCR genes rearrange. This recombination involves specific genes in the variable (V), diversity (D), and joining (J) regions of the TCR gene. The recombination occurs in the germ line sequences of developing T cells and contributes to the diversity of TCRs.
- Similar to B cells, T cells can undergo N region diversification, which increases the diversity of TCRs. The enzyme deoxynucleotidyl transferase inserts additional nucleotides, expanding the pool of TCRs that can be generated.
- During childhood and adolescence, the thymus is most active and produces a significant number of T cells. However, thymus atrophy occurs in adulthood, suggesting that the majority of T cells are generated before reaching puberty.
- T cell lineage development begins in the thymic cortex and concludes in the thymic medulla. The thymus plays a crucial role in both training and regulating the development of T cells. The differentiation of T cells in the thymus involves the expression of CD4 and CD8 markers.
- Thymocytes, the developing T cells, initially lack the CD4 and CD8 markers and are referred to as double-negative thymocytes. The rearrangement of the TCR gene is an important developmental stage that commits the cells to either the αβ T cell or γδ T cell lineage.
- As the T cells progress in their development, they become double-positive thymocytes, expressing both CD4 and CD8 markers. The process of positive selection occurs when the developing αβ TCR recognizes both major histocompatibility complex (MHC) molecules and the peptide epitopes presented by MHC. Positive selection allows the cells to continue maturation.
- During positive selection, CD4 T cells mature when they recognize MHC class II molecules, while CD8 T cells mature when they recognize MHC class I molecules. The cells undergo a process known as thymic commitment, leading to the expression of either CD4 or CD8 markers.
- After positive selection, the cells go through a process called negative selection, which tests their ability to respond to self-antigens. This process helps eliminate T cells that may cause autoimmune reactions.
- The T cells that successfully complete the maturation process are released into the population as mature T cells, ready to recognize and respond to antigens encountered in the periphery.
- In summary, T cell development involves the differentiation of common lymphoid progenitor cells in the bone marrow, migration to the thymus, gene rearrangements, thymic selection, and maturation. The thymus plays a critical role in training and regulating T cell development. Positive and negative selection processes determine the fate of developing T cells, ensuring the generation of a diverse and self-tolerant T cell repertoire.
1. Maturation
- Maturation is a crucial process in T cell development that occurs in the thymus. It involves the differentiation and progression of thymocytes through various stages, leading to the generation of mature T cells capable of recognizing and responding to specific antigens.
- When thymocytes enter the thymus, they encounter the thymic epithelium and progress to the early thymic progenitor (ETP) stage. At this stage, the cells lack the CD4 and CD8 co-receptors but express CD44 and do not express CD25. They are also positive for the ckit marker.
- The microenvironment within the thymus plays a vital role in directing the development of thymocytes towards the T cell lineage and restricting their potential to differentiate into myeloid and dendritic cells.
- The thymus consists of four major compartments, each performing distinct functions and regulating different stages of T cell development. These compartments are the subcapsular zone, cortex, medulla, and corticomedullary junction.
- Thymocyte development progresses through different stages within these compartments, and the expression of cell-surface markers changes accordingly. The thymocytes can be classified into double-negative (DN) cells, which lack CD4 and CD8 co-receptors.
- DN cells can be further divided into four stages. DN1 cells, identified by high levels of CD117, represent the ETPs and account for a small fraction of the total thymic T cell pool. DN1 cells migrate from the corticomedullary junction into the deeper cortex, moving towards the subcapsular region.
- Within the cortex, DN1 cells differentiate into DN2 thymocytes, characterized by the expression of CD24, CD25, CD44, and CD117. These cells undergo gene rearrangements and respond to cytokines like IL-7.
- The DN2 thymocytes then progress to the DN3 stage, where they express an invariant α-chain called pre-Tα. Gene rearrangement, together with the presence of the invariant chain, signals the continuation of T cell maturation.
- At the DN3 stage, the cells mature into DN4 and upregulate the expression of both CD4 and CD8, acquiring a double positive status. This maturation process is crucial for the subsequent positive and negative selection processes.
- The survival and differentiation of double-positive thymocytes depend on the specificity and binding strength of their αβ T cell receptors. Positive selection ensures that thymocytes expressing T cell receptors capable of recognizing self-antigens presented by major histocompatibility complex molecules can mature further.
- On the other hand, negative selection eliminates thymocytes with T cell receptors that strongly recognize self-antigens, preventing the development of autoreactive T cells.
- Overall, the process of maturation in T cell development involves the sequential differentiation of thymocytes through distinct stages, accompanied by changes in cell-surface marker expression. Positive and negative selection further refine the T cell repertoire, leading to the generation of functional and self-tolerant mature T cells.
a. Positive Selection
- Positive selection is a critical process that occurs during T cell development in the thymus. It involves the interaction of double-positive T cells (expressing both CD4 and CD8 co-receptors) with self-antigens presented by thymic cortical epithelial cells.
- Thymic cortical epithelial cells express a diverse array of self-antigens on major histocompatibility complex (MHC) molecules. These self-antigens are derived from proteins that are normally present in the body’s own cells.
- During positive selection, double-positive T cells migrate to the cortex of the thymus, where they come into contact with the self-antigens displayed by the thymic cortical epithelial cells. This interaction is crucial for determining the fate of the developing T cells.
- The strength and specificity of the interaction between the T cell receptor (TCR) on the double-positive T cells and the self-antigen-MHC complex determine the outcome of positive selection. T cells that interact with the self-antigen-MHC complex too weakly or not at all are unlikely to receive the necessary survival signals and are eliminated through apoptosis (programmed cell death).
- On the other hand, T cells that interact strongly with the self-antigen-MHC complex are positively selected to continue their maturation process. The specific outcome of positive selection depends on whether the T cell expresses CD4 or CD8 co-receptors.
- CD4+ T cells, also known as helper T cells, interact preferentially with MHC class II molecules. These T cells receive survival signals when they recognize self-antigens presented by MHC class II molecules on the thymic cortical epithelial cells. As a result, CD4+ T cells that successfully interact with self-antigens on MHC class II molecules are positively selected to progress in their development.
- CD8+ T cells, also called cytotoxic T cells, have a preference for MHC class I molecules. Double-positive T cells that interact effectively with self-antigens presented by MHC class I molecules receive survival signals and are positively selected to continue their maturation process as CD8+ T cells.
- It is important to note that positive selection is a dynamic process, and only a fraction of developing thymocytes successfully undergo this selection process. Many thymocytes undergo apoptosis during positive selection, ensuring that only T cells capable of recognizing self-antigens presented on MHC molecules with appropriate affinity are allowed to progress.
- In summary, positive selection in the thymus plays a crucial role in shaping the T cell repertoire by promoting the survival and maturation of T cells that can interact appropriately with self-antigens presented on MHC molecules. This process ensures the generation of functional T cells capable of recognizing foreign antigens in the context of self-MHC molecules.
b. Negative Selection
- Negative selection is a crucial process that occurs during T cell development in the thymus. It serves to eliminate thymocytes (developing T cells) that have a high affinity for self-antigens, preventing the generation of T cells that could potentially cause autoimmune reactions.
- After undergoing positive selection in the thymic cortex, the surviving double-positive T cells migrate to the medulla of the thymus, where negative selection takes place. In the medulla, these T cells encounter a diverse array of self-antigens presented by medullary thymic epithelial cells and other antigen-presenting cells.
- During negative selection, T cells that recognize self-antigens with a high affinity receive apoptotic signals. This leads to programmed cell death, or apoptosis, of the self-reactive thymocytes. The elimination of these potentially harmful T cells helps maintain self-tolerance and prevents autoimmune responses.
- It is important to note that negative selection does not eliminate all T cells that react with self-antigens. Some of the self-reactive T cells undergo a process called “clonal diversion” or “lineage diversion.” Instead of being eliminated, these cells are redirected to become regulatory T cells (Tregs). Tregs play a crucial role in suppressing excessive immune responses and maintaining immune tolerance.
- The cells that successfully complete the negative selection process, either by avoiding strong self-antigen recognition or by being diverted to the Treg lineage, are allowed to mature further and exit the thymus as mature naïve T cells. These mature T cells are characterized by their ability to recognize foreign antigens presented by antigen-presenting cells in peripheral tissues.
- Negative selection, together with positive selection, plays a crucial role in shaping the T cell repertoire, ensuring the development of functional T cells capable of recognizing foreign antigens while maintaining self-tolerance. The balance between positive and negative selection processes helps maintain a diverse and appropriately regulated immune system.
- In summary, negative selection in the thymus eliminates developing T cells that recognize self-antigens too strongly, preventing the generation of potentially harmful autoreactive T cells. Some self-reactive T cells are diverted to become regulatory T cells, while the rest undergo apoptosis. The surviving T cells that pass negative selection exit the thymus as mature naïve T cells, ready to participate in immune responses while maintaining tolerance to self-antigens.
2. Activation
- Activation of T cells is a crucial step in initiating an effective immune response against specific antigens. Once mature naïve T cells leave the thymus and enter the bloodstream, they circulate throughout the body until they encounter antigen-presenting cells (APCs) displaying their specific antigen.
- The activation of CD4+ T cells occurs through a two-step process. First, the T cell receptor (TCR) on the surface of CD4+ T cells interacts with the antigenic peptide presented by major histocompatibility complex class II (MHC-II) molecules on the APC. This engagement provides the initial signal for activation. Similarly, CD8+ T cells are activated by recognizing antigens presented on MHC class I molecules.
- However, the initial TCR signaling alone is not sufficient for full T cell activation. The second signal, known as co-stimulation, is required to provide additional activation signals to T cells. Co-stimulatory molecules, such as CD28 or ICOS, on the T cell surface interact with ligands, such as CD80 and CD86, present on the APCs. This co-stimulatory signal ensures that T cell activation occurs only in the presence of an infection or pathogen, preventing inappropriate responses to self-antigens.
- Naïve T cells primarily express CD28 as a co-stimulatory receptor, and its interaction with CD80 and CD86 on APCs enhances T cell activation. This two-step activation process involving TCR signaling and co-stimulation helps ensure specificity and control over the immune response.
- In addition to TCR engagement and co-stimulation, cytokines play a vital role in T cell activation. Cytokines are small signaling molecules released by various cells of the immune system. They provide additional signals that influence the fate and differentiation of T cells, particularly helper T cells. Different cytokines can drive CD4+ T cells to differentiate into distinct subsets, such as T helper 1 (Th1), Th2, Th17, or regulatory T cells (Tregs), each with specific functions in the immune response.
- The cytokine milieu during T cell activation guides the development of specific T cell subsets, determining their effector functions and the type of immune response they will initiate. This cytokine signaling helps shape the adaptive immune response and enables T cells to fulfill specialized roles in combating infections, promoting inflammation, or regulating immune reactions.
- In summary, T cell activation involves a two-step process: initial recognition of antigen by the TCR and subsequent co-stimulation provided by interactions between co-stimulatory molecules on T cells and ligands on APCs. Cytokines also play a critical role in shaping T cell responses. This coordinated activation process ensures specific immune responses against antigens while maintaining self-tolerance and avoiding unnecessary immune reactions.
3. Differentiation
- Differentiation is a critical process in T cell development that determines the fate and function of T cells based on their lineage commitment. This commitment occurs during the double-positive thymocyte stage when T cells decide whether to become CD8+ cytotoxic T cells or CD4+ helper T cells.
- Lineage commitment involves changes in genomic organization and gene expression, leading to the silencing of certain genes and the activation of genes associated with specific lineage functions. The exact mechanisms underlying T cell differentiation are still being actively studied, but a prevailing model suggests that it is influenced by the affinity of the T cell receptor (TCR) for either MHC class I or MHC class II molecules.
- During positive selection in the thymus, CD4+CD8+ double-positive thymocytes interact with both MHC class I and MHC class II molecules presented by thymic cortical epithelial cells. The strength of these interactions between the TCR and the MHC-peptide complexes plays a crucial role in lineage commitment. The TCR affinity towards self-antigens presented by MHC molecules helps determine whether a double-positive thymocyte will differentiate into a CD8+ cytotoxic T cell or a CD4+ helper T cell.
- If the TCR has a higher affinity for MHC class I molecules, the thymocyte is more likely to differentiate into a CD8+ cytotoxic T cell, which plays a crucial role in targeting infected or abnormal cells for elimination. Conversely, if the TCR has a higher affinity for MHC class II molecules, the thymocyte is more likely to become a CD4+ helper T cell, which coordinates immune responses by providing help to other immune cells.
- The differentiation process involves complex molecular events, including changes in gene expression and epigenetic modifications that regulate the development of specific T cell lineages. These events lead to the acquisition of distinct phenotypic and functional characteristics associated with each T cell subset.
- It is important to note that lineage commitment is not solely determined by TCR affinity for MHC molecules, and additional factors, such as cytokines and co-stimulatory signals, also contribute to the differentiation process. The cytokine milieu in the microenvironment influences the development of different subsets of CD4+ helper T cells, including Th1, Th2, Th17, and regulatory T cells (Tregs), each characterized by their unique cytokine secretion profiles and immune functions.
- In summary, T cell differentiation involves lineage commitment during the double-positive thymocyte stage, where T cells decide whether to become CD8+ cytotoxic T cells or CD4+ helper T cells. This decision is influenced by the affinity of the TCR for MHC class I or MHC class II molecules. The differentiation process includes changes in gene expression and is regulated by various molecular and environmental cues. Understanding T cell differentiation is crucial for unraveling the complexity of the immune system and developing targeted therapeutic interventions.
Positive Selection of T Cells
- Positive selection is a crucial process in T cell development that occurs in the thymus, where T cells are exposed to self-antigens presented on the surface of antigen-presenting cells (APCs). This selection process ensures that T cells can recognize and interact with major histocompatibility complex (MHC) molecules, which are essential for effective immune responses.
- During positive selection, double-positive αβ T cells, which express both CD4 and CD8 co-receptors, interact with cortical epithelial cells that express either MHC class I or MHC class II molecules, along with self-peptides. The goal is to test whether the T cell receptor (TCR) on the double-positive T cell can bind with sufficient affinity to MHC molecules.
- If the TCR on the double-positive T cell interacts weakly or fails to bind with self-MHC molecules within a specific timeframe, usually 3-4 days, the T cell undergoes apoptosis and is eliminated in the thymus cortex. On the other hand, if the TCR interacts strongly with self-MHC molecules, the T cell receives a survival signal and proceeds to the next stage of development.
- The positive selection process is important for shaping the T cell repertoire by allowing only T cells with TCRs capable of recognizing self-MHC molecules to survive. This ensures that mature T cells have the capacity to recognize foreign antigens presented on self-MHC molecules during immune responses.
- Studies using experimental models, such as radiation chimeras and TCR transgenic mice, have provided insights into the mechanisms of positive selection. Radiation chimeras, where bone marrow cells from one mouse strain are transplanted into another strain with damaged hematopoietic cells, have demonstrated that T cells can recognize antigens presented solely on host MHC molecules.
- TCR transgenic mice, which express TCRs limited to a specific MHC allele, have further confirmed the role of positive selection. Class I TCR transgenic mice predominantly produce CD8+ cytotoxic T cells, while Class II TCR transgenic mice primarily generate CD4+ helper T cells. This supports the notion that positive selection on Class I MHC molecules leads to the development of CD8+ T cells, while positive selection on Class II MHC molecules promotes the formation of CD4+ T cells.
- The process of positive selection is influenced by various factors, including the expression of co-receptors CD4 and CD8, as well as the presence of specific peptides presented by thymic epithelial cells. Co-receptor binding to MHC molecules is crucial for signal transduction and effective positive selection.
- Peptides presented by thymic epithelial cells play a role in positive selection. For example, the presence of the HLA-DM molecule, which aids in the removal of CLIP peptides from MHC class II molecules, can affect positive selection outcomes. Studies have shown that mice deficient in HLA-DM-like molecules exhibit alterations in CD4+ T cell development and specificity.
- The precise mechanisms that govern positive selection and determine the fate of T cells, whether they become CD4+ or CD8+ T cells, are still under investigation. It is believed that factors such as TCR affinity, co-receptor signaling, and interactions with self-peptides all contribute to the process. The complex interplay of these factors ensures the generation of a diverse and functional T cell repertoire capable of mounting appropriate immune responses.
- Understanding positive selection is essential not only for unraveling the intricacies of T cell development but also for advancements in areas such as bone marrow transplantation, where compatibility between donor and recipient MHC alleles is crucial for the successful reconstitution of the immune system.
Negative Selection of T Cells
- Negative selection of T cells is a crucial process that occurs in the thymus to eliminate T cells with high-affinity interactions with self-antigens. This selection mechanism ensures the development of a functional immune system while preventing the generation of self-reactive T cells that could lead to autoimmunity.
- After passing through positive selection, T cells encounter antigen-presenting cells (APCs) such as macrophages and dendritic cells at the cortico-medullary junction of the thymus. These APCs express MHC-self peptide complexes, allowing them to interact with T cell receptors (TCRs) on developing T cells.
- During negative selection, T cells with high-affinity interactions with self-peptide-MHC complexes undergo apoptosis. This process is essential for removing potentially harmful T cells that could recognize and attack self-tissues. Negative selection has been demonstrated using transgenic mice and bone marrow chimeras.
- Bone marrow chimeras, where bone marrow from one mouse strain is transplanted into irradiated recipients of a different strain, have provided evidence for negative selection. These experiments showed that T cells are tolerant not only to host MHC but also to donor MHC, indicating the effective elimination of self-reactive T cells through negative selection.
- Additionally, studies using mice expressing endogenous superantigens have shed light on negative selection. Superantigens bind to TCR Vβ regions and MHC outside of the typical peptide-binding location, resulting in strong signals and cytokine secretion by mature Th cells. T cells containing Vβ segments that bind the superantigen are programmed to undergo cell death, ensuring the elimination of self-reactive T cells.
- Negative selection also relies on signals from bone marrow-derived APCs. The signals received during positive and negative selection must be distinct to allow different types of T cells to survive and exit the thymus. It has been proposed that negative selection requires higher avidity interactions between TCRs and self-peptide-MHC complexes compared to positive selection.
- Experimental studies have shown that increasing peptide presentation initially increases the number of T cells produced (positive selection), but excessive peptide expression leads to a decrease in T cell numbers (negative selection). This supports the concept of differential avidity, where positive and negative selection signals have distinct avidity thresholds.
- Moreover, the differential signaling hypothesis suggests that positive and negative selection transmit qualitatively distinct signals. Agonist peptides, which activate T cells, may transmit positive selection signals, while antagonist peptides, which provide incomplete signals, prevent T cell activation by agonist peptides, potentially leading to positive selection. However, this has been observed primarily in CD8 cells rather than CD4 cells.
- Negative selection is a crucial process in establishing peripheral tolerance. It ensures the removal of self-reactive T cells, reducing the risk of autoimmune diseases. By maintaining a balance between displaying pathogen peptides and eliminating self-reactive T cells, negative selection plays a crucial role in shaping the immune system’s functionality and self-tolerance.
Antigen Recognition By T Cells
- Major histocompatibility complex (MHC), or human leukocyte antigen (HLA) in humans, is an antigen presentation protein that T lymphocytes can only identify when they are presented on the surface as short peptides.
- Only professional antigen-presenting cells (APCs; B cells, dendritic cells (DCs), and macrophages) express MHC class II molecules, but MHC class I molecules are present on all nucleated cells. The choice of MHC to which a peptide will be loaded depends on the processing method used.
The HLA Locus
- A human’s HLA locus is located on the chromosome 6 short arm. The HLA-A, HLA-B, and HLAC loci make up the class I region. The D region, which is part of class II, is further broken down into the HLA-DP, HLA-DQ, and HLA-DR subregions.
- Class III proteins, which have no structural homology to either class I or class II molecules, are encoded by a region between the class I and class II loci.
- Complement proteins, tumour necrosis factor, and lymphotoxin are all examples of class III compounds.
- T cell recognition of foreign antigen and “self”/”non-self” discrimination rely heavily on the highly polymorphic class I and class II MHC components.
- In the event of a transplant, any MHC class I or class II molecules not already present in the recipient are recognised as foreign antigens and attacked accordingly.
- There is substantial allotypic polymorphism present in all MHC molecules; that is, there are differences in the sequences of some parts of the molecules from one individual to the next.
- Having the same allotype at all genes encoding MHC molecules is an extremely rare occurrence between two unrelated persons.
- All nucleated cells codominantly express the three MHC class I molecules, which differ from one another in amino acid sequence but are otherwise highly conserved.
- To have “codominant expression,” both copies of the genes producing these proteins must be active.
- Class II MHC molecules are unique in that their expression features a complex blend of homologous and heterologous αβ dimers, which together represent proteins from both parents. Subunit genes (both α and β ) are highly polymorphic on a species level.
- In contrast to the functional equivalence shared by homologous dimers, heterologous dimers are specific to the F1 genotype and are not shared with the parental class II molecules.
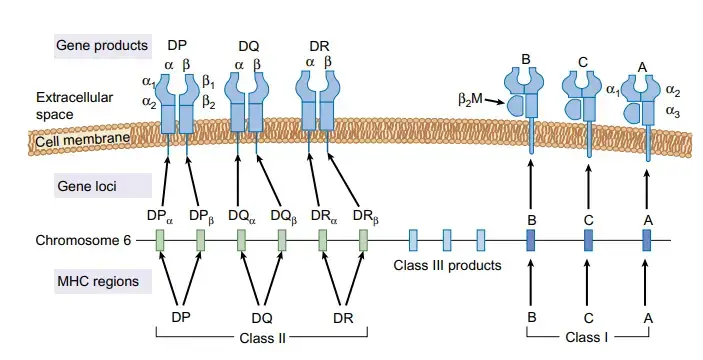
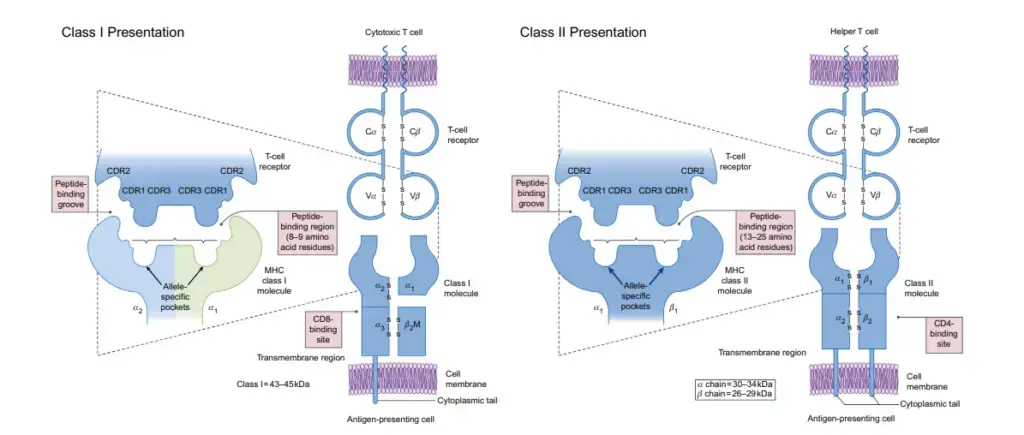
MHC Class I
- Class I MHC molecules recognise and bind peptides from cytosol-processed antigens, such as viral proteins synthesised by the host cell. The proteasome is a structure that breaks down proteins in the body spontaneously.
- The TAP (transporters associated with antigen processing) protein is responsible for the degradation and transport of antigens to the endoplasmic reticulum (ER), where the peptides are further processed and loaded onto the MHC class I.
- Binding of the peptide to MHC class I is followed by stabilisation of the complex by a β2-macroglobulin molecule and subsequent export to the cell membrane.
- CD8 T cells (cytotoxic T cells) specific for the attached peptide can now identify the full surface molecule.
MHC Class II
- Exogenous peptides from organelle-processed antigens are bound by MHC class II molecules.
- After engulfing an extracellular antigen by phagocytosis or endocytosis, APCs wrap it in a vesicle within the cell.
- In order to break down the antigen into smaller peptide fragments, the APCs get acidified upon activation, activating proteases in the process.
- Peptide-containing vesicles are joined with MHC class II protein-containing vesicles.
- Peptide specific CD4 T cells (helper T cells) can recognise it once it has fused with MHC class II molecules and been transported to the cell membrane surface.
Diseases Involving T Cells
Diseases that target T cells, a crucial part of the immune system, can have devastating effects very fast. T cell lymphoma and T cell mediated rejection are two further examples of such disorders that might have fatal consequences. As of right now, there is no cure for these conditions. Recent successes in stem cell study and other medicines, however, have opened up promising new doors.
Human Immunodeficiency Virus (HIV)
- HIV is a retrovirus that infects the immunological and nervous systems and has a spherical shape with a liquid envelope.
- The CD4 molecule (together with other receptors, such as CCR5 and CXCR4) on macrophages and CD4+ T cells is used as a portal for cell entrance.
- By binding to these receptors, HIV is able to fuse with the cell membrane and then inject its genome into the cytoplasm.
- An intracellular kinase gene called nef can be found in the genome. This may lead to the activation of afflicted T cells, viral replication, and increased infectiousness. It also causes a decrease in CD4+ and MHC molecules within the infected cells.
- Once the genome enters the cell, it undergoes reverse transcription to produce complementary DNA (cDNA). This cDNA is efficiently integrated into the genome of proliferating T cells, where it can remain dormant for months to years.
- Transcription of the proviral DNA can also result in the production of viral particles on the membrane of an infected cell, which can trigger the cell’s programmed death response.
- Memory In the early stages of HIV infection, when substantial cell death rates are present, the vast reservoir of lymphocytes is quickly depleted because CD4+ T cells are the first to be infected. At this stage, the virus has already spread throughout the body and triggered an immunological reaction.
- Through this mechanism, dendritic cells are enlisted, which then consume the infected T cells. Following antigen processing, dendritic cells transmit the modified antigen to functional CD4+ T cells. The viral infection then spreads swiftly throughout the body.
- Helper T cells, macrophages, and dendritic cells are then infected. Additional helper T cells then go on the offensive against the infected T cells, activating the immune system in two ways: humoral and cell-mediated.
- When T cells are depleted, a vicious loop is created. While the immune response isn’t yet at full strength, it can nevertheless fend off most new pathogens in this early stage.
- However, HIV relentlessly replicates and kills off the immune cells it infects in the subsequent phase. During clinical latency, the number of CD4+ T cells in the bloodstream gradually decreases.
- Eventually, there won’t be enough CD4+ cells to go around since they’ll be dying off quicker than they can multiply. Reduced CD4+ T-cell counts lead to the onset of acquired immunodeficiency syndrome (AIDS).
- Because of the compromised immune system, the body is more vulnerable to future infections.
- Therefore, HIV/AIDS is not fatal on its own. Patients with HIV/AIDS, however, do not mount a sufficient immune response to fend off illness.
- HIV has no definitive treatment available at this time. In contrast, the most effective treatments focus on avoiding the disease altogether or at least delaying its progression.
- Antiretroviral medication has been relatively effective in prolonging the health of HIV patients. Replacement of the lost white blood cells with HIV-resistant variants may, however, be the key to a complete cure, as suggested by new stem cell research.
T Cell Lymphoma
- T cell lymphoma is a kind of cancer in which T cells are targeted. It is initially produced by the infection of the human T cell leukaemia virus type 1 (HTLV-1), a type of retrovirus.
- It can develop in transverse myelitis, which is a disorder that leads in demyelination of the central nervous system.
- Typically, people presenting with T cell lymphoma have skin lesions, lymphadenopathy, hepatosplenomegaly, hypercalcemia, and different malignancies in the blood. The cells infected also exhibit large amounts of CD25.
- As an exceedingly aggressive tumour, patients typically last 8 months past diagnosis. Motivated to overcome this bad prognosis, various investigations have been done to develop effective treatments, including monoclonal antibodies, histone deacetylase inhibitors, anti-metabolites, and more immunomodulary medicines.
T Cell Mediated Rejection
- Transplanted organs can be rejected in the new body due to cell- mediated rejection and/or antibody- mediated rejection.
- This is usually when the host’s T cells do not identify the new organ’s cells as its own, resulting in immune system activation.
- Cytotoxic T cells damage the new organ’s cells, which can lead to parenchymal and endothelial cell death. CD4+ T cells stimulate inflammation, allowing lymphocytes and macrophages to fill the new organ.
- Additionally, microphages can target the cell and blood arteries, leading to reduced oxygen delivery in the organ.
- In order to safeguard the new organ, transplant recipients have to take immunosuppressive medicines to prevent their immune systems from activating.
- Typically, patients take these medicines for as long as they get the transplant. However, recent studies may discover that mingling the white blood cells from the suitable organ donor and the patient minimises organ reject.
- In this study, several of the patients were able to quit immunosuppressive drug treatments without organ rejection for at least two years.
Function of T cells in Immune system
- T cells play a critical role in the immune system, particularly in the adaptive immune response. They contribute to various aspects of immune function, including direct destruction of infected cells, cytokine production, immune cell activation, and regulation of immune responses.
- One of the key functions of T cells is their ability to recognize antigens. T cells express receptors known as T cell receptors (TCRs) on their surface, which enable them to recognize specific antigens presented by antigen-presenting cells (APCs). This recognition allows T cells to identify infected or abnormal cells and initiate an immune response against them.
- T cells are involved in cell-mediated immunity, where they directly destroy infected host cells. Once activated by antigen recognition, cytotoxic T cells (also known as CD8+ T cells) can directly kill infected cells by releasing cytotoxic molecules, such as perforin and granzymes, or by triggering apoptosis in the target cells.
- In addition to their cytotoxic function, T cells also have a role in cytokine production. Upon activation, T cells can secrete a wide range of cytokines that help regulate immune responses. Cytokines produced by T cells can have various effects, including activating other immune cells, promoting inflammation, enhancing immune responses, or dampening immune reactions.
- T cells are instrumental in the activation of other immune cells. They can interact with and provide necessary signals to B cells, which are responsible for antibody production, enhancing the antibody response. T cells can also interact with macrophages and dendritic cells, promoting their activation and enhancing their ability to present antigens to other immune cells.
- Another critical function of T cells is the modulation of immunological responses. Regulatory T cells (Tregs) are a specialized subset of T cells that play a crucial role in maintaining immune tolerance and preventing excessive immune responses. Tregs help regulate the activity of other T cells and prevent them from attacking self-tissues, thus contributing to self-tolerance and preventing autoimmune diseases.
- T cells also play a role in immunological memory. Upon encountering an antigen, some T cells differentiate into memory T cells, which can persist for long periods and quickly respond to subsequent encounters with the same antigen. This memory response allows for a more rapid and robust immune response upon re-exposure to a pathogen, leading to more effective clearance of the infection.
- Overall, T cells are essential for the establishment, maintenance, and regulation of immunological responses. Their ability to recognize antigens, kill infected cells, produce cytokines, activate other immune cells, and modulate immune responses contributes to the overall effectiveness of the immune system in defending against pathogens and maintaining immune homeostasis.
Chimeric antigen receptors (CAR) T cell therapy
- Chimeric antigen receptor (CAR) T cell therapy is an innovative approach to cancer treatment that harnesses the power of the immune system. This therapy involves genetically modifying a patient’s T cells to express chimeric antigen receptors on their surface, enabling them to recognize and target specific cancer cells.
- CAR T cell therapy has shown significant success in the treatment of hematologic malignancies, such as certain types of leukemia and lymphoma. The targeted antigen in many of these cases is CD19, a protein found on the surface of precursor and mature B cells. By engineering T cells to express CARs that recognize CD19, the therapy can effectively target and eliminate cancerous B cells, providing a potentially curative treatment option for B cell malignancies.
- Clinical trials have demonstrated remarkable outcomes for patients receiving CAR T cell therapy for hematologic cancers. Many patients who had previously exhausted standard treatment options, including chemotherapy and stem cell transplantation, have achieved complete remissions and long-term survival with CAR T cell therapy.
- While CAR T cell therapy has shown great success in hematologic cancers, its application in solid tumors is more challenging. Solid tumors often have a more complex tumor microenvironment, which can impede the effectiveness of CAR T cells. The lack of specific tumor antigens that are unique to cancer cells further complicates the targeting process.
- Efforts are underway to overcome these challenges and improve the efficacy of CAR T cell therapy for solid tumors. Researchers are exploring different strategies, such as identifying novel tumor antigens and engineering CAR T cells with enhanced tumor penetration capabilities. Additionally, combination therapies involving CAR T cells and other immunotherapies, such as checkpoint inhibitors, are being investigated to enhance the overall anti-cancer response.
- While the efficacy of CAR T cell therapy in solid tumors is still being optimized, there have been promising results in certain types of cancers, including certain types of sarcoma and neuroblastoma. Ongoing research and clinical trials are continuously expanding our understanding of CAR T cell therapy and its potential applications in various cancer types.
- In summary, CAR T cell therapy is a groundbreaking immunological approach to cancer treatment. By genetically modifying T cells to express chimeric antigen receptors, this therapy enables targeted recognition and elimination of cancer cells. While its success has been remarkable in hematologic malignancies, further advancements are needed to enhance its efficacy in solid tumors. Nonetheless, CAR T cell therapy represents a significant advancement in the field of cancer immunotherapy and offers hope for improved outcomes in cancer patients.
FAQ
What are T cells?
T cells, also known as T lymphocytes, are a type of white blood cell that plays a crucial role in the immune system’s adaptive response to pathogens, foreign substances, and cancer cells.
What is the function of T cells?
T cells have several functions in the immune system. They can directly kill infected host cells or cancer cells, activate other immune cells, produce cytokines to regulate immune responses, and contribute to the development of immunological memory.
How do T cells recognize antigens?
T cells express antigen receptors called T-cell receptors (TCRs) on their surface. These receptors can recognize specific antigens presented by major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells.
What is the difference between CD4 and CD8 T cells?
CD4 and CD8 are co-receptors expressed on the surface of T cells. CD4 T cells, also known as helper T cells, assist other immune cells and coordinate immune responses. CD8 T cells, also called cytotoxic T cells, directly kill infected cells or cancer cells.
How do T cells develop and mature?
T cells are produced in the bone marrow from hematopoietic stem cells and then migrate to the thymus, where they undergo a process of maturation and education. In the thymus, T cells undergo positive and negative selection to ensure they can recognize foreign antigens while tolerating self-antigens.
What is the role of T cells in immune memory?
T cells play a crucial role in the establishment of immunological memory. Once they encounter an antigen, some T cells differentiate into memory T cells, which persist in the body after the infection resolves. These memory T cells can mount a faster and stronger immune response upon re-exposure to the same antigen.
What are regulatory T cells?
Regulatory T cells, or Tregs, are a subset of T cells that help maintain immune tolerance and prevent excessive immune responses. They suppress the activity of other immune cells and play a role in preventing autoimmune diseases.
Can T cells be used in cancer therapy?
Yes, T cells can be engineered and used in cancer therapy. Chimeric antigen receptor (CAR) T cell therapy is an example where T cells are genetically modified to express receptors that target specific antigens on cancer cells, enabling them to recognize and eliminate the cancer cells.
How do T cells interact with B cells?
T cells and B cells cooperate in the immune response. T cells provide help to B cells by activating them and promoting the production of antibodies. This interaction is crucial for the generation of effective antibody responses against pathogens.
What happens if T cells become overactive or dysregulated?
Overactive or dysregulated T cell responses can lead to various immune-related disorders, including autoimmune diseases, allergies, and chronic inflammatory conditions. In these cases, the immune system mistakenly targets self-antigens or overreacts to harmless substances.
References
- Heath, W. R. (1998). T Lymphocytes. Encyclopedia of Immunology, 2341–2343. doi:10.1006/rwei.1999.0588
- Actor, J. K. (2014). T Lymphocytes. Introductory Immunology, 42–58. doi:10.1016/b978-0-12-420030-2.00004-4
- Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018 Jul;8(4):539-551. doi: 10.1016/j.apsb.2018.03.001. Epub 2018 Apr 5. PMID: 30109179; PMCID: PMC6090008.
- Kumar BV, Connors TJ, Farber DL. Human T Cell Development, Localization, and Function throughout Life. Immunity. 2018 Feb 20;48(2):202-213. doi: 10.1016/j.immuni.2018.01.007. PMID: 29466753; PMCID: PMC5826622.
- Koch U, Radtke F. Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol. 2011;27:539-62. doi: 10.1146/annurev-cellbio-092910-154008. Epub 2011 Jul 5. PMID: 21740230.
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. T Cells and MHC Proteins. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26926/
- https://teachmephysiology.com/immune-system/cells-immune-system/t-cells/
- https://my.clevelandclinic.org/health/body/23547-cytotoxic-t-cells
- https://microbenotes.com/t-cell-t-lymphocyte/
- https://www.akadeum.com/blog/different-types-of-t-cells/
- https://www2.nau.edu/~fpm/immunology/Exams/Tcelldevelopment-401.html
- https://lymphoma.org/understanding-lymphoma/aboutlymphoma/nhl/t-cell-lymphoma/
- https://www.celiackidsconnection.org/2018/05/06/what-are-the-different-types-of-t-cells/
- https://biologydictionary.net/t-cells/
- https://www.news-medical.net/health/What-are-T-Cells.aspx