In the early 20th century there was no treatment to treat infections caused by bacteria. This included tuberculosis and pneumonia, as well as the rheumatic and gonorrhea diseases, and infections of the urinary tract. However, in 1929 the the bacteriologist Alexander Fleming discovered the first real antibiotic, penicillin, heralding a new era of medical science.
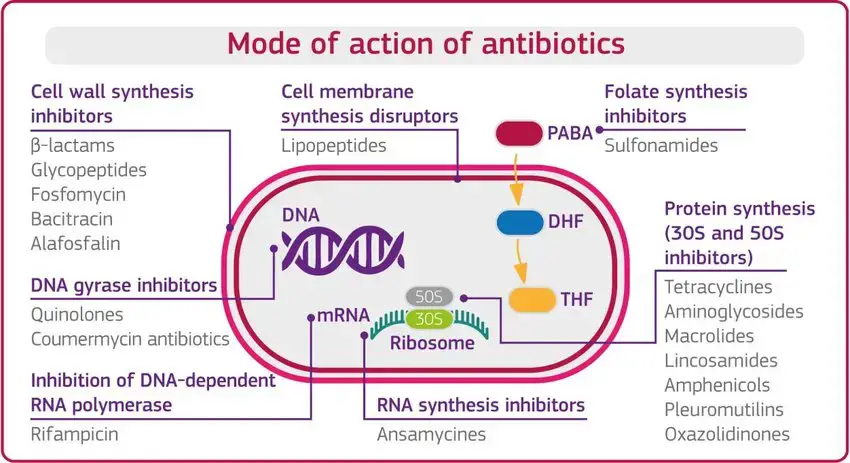
Since then, researchers have discovered an array of antibiotics that fight bacteria in a variety ways.
Many antibiotics, like penicillin, attack the cells wall that bacteria use to live. In particular, they block bacteria from synthesising an molecule within the cell wall known as peptidoglycan. It gives the wall the strength needed to endure within the body of the individual.
However, there are many ways to hinder the synthesis of peptidoglycanvancomycin for instance can also inhibit peptidoglycan however not in the way as penicillin.
Inhibition of Cell Wall Biosynthesis by Antibiotics
Antibiotics are substances that are specifically designed to destroy cells. The term”antibiotic” is often employed interchangeably with antibacterial, however antiviral, antifungal and other antineoplastic compounds are also known as antibiotics. Antibacterial actions generally fall within one of three mechanisms which are based on the suppression or control of the enzymes that are involved in cell wall biosynthesis nucleic acid metabolism , repair or protein synthesis in turn. A lot of the cellular functions that antibiotics target are the most effective when cells multiply. There is usually an overlap in these functions among prokaryotic bacterial cells as well as mammalian cells of the eukaryotic species it’s not unusual that certain antibiotics have also been proven to be effective in the fight against cancer.
Inhibition of Cell Wall Biosynthesis
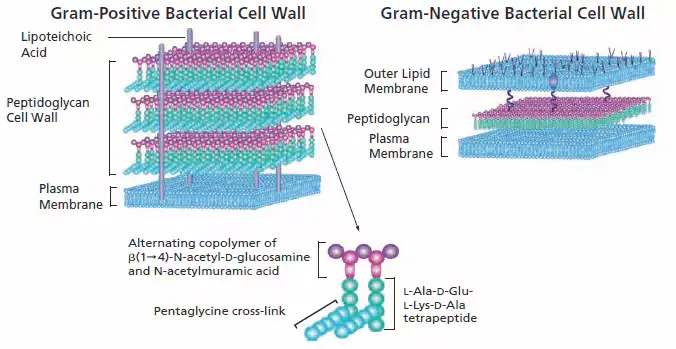
In structure, bacteria are similar to primitive plants because the cells are enclosed by an inner peptidoglycan cellular wall, in addition to an inner plasma membrane as well as in Gram-negative bacteria, an outer bilayer of lipids. Certain antibacterials hinder the production of cell walls and weaken the peptidoglycan scaffold inside the bacterial wall, so that its structural integrity is eventually compromised. Because mammalian cells possess plasma membranes but do not have the structure of the peptidoglycan wall and this class of antibacterials targets bacteria without having a significant adverse effect on the mammalian cells that make up the host.
Peptidoglycan formation begins in the cytoplasm , with the production of a muramyl precursor that contains a terminal D-Ala-Dala. Certain antibiotics hinder the process of synthesis of the primary building block of peptidoglycans. For instance,
D-cycloserine
D-cycloserine blocks two enzymes that are involved in the precursor synthesisprocess, stopping both the conversion of L-alanine to D-alanine via racemase and the synthesis of D-alanyl D-alanine via D-Ala-Dal ligase. Within the cytoplasm, muramyl pentapeptide is linked to an UDP-glucosamine water-soluble moiety.
In the next phase of peptidoglycan formation the muramyl pentapeptide N acetylglucos changed to an C55 undecaprenyl phosphate following an UMP release and forms an Lipid I intermediate.
β-Lactam antibiotics
β-Lactam antibiotics, such as monobactams, cephalosporins and penicillins and carbapenems, are distinguished by a lactam ring within their molecular structure. They interfere with the production of the collagen layer on bacteria’s cell walls. They block the transpeptidase and transglycosylase or carboxypeptidase functions of specific membrane-bound penicillin-binding proteins (PBPs) which facilitate cross-linking between cells’ wall components in the final phase of bacterial cell wall formation. The process is initiated through adhering to the terminus D-Ala-Dala in the structure of the peptidoglycan that is lengthening and then rapidly acylating the serine that is the active site of the PBP. This is then deacylation is very slow that disables the PBP.
When cells expand and expands, it’s not able to produce cells with more wall thickness to support the growing. This means that the pressure within the cell will force the plasma membrane away from the weak point inside the cell wall, much like balloons, and eventually burst. Because the creation of the division furrow in order to generate new daughter cells is dependent on the capability to synthesize the new cell wall cells are unable to squeeze out the extra cell’s cytoplasmic material. A spheroplast that is extremely fragile is created when the cell wall completely disappears. In this situation bacteria lose control of their shape and replication of a large portion of their metabolic and genetic material disrupts their homeostasis which causes cell death.
Bacteria can develop resistance to β-lactam antibiotics through the production of the enzyme β-lactamase. It targets the β-lactam ring in order to inhibit the antibiotic. Over 1,000 unique β-lactamases have already been identified. To overcome this resistance β-lactam antibiotics are typically administered with β-lactamase inhibitors. Efflux, as well as modification or elimination of porin function could also be a factor in resistance to certain β-lactams combination with β-lactamase production that results in resistance to almost all β-lactams. Certain bacteria are also capable of developing new PBPs that are not suited for β-lactams. The best-known example of this strategy for resistance occurs with methicillin-resistant S. aureus (MRSA) that evolved a PBP with low affinity for all but a few recently developed β-lactams.
Tunicamycin
Tunicamycin hinders the conversion of the undecaprenylphosphate into the intermediate lipid I, thereby stopping the process of completing the structure of the peptidoglycan. Another glycosylation procedure completes the peptidoglycan structure, after which it is transferred through it’s C55 liquid tail, which is then transported to the membrane’s periplasmic surface which is where the peptidyglycan component becomes embedded into the wall of the cell.
Bacitracin
Bacitracin blocks lipid phosphatase and prevents the release of the peptidoglycan’s finished form from its C55 carrier of lipids.
A variety of transpeptidases and transglycosylases link to form new peptidoglycan structure with the cells’ the peptidoglycan matrix. The uniqueness of antibacterials b-lactam can be explained by their capability to inhibit transpeptidase enzymes as well as stop the formation of the peptidoglycan peptidoglycan layers in the Gram-positive as well as Gram-negative bacteria. B-Lactam molecules due to their structural resemblance to the D-alanyl-Dalanine group in the peptidoglycan structure compete for binding sites for transpeptidases. When first introduced to the market penicillin, a b-lactam antibiotic, was regarded as an “magic bullet” because of its ability to fight bacteria without harming patients.
Vancomycin
Vancomycin is a glycopeptide antibiotic that has a much bigger structure also blocks cell wall formation through interfering in transglycosylases. Its efficacy is restricted to Gram-positive bacteria as it is ineffective at piercing the cytoplasmic outer membrane of Gram-negative bacteria because of its massive size contrasted to penicillin.
fosfomycin
The phosphonic acid fosfomycin blocks the development of vast variety of bacteria through blocking the enolpyruvyltransferase MurA that catalyzes the initial step in the synthesis of peptidoglycans that is crucial for the formation of cell walls. It is absorbed into cells via glycerol-3-phosphate and hexose-6-phosphate transportation systems. A defect in these transporters which result in increased efflux or reduced the rate of cellular absorption can result in resistance. Particular murA genetic mutations which result in an enzyme that has a lower fosfomycin affinity or an increased expression of MurA that impedes the effectiveness fosfomycin to inhibit growth are also known to cause resistance. Fosfomycin-inactivating enzymes have also been documented.
Glycopeptides and lipoglycopeptides
Lipoglycopeptides and Glycopeptides consist of glycosylated polycyclic and cyclic nonribosomal peptides which interfere with cell wall development by creating a complex between an antibiotic as well as the C-terminal D.Ala-D.Ala dipeptide from the nascent peptidoglycan that is located on the surface of the cell membranes from Gram positive bacteria. The creation of this complex blocks the transpeptidation and transglycosylation reactions required for the completeness of the peptidoglycan chains, leading to an ineffective cell wall, and consequent cell death.
They are too large to be able to traverse the porin channels that are found within the outer membrane that Gram-negative bacteria. In addition to its interaction with D-Ala-D-Ala, some antibiotics in this group bind to lipid II, a cell wall precursor on the cytoplasmic side of the cell membrane that must translocate across the cell membrane to deliver and incorporate its disaccharide-pentapeptide monomer for cross-linking into peptidoglycan.
This process results in the membrane’s depolarization, and ultimately disintegration that causes cell death. Because glycopeptides are able to target the outer layer of the cell wall They do not need to cross a membrane and are not able to interfer with enzymes. The mechanisms that are known to be resistant to glycopeptides are a result of structural changes within the substrates of enzymes that contain the amino acids that are the final ones in the precursors of pentapeptides. The most significant modifications are the replacement of the C-terminal D’Ala with D-lactate or Dserine, which results in D-Ala D-Lac or D-Ala D-S sequences of peptides that have reduced affinity for binding to the antibiotic.
Mechanism of Cell Wall Biosynthesis Inhibition by Antibiotics
Bacteria, akin to primitive plants, possess a unique cell wall structure. This cell wall, primarily composed of peptidoglycan, provides essential rigidity and shape. However, certain antibiotics can disrupt the biosynthesis of this critical component, leading to bacterial cell death.
Peptidoglycan Synthesis
Peptidoglycan synthesis starts in the cytoplasm with the creation of muramyl pentapeptide precursors. These precursors include a terminal D-Ala-D-Ala dipeptide. Various antibiotics target this initial stage. For instance, D-cycloserine inhibits enzymes responsible for converting L-alanine to D-alanine and forming the D-Ala-D-Ala dipeptide. This inhibition halts the synthesis of the peptidoglycan building blocks.
Lipid Carrier and Assembly
In the next phase, muramyl pentapeptide N-acetylglucosamine transfers to a lipid carrier, C55 undecaprenyl phosphate, forming a lipid I intermediate. This step is crucial for peptidoglycan assembly. Tunicamycin, for example, obstructs the formation of this lipid intermediate, stalling peptidoglycan synthesis. Subsequently, the completed peptidoglycan unit, attached to its lipid carrier, is transported to the periplasmic surface, where it integrates into the cell wall.
Integration and Cross-linking
Several enzymes, including transpeptidases and transglycosylases, facilitate the integration of new peptidoglycan units into the existing cell wall matrix. β-lactam antibiotics, such as penicillin, target these transpeptidase enzymes. By mimicking the D-alanyl-D-alanine structure, β-lactams competitively inhibit these enzymes, preventing the formation of cross-links within the peptidoglycan layer. This inhibition compromises the cell wall’s integrity, leading to cell lysis.
Glycopeptide Antibiotics
Glycopeptide antibiotics, like vancomycin, also inhibit cell wall synthesis but operate differently. They bind to the D-Ala-D-Ala termini of peptidoglycan precursors, obstructing the transglycosylation and transpeptidation steps necessary for cell wall construction. Due to their larger size, glycopeptides are effective primarily against Gram-positive bacteria, as they cannot penetrate the outer membrane of Gram-negative bacteria.
Resistance Mechanisms
Bacteria can develop resistance to these antibiotics through several mechanisms:
- β-Lactamases: These enzymes hydrolyze the β-lactam ring, rendering the antibiotic ineffective.
- Efflux Pumps: Increase the expulsion of the antibiotic from the bacterial cell.
- Porin Modifications: Alterations that reduce antibiotic uptake.
- Modified Penicillin-Binding Proteins (PBPs): Reduced affinity for β-lactams, seen in methicillin-resistant Staphylococcus aureus (MRSA).
- Altered Target Sites: Changes in the D-Ala-D-Ala structure, such as the replacement with D-Ala-D-Lactate, reduce glycopeptide binding.
Phosphonic Acid Antibiotics
Fosfomycin, a phosphonic acid antibiotic, targets the enzyme MurA, which catalyzes the first step in peptidoglycan synthesis. By inhibiting MurA, fosfomycin prevents the formation of the cell wall. Resistance can arise from mutations in the murA gene, changes in transport systems, or enzymatic inactivation of fosfomycin.
References
- Sanseverino, Isabella & Navarro, Anna & Loos, Robert & Marinov, Dimitar & Lettieri, Teresa. (2018). State of the Art on the Contribution of Water to Antimicrobial Resistance. 10.2760/771124.
- Beta-Lactam Antibiotics & Other Cell Wall Synthesis Inhibitors. In: Trevor AJ, Katzung BG, Kruidering-Hall M. eds. Katzung & Trevor’s Pharmacology: Examination & Board Review, 11e. McGraw Hill; 2015. Accessed February 17, 2022. https://accesspharmacy.mhmedical.com/content.aspx?bookid=1568§ionid=95703865
- Romaniuk JA, Cegelski L. Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Philos Trans R Soc Lond B Biol Sci. 2015;370(1679):20150024. doi:10.1098/rstb.2015.0024
- Nami Katayama, Hiroyoshi Takano, Motoji Sugiyama, Susumu Takio, Atsushi Sakai, Kan Tanaka, Haruko Kuroiwa, Kanji Ono, Effects of Antibiotics that Inhibit the Bacterial Peptidoglycan Synthesis Pathway on Moss Chloroplast Division, Plant and Cell Physiology, Volume 44, Issue 7, 15 July 2003, Pages 776–781, https://doi.org/10.1093/pcp/pcg096
- Inhibiting Cell Wall Synthesis. (2021, January 4). https://bio.libretexts.org/@go/page/11931
- https://www.livescience.com/44201-how-do-antibiotics-work.html