What are Amino Acids?
- Amino acids are a distinct group of organic compounds that set themselves apart from other natural substances based on their unique chemical and biochemical properties. They possess ampholytic properties, meaning they can act as both acids and bases, and they play a crucial role as constituents of proteins. Amino acids are characterized by the presence of an aliphatic primary amino group in the α position to the carboxyl group within a carboxylic acid structure, along with a specific stereochemistry.
- Proteins, which are vital for the proper functioning of living organisms, are synthesized from a set of 20 amino acids. This process is governed by strict genetic control, making amino acids the fundamental units of proteins. While there are over 300 amino acids found in nature, only the 20 standard amino acids are incorporated into proteins due to their genetic coding. The remaining amino acids are categorized as non-protein amino acids, either as modified residues occurring after protein synthesis through posttranslational modifications or as amino acids present in organisms but not as protein constituents.
- Amino acids consist of carbon, hydrogen, and nitrogen atoms, forming organic compounds. They serve as monomers or building blocks for proteins, which make them essential biomolecules. Proteins, unlike other biomolecules such as carbohydrates, nucleic acids, and lipids, were initially recognized for their biological roles in organisms. These biomolecules possess well-defined physicochemical properties. The linear sequence of amino acids, referred to as the one-dimensional protein structure, governs the formation of the protein’s three-dimensional structure. This structure, in turn, determines its interactions with other molecules.
- The understanding of amino acids began over a century ago with the observation that proteins, when exposed to hydrolysis by boiling acids, break down into smaller molecular substances. The first amino acid to be discovered and isolated was Glycine, obtained from gelatin. Subsequently, other amino acids like cystine, asparagine, leucine, and threonine were identified, with threonine being the final of the 20 common amino acids to be discovered in 1935. By 1983, approximately 500 amino acids had been identified through various studies. However, only 20 amino acids were found to be present in the genetic code, making them the essential building blocks of proteins.
- In summary, amino acids are a distinctive group of organic compounds composed of carbon, hydrogen, and nitrogen. They act as monomers for proteins and play a fundamental role in the functioning of living organisms. The linear sequence of amino acids determines the three-dimensional structure of proteins, influencing their interactions with other molecules. Over time, extensive research has led to the discovery of numerous amino acids, but only 20 are incorporated into proteins as per the genetic code. Understanding the structures, functions, and applications of amino acids provides valuable insights into their significance in the real world.
Definition of Amino Acids
Amino acids are organic compounds that serve as the building blocks of proteins. They consist of carbon, hydrogen, and nitrogen atoms and play essential roles in the proper functioning of living organisms.
List of 20 Amino acids with the chemical formula
| Alanine | C3H7NO2 | Leucine | C6H13NO2 |
| Aspartic Acid | C4H7NO4 | Lysine | C6H14N2O2 |
| Asparagine | C4H8N2O3 | Methionine | C5H11NO2S |
| Arginine | C6H14N4O2 | Proline | C5H9NO2 |
| Cytosine | C4H5N3O | Phenylalanine | C9H11NO2 |
| Cysteine | C3H7NO2S | Serine | C3H7NO3 |
| Glycine | C2H5NO2 | Tyrosine | C9H11NO3 |
| Glutamine | C5H10N2O3 | Threonine | C4H9NO3 |
| Histidine | C6H9N3O2 | Tryptophan | C11H12N2O2 |
| Isoleucine | C6H13NO2 | Valine | C5H11NO2 |
Physical properties of amino acids
- Color and Crystalline Solid: Amino acids are colorless and exist as crystalline solids.
- High Melting Point: All amino acids have a melting point greater than 200°C, indicating their stability at high temperatures.
- Solubility: Amino acids are generally soluble in water. However, their solubility varies depending on the R-group and the pH of the solvent. They are slightly soluble in alcohol and show limited solubility in methanol, ethanol, and propanol.
- Decomposition: When exposed to high temperatures, amino acids undergo decomposition.
- Optical Activity: Except for glycine, all amino acids are optically active due to the presence of asymmetric α-carbon atoms.
- Peptide Bond Formation: Amino acids can form peptide bonds by connecting their amino and carboxylate groups. Peptide bonds are covalent bonds formed between the alpha-amino group of one amino acid and the alpha-carboxyl group of another. These bonds are planar and partially ionic in nature.
Additional Information:
- Taste: Some amino acids exhibit specific tastes. For example, glycine, alanine, and valine may taste sweet, leucine is tasteless, while arginine and isoleucine can taste bitter.
- Zwitterions and Isoelectric Point: Amino acids can exist as zwitterions, which are hybrid molecules containing both positive and negatively charged groups. The term “Zwitter” comes from the German word meaning “hybrid.” At normal pH levels in cells, the carboxyl group donates a proton to the amino group within the same molecule. The pH at which an amino acid carries no net electrical charge is called the isoelectric point.
- Titration Curve of Glycine: Glycine, being the simplest amino acid, is optically inactive as it lacks an asymmetric carbon atom. Acid-base titration involving glycine includes the gradual addition or removal of protons to study its behavior in solution.
| Amino Acid | Code | Hydropathy | Charge | pKa, NH2 | pKa, COOH | pK(R) | Solubility |
|---|---|---|---|---|---|---|---|
| Arginine | R | hydrophilic | + | 9.09 | 2.18 | 13.2 | 71.8 |
| Asparagine | N | hydrophilic | N | 8.8 | 2.02 | 2.4 | |
| Aspartate | D | hydrophilic | – | 9.6 | 1.88 | 3.65 | 0.42 |
| Glutamate | E | hydrophilic | – | 9.67 | 2.19 | 4.25 | 0.72 |
| Glutamine | Q | hydrophilic | N | 9.13 | 2.17 | 2.6 | |
| Lysine | K | hydrophilic | + | 8.9 | 2.2 | 10.28 | |
| Serine | S | hydrophilic | N | 9.15 | 2.21 | 36.2 | |
| Threonine | T | hydrophilic | N | 9.12 | 2.15 | freely | |
| Cysteine | C | moderate | N | 10.78 | 1.71 | 8.33 | freely |
| Histidine | H | moderate | + | 8.97 | 1.78 | 6 | 4.19 |
| Methionine | M | moderate | N | 9.21 | 2.28 | 5.14 | |
| Alanine | A | hydrophobic | N | 9.87 | 2.35 | 15.8 | |
| Valine | V | hydrophobic | N | 9.72 | 2.29 | 5.6 | |
| Glycine | G | hydrophobic | N | 9.6 | 2.34 | 22.5 | |
| Isoleucine | I | hydrophobic | N | 9.76 | 2.32 | 3.36 | |
| Leucine | L | hydrophobic | N | 9.6 | 2.36 | 2.37 | |
| Phenylalanine | F | hydrophobic | N | 9.24 | 2.58 | 2.7 | |
| Proline | P | hydrophobic | N | 10.6 | 1.99 | 1.54 | |
| Tryptophan | W | hydrophobic | N | 9.39 | 2.38 | 1.06 | |
| Tyrosine | Y | hydrophobic | N | 9.11 | 2.2 | 10.1 | 0.038 |
Chemical Properties of amino acids
- Zwitterionic Property: Amino acids exhibit zwitterionic properties, meaning they contain functional groups with both positive and negative charges. The presence of an amine group (basic) and a carboxylic group (acidic) allows amino acids to exist as zwitterions. The amine group accepts a hydrogen ion (H+) from the carboxylic group, resulting in a neutral zwitterion with no net charge. This is the predominant form of amino acids in solution.
- Amphoteric Property: Amino acids display amphoteric behavior, acting as both acids and bases due to the presence of amine and carboxylic groups. The amine group can act as a base, accepting protons, while the carboxylic group can act as an acid, donating protons. This dual nature allows amino acids to participate in various chemical reactions.
- Ninhydrin Test: The ninhydrin test is used to detect the presence of α-amino acids in a protein solution. When ninhydrin solution is added and heated, a violet color is formed, indicating the presence of α-amino acids.
- Xanthoproteic Test: The xanthoproteic test is employed to identify aromatic amino acids, such as tyrosine, tryptophan, and phenylalanine, in a protein solution. Nitric acid reacts with the benzoid radicals present in the amino acid chain, resulting in a yellow coloration.
- Reaction with Sanger’s Reagent: Sanger’s reagent, also known as 1-fluoro-2,4-dinitrobenzene, reacts with free amino groups in the peptide chain under mild alkaline conditions and cold temperatures. This reaction allows for the identification and labeling of amino acids.
- Reaction with Nitrous Acid: Nitrous acid reacts with the amino group of amino acids, liberating nitrogen gas and forming the corresponding hydroxyl group. This reaction can be utilized for specific chemical modifications and derivatization of amino acids.
Amino acid wheel

Structure of Amino acids
The structure of amino acids follows a general pattern: H2NCHRCOOH. It can be visually represented as:

There are 20 naturally occurring amino acids, and despite their differences, they share common structural features. These include an amino group (-NH3+), a carboxylate group (-COO-), and a hydrogen atom, all bonded to the same carbon atom known as the α-carbon. The distinguishing feature among amino acids is their unique side chain, also referred to as the R group.
The four groups attached to the α-carbon of each amino acid are as follows:
- Amino group (-NH3+): This group consists of a nitrogen atom bonded to three hydrogen atoms. It is responsible for the basic properties of amino acids.
- Carboxyl group (COOH): This group contains a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-OH). It imparts acidic properties to amino acids.
- Hydrogen atom: A single hydrogen atom is attached to the α-carbon.
- Side chain (R): The side chain, also known as the variable or unique group, distinguishes each amino acid from one another. It can be a simple alkyl group or a complex functional group, contributing to the diversity of amino acids.
The structural variation of amino acids, particularly in their side chains, plays a crucial role in determining their properties, functions, and interactions within biological systems. The unique combination of these four groups defines the distinct characteristics and roles of each amino acid in protein synthesis, enzymatic activity, and other biochemical processes.

Structure of 20 Amino acids with their chemical formula
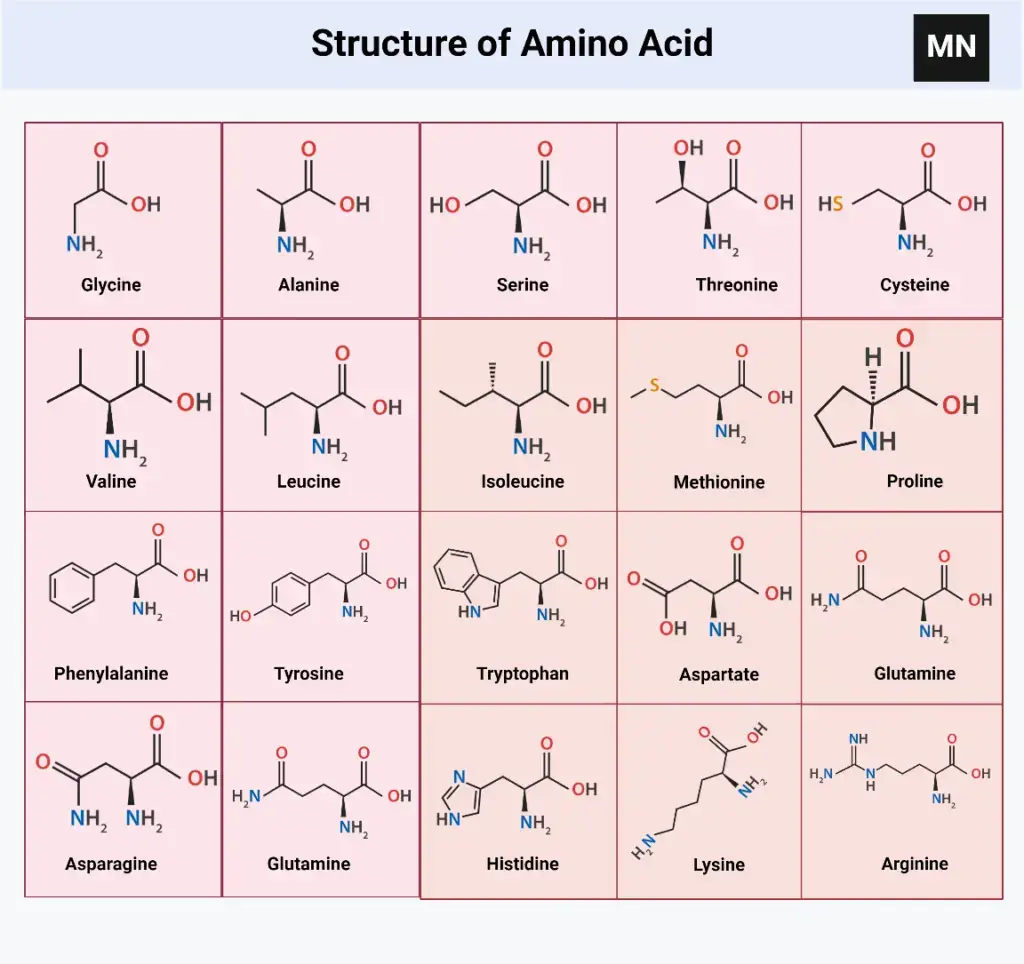
Amino acid classification based on the structure
Based on their chemical structure and physical nature, an exhaustive classification of amino acids can be made. Each amino acid is given a symbol or 3 letter name. These symbols are used to represent amino acids in the protein structure. Seven distinct groups are made up of the 20 amino acids in proteins.
- Amino acids with aliphatic side chains: Monoamine monocarboxylic acid is an amino acid that has aliphatic side chain. This group includes the most basic amino acids, such as glycine (alanine), valine, leucine, leucine, and valine. The branched side chains of the last three amino acids (Leu and Val) are what we call branched chain amino acid.
- Hydroxyl group containing amino acids: Tyrosine, threonine, and serine are examples of hydroxyl groups containing amino acid. Tyrosine, which is aromatic in nature, is usually considered to be under aromatic amino acids.
- Sulfur containing amino acids: Cysteine containing a sulfhydryl and methionine containing a thioether are two of the amino acids that were incorporated into protein synthesis. Cystine is another importa nt, sulfur-containing amino acid. It is made by the condensation of two cysteine molecules.
- Acidic amino acids and their amides: Aspartic and glutamic acid are dicarboxylic monoamino acid acids, while asparagine or glutamine are the respective amide derivatives. These four amino acids have distinct codons that allow them to be incorporated into proteins.
- Basic amino acids: The dibasic monocarboxylic acid dibasic lysine (with guanidino) and arginine with imidazole (with imidazole) are the three basic amino acids. They are very basic in nature.
- Aromatic amino acids: Phenylalanine (with an indole-ring), tyrosine, and tryptophan are all aromatic amino acids. Histidine could also be included in this category.
- Imino acids: A unique amino acid is proline containing the pyrrolidine rings. Instead of the amino group (NH2) found in other amino acid, it has an imino (NH) instead. Proline is a D-imino acid.
- Heterocyclic amino acids: Histidine, tryptophan and proline.
Classification of amino acids based on polarity
Based on their polarity, amino acids can be divided into four groups. For protein structure, polarity is crucial.
- Non-polar amino acids: Also known as hydrophobic (water hateing), these amino acids are non-polar. They do not have a charge on the R’ group. This group includes the following amino acids: alanine (leucine), isoleucine; valine; methionine; phenylalanine; tryptophan, proline.
- Polar amino acids with no charge on ‘R’ group: These amino acid have no charge on their “R” group. However, they possess groups like hydroxyl or sulfhydryl and are involved in hydrogen bonding proteins. Glycine, where R = H, is also included in this category. This group includes glycine (where R = H), serine, threonine and cysteine as well as glutamine, asparagine, tyrosine and threonine.
- Polar amino acids with positive ‘R’ group: This group includes the three amino acids histidine, arginine, and lysine.
- Polar amino acids with negative ‘R’ group: This group includes the dicarboxylic monoamino acid– aspartic and glutamic acids.
Classification of amino acids on the basis of nutrition
These 20 amino acids are necessary for the synthesis and other biological functions. All 20 amino acids are not required to be consumed in the diet. Amounts of amino acids can be divided into two groups based on nutritional requirements: essential and non-essential.
1. Essential or indispensable amino acids
Essential or indispensable amino acids play a crucial role in the body as they cannot be synthesized internally and must be obtained through dietary sources. These amino acids are essential for protein synthesis and various physiological functions. Here are the nine essential amino acids:
- Histidine: Histidine is important for the growth and repair of tissues, as well as the production of red and white blood cells. It can be found in foods like meat, poultry, fish, dairy products, and grains.
- Isoleucine: Isoleucine is involved in muscle metabolism, immune function, and energy regulation. Dietary sources of isoleucine include meat, fish, eggs, dairy products, legumes, and seeds.
- Leucine: Leucine plays a key role in muscle protein synthesis, wound healing, and regulation of blood sugar levels. It can be found in foods like meat, poultry, fish, dairy products, legumes, and nuts.
- Lysine: Lysine is essential for growth, collagen formation, calcium absorption, and the production of hormones, enzymes, and antibodies. Good sources of lysine include meat, poultry, fish, dairy products, legumes, and certain grains.
- Methionine: Methionine is involved in protein synthesis, metabolism, and the production of important molecules like glutathione. Dietary sources of methionine include meat, poultry, fish, dairy products, eggs, and certain plant foods.
- Phenylalanine: Phenylalanine is necessary for the production of neurotransmitters like dopamine, norepinephrine, and epinephrine. It can be obtained from foods such as meat, fish, eggs, dairy products, legumes, and certain grains.
- Threonine: Threonine plays a role in protein synthesis, immune function, and the maintenance of healthy skin and connective tissues. It can be found in foods like meat, poultry, fish, dairy products, eggs, and certain nuts and grains.
- Tryptophan: Tryptophan is a precursor for the production of serotonin, a neurotransmitter involved in mood regulation and sleep. Good sources of tryptophan include meat, poultry, fish, dairy products, eggs, legumes, and certain seeds.
- Valine: Valine is important for muscle metabolism, tissue repair, and the maintenance of nitrogen balance in the body. Dietary sources of valine include meat, poultry, fish, dairy products, legumes, and certain grains.
To ensure an adequate intake of these essential amino acids, it is important to include a variety of protein-rich foods in the diet, such as animal products, legumes, nuts, seeds, and whole grains.
Functions of Essential Amino acids
Essential amino acids play crucial roles in various physiological functions. Here are some unique functions of essential amino acids:
- Phenylalanine: Supports a healthy nervous system and helps enhance memory power.
- Valine: Acts as an important component in promoting muscle growth and repair.
- Threonine: Supports the functions of the immune system, promoting immune cell development and activity.
- Tryptophan: Involved in the production of vitamin B3 (niacin) and serotonin hormones, which regulate appetite, sleep, and mood.
- Isoleucine: Plays a vital role in the formation of hemoglobin, stimulates insulin synthesis by the pancreas, and aids in the transport of oxygen to various body tissues.
- Methionine: Used in the treatment of kidney stones, helps maintain healthy skin, and contributes to the control of pathogenic bacteria.
- Leucine: Promotes protein synthesis, muscle growth, and the release of growth hormones.
- Lysine: Essential for the formation of antibodies, hormones, and enzymes. It is also involved in the development and fixation of calcium in bones.
- Histidine: Plays a role in enzymatic processes and is involved in the synthesis of red blood cells (erythrocytes) and white blood cells (leukocytes).
These functions highlight the diverse roles of essential amino acids in supporting various bodily processes, including muscle growth, immune function, neurotransmitter production, hormone regulation, and cell development. Ensuring an adequate intake of these essential amino acids through a balanced diet is crucial for overall health and well-being.
2. Non-essential or dispensable amino acids
Non-essential amino acids are amino acids that can be synthesized by the body and, therefore, do not need to be obtained from dietary sources. These amino acids play important roles in various physiological functions. Here are some examples of non-essential amino acids:
- Arginine: Arginine is involved in immune function, wound healing, hormone release, and the production of nitric oxide, which helps relax blood vessels. Dietary sources of arginine include meat, poultry, fish, dairy products, nuts, and seeds.
- Aspartic acid: Aspartic acid plays a role in the synthesis of other amino acids and is involved in energy production and the functioning of the nervous system. It can be found in both animal and plant protein sources.
- Glutamic acid: Glutamic acid is important for brain function, as it acts as a neurotransmitter. It is also involved in energy metabolism and the synthesis of other amino acids. Glutamic acid is found in protein-rich foods like meat, poultry, fish, dairy products, and certain plant sources.
- Asparagine: Asparagine plays a role in protein synthesis, the formation of certain molecules in the body, and the functioning of the nervous system. It can be obtained from various dietary sources, including meat, poultry, fish, dairy products, legumes, and certain grains.
- Glutamine: Glutamine is the most abundant amino acid in the body and plays a crucial role in cell growth, immune function, and gut health. It can be found in meat, poultry, fish, dairy products, legumes, and certain plant foods.
- Proline: Proline is involved in the synthesis of collagen, the structural protein that provides support to various tissues in the body. Dietary sources of proline include meat, poultry, fish, dairy products, and certain plant foods.
- Glycine: Glycine is important for the synthesis of proteins, DNA, and other molecules in the body. It also plays a role in the central nervous system and helps regulate sleep. Glycine can be obtained from meat, poultry, fish, dairy products, legumes, and certain plant sources.
- Serine: Serine is involved in the synthesis of proteins, phospholipids, and other important molecules in the body. It can be found in meat, poultry, fish, dairy products, legumes, and certain grains.
- Tyrosine: Tyrosine is a precursor for the production of neurotransmitters such as dopamine, epinephrine, and norepinephrine. It can be obtained from various dietary sources, including meat, poultry, fish, dairy products, and certain plant foods.
- Cysteine: Cysteine plays a role in the synthesis of proteins, the antioxidant defense system, and the detoxification of harmful substances in the body. Dietary sources of cysteine include meat, poultry, fish, dairy products, and certain plant foods.
- Alanine: Alanine is involved in energy metabolism, the synthesis of glucose, and the regulation of blood sugar levels. It can be found in meat, poultry, fish, dairy products, legumes, and certain grains.
- Ornithine: Ornithine is involved in the urea cycle, which helps remove ammonia from the body. It can be synthesized from arginine and is also found in certain dietary sources like meat, poultry, fish, and dairy products.
These non-essential amino acids are synthesized by the body, but dietary sources containing these amino acids can still enhance their availability and support their functions. Including a variety of protein-rich foods in the diet ensures an adequate supply of these non-essential amino acids for optimal physiological function.
Functions of Non-Essential Amino acids
Non-essential amino acids also contribute to various physiological functions in the body. Here are unique functions of non-essential amino acids:
- Alanine: Aids in detoxification, glucose production, and the synthesis of other amino acids.
- Cysteine: Acts as an antioxidant, contributes to collagen production, and affects skin texture and elasticity.
- Glutamine: Supports healthy brain function and is essential for the synthesis of DNA and RNA.
- Glycine: Plays a role in proper cell growth, wound healing, and acts as a neurotransmitter.
- Glutamic acid: Functions as a neurotransmitter and is involved in the development and functioning of the brain.
- Arginine: Promotes protein and hormone synthesis, aids in kidney detoxification, wound healing, and supports a healthy immune system.
- Tyrosine: Essential for the production of thyroid hormones (T3 and T4), synthesis of neurotransmitters, and melanin pigments found in eyes, hair, and skin.
- Serine: Contributes to muscle growth and the synthesis of immune system proteins.
- Asparagine: Involved in nitrogen transportation into cells, synthesis of purines and pyrimidines for DNA production, nervous system development, and enhancing stamina.
- Aspartic acid: Plays a significant role in metabolism and promotes the synthesis of other amino acids.
- Proline: Essential for tissue repair, collagen formation, prevention of arterial thickening (arteriosclerosis), and regeneration of skin.
These functions highlight the diverse roles of non-essential amino acids in various biological processes, ranging from brain function and tissue repair to hormone synthesis and immune system support. While non-essential amino acids can be synthesized by the body, ensuring an adequate intake of essential and non-essential amino acids through a balanced diet is important for overall health and well-being.
Amino acid classification based on their metabolic fate
Amino acids, the building blocks of proteins, play crucial roles in various metabolic processes within the human body. One way to categorize amino acids is based on their metabolic fate, specifically their contribution to glucose formation or the production of ketone bodies. This classification helps us understand how amino acids are utilized and metabolized by the body. Let’s explore the different categories of amino acids based on their metabolic fate:
- Glucogenic amino acids: These amino acids serve as precursors for gluconeogenesis, the process of glucose formation. Glucose is an essential energy source for the body, especially during fasting or periods of low carbohydrate intake. Glucogenic amino acids can be converted into intermediates that participate in gluconeogenesis. The following amino acids fall into this category:
- Glycine
- Alanine
- Serine
- Aspartic acid
- Asparagine
- Glutamic acid
- Glutamine
- Proline
- Valine
- Methionine
- Cysteine
- Histidine
- Arginine
When the body requires glucose, these amino acids can be broken down to produce energy or be converted into glucose molecules to meet the body’s energy demands.
- Ketogenic amino acids: These amino acids are primarily metabolized to form ketone bodies. Ketone bodies are produced when the body breaks down fatty acids for energy, typically during prolonged fasting or low-carbohydrate diets. The two amino acids classified as ketogenic are:
- Leucine
- Lysine
The breakdown of these amino acids leads to the production of ketone bodies, which can serve as an alternative fuel source for certain tissues, such as the brain, during periods of limited glucose availability.
- Both glucogenic and ketogenic amino acids: This category includes amino acids that can be metabolized to form precursors for both ketone bodies and glucose. The amino acids in this group are:
- Isoleucine
- Phenylalanine
- Tryptophan
- Tyrosine
These amino acids can be converted into intermediates that contribute to both gluconeogenesis and ketogenesis. Their metabolic fate depends on various factors such as the body’s energy needs, hormonal regulation, and overall metabolic state.
In conclusion, amino acids can be classified based on their metabolic fate. Glucogenic amino acids act as precursors for glucose formation, ketogenic amino acids are broken down to form ketone bodies, and some amino acids can contribute to both glucose and ketone body production. Understanding these classifications helps us comprehend the diverse roles of amino acids in energy metabolism and their significance in maintaining overall physiological function.
Synthesis of amino acids
Chemical synthesis
Mutant bacteria is often used to produce amino acids commercially. Enzymatic conversions are possible with synthetic intermediates. 2-Aminothiazoline-4-carboxylic acid is an intermediate in one industrial synthesis of L-cysteine for example. The addition of ammonia fumarate to fumarate via a lyase produces aspartic acid.
Biosynthesis
The first form of nitrogen in plants is glutamate. This organic compound is formed from alphaketoglutarate in the mitochondrion. Transaminases are used by plants to transfer the amino group of other amino acids from glutamate into another alpha-keto. Aspartate aminotransferase, for example, converts glutamate to oxaloacetate and alpha-ketoglutarate to aspartate. Transaminases are also used in other organisms for amino acid synthesis.
Modifications to standard amino acids can often create nonstandard amino acids. For example, homocysteine is formed through the transsulfuration pathway or by the demethylation of methionine via the intermediate metabolite S-adenosylmethionine, while hydroxyproline is made by a post translational modification of proline.
Many uncommon amino acids are synthesized by plants and microorganisms. Some microbes produce 2-aminoisobutyric and lanthionine which are sulfide-bridged alanine derivatives. These amino acids can be found in peptidic-lantibiotics like alamethicin. [126] However, in plants, 1-aminocyclopropane-1-carboxylic acid is a small disubstituted cyclic amino acid that is an intermediate in the production of the plant hormone ethylene.
Physicochemical Properties of Amino Acids
1. Stereochemistry
- Stereochemistry refers to the study of the three-dimensional arrangement of atoms within a molecule and how it affects the molecule’s properties and interactions. In the context of amino acids, stereochemistry plays a crucial role in their structure and function.
- In the case of amino acids, they exist predominantly in the L-configuration, which was established by Emil Fischer. L-configuration refers to the spatial arrangement of the amino acid molecules as superimposable mirror images of each other. This configuration is significant because L-amino acids are the primary building blocks involved in protein synthesis during the translation process.
- However, it is worth noting that there are rare instances where D-amino acids can be found in certain proteins. These D-amino acids have a different spatial arrangement and are mirror images of the L-amino acids.
- The Fischer convention is a notation system used to describe the stereochemistry of molecules. It relates the configuration of a chiral center to the arrangement of groups around it in comparison to the structure of glyceraldehyde. For alpha-amino acids, the positioning of the amino, carboxyl, R, and H groups around the carbon atom is related to the arrangement of the hydroxyl, aldehyde, CH2OH, and H groups, respectively, in glyceraldehyde.
- Understanding stereochemistry is crucial in biochemistry, as it influences the folding, structure, and function of proteins and other biomolecules. The spatial arrangement of atoms within a molecule can impact how molecules interact with each other, enzymes, receptors, and other biological systems, ultimately affecting their biological activity.
2. Peptide bond formation
1. Stereochemistry
- Stereochemistry refers to the study of the three-dimensional arrangement of atoms within a molecule and how it affects the molecule’s properties and interactions. In the context of amino acids, stereochemistry plays a crucial role in their structure and function.
- In the case of amino acids, they exist predominantly in the L-configuration, which was established by Emil Fischer. L-configuration refers to the spatial arrangement of the amino acid molecules as superimposable mirror images of each other. This configuration is significant because L-amino acids are the primary building blocks involved in protein synthesis during the translation process.
- However, it is worth noting that there are rare instances where D-amino acids can be found in certain proteins. These D-amino acids have a different spatial arrangement and are mirror images of the L-amino acids.
- The Fischer convention is a notation system used to describe the stereochemistry of molecules. It relates the configuration of a chiral center to the arrangement of groups around it in comparison to the structure of glyceraldehyde. For alpha-amino acids, the positioning of the amino, carboxyl, R, and H groups around the carbon atom is related to the arrangement of the hydroxyl, aldehyde, CH2OH, and H groups, respectively, in glyceraldehyde.
- Understanding stereochemistry is crucial in biochemistry, as it influences the folding, structure, and function of proteins and other biomolecules. The spatial arrangement of atoms within a molecule can impact how molecules interact with each other, enzymes, receptors, and other biological systems, ultimately affecting their biological activity.

2. Peptide bond formation
- Peptide bond formation is a crucial process in protein synthesis, determining the structure and function of proteins. It involves the covalent attachment of amino acids through a condensation reaction.
- During peptide bond formation, the carboxyl group (-COOH) of one amino acid reacts with the amino group (-NH2) of another amino acid. This condensation reaction results in the release of a water molecule, and the formation of a peptide bond between the carbonyl carbon of one amino acid and the nitrogen of the other amino acid.
- When fewer than 50 amino acids are linked together by peptide bonds, the resulting chain is called an oligopeptide. Chains of more than 50 amino acids are known as polypeptides, and proteins are formed by the combination of multiple polypeptides. In this context, individual amino acids are referred to as amino acid residues.
- It is important to note that the acid-base properties of individual amino acids, such as the ability to donate or accept protons, are lost in proteins due to the involvement of the carboxyl group in peptide bond formation. Instead, the overall acidity or basicity of proteins is determined by the ionization characteristics of the individual R groups of the amino acid components. These R groups can be charged, polar, or nonpolar, contributing to the overall ionization behavior of proteins.
- Overall, peptide bond formation is a critical process that links amino acids together, forming the backbone of proteins. The specific sequence and arrangement of amino acids in a protein chain determine its structure and function, playing a fundamental role in various biological processes.

3. Optical properties
- Amino acids, with the exception of glycine, exhibit a property known as optical activity. This optical activity arises from the presence of chiral carbons in the amino acid molecules. Chiral carbons are carbons that have four different groups attached to them, resulting in a non-superimposable mirror image of the molecule.
- The asymmetry created by these chiral carbons gives rise to enantiomers, which are molecules that are mirror images of each other but cannot be superimposed. Enantiomers have identical physical and chemical properties except for their interaction with plane-polarized light.
- Glycine, however, does not possess a chiral carbon. It has two hydrogen atoms attached to its alpha-carbon, making it optically inactive.
- When performing ordinary chemical synthesis, it is important to consider the concept of enantiomers. Chemical or physical processes generally do not have a stereochemical bias, which means that during the synthesis of chiral molecules, racemic mixtures are often produced. Racemic mixtures contain equal amounts of both the right-handed (D-enantiomer) and left-handed (L-enantiomer) forms of the molecule.
- Understanding the optical properties and stereochemistry of amino acids and other molecules is essential in fields such as pharmaceuticals, biochemistry, and materials science, as it influences the behavior and interactions of these compounds in biological systems and chemical reactions.

4. Acid-base properties
- Amino acids exhibit both acid and base properties, making them amphoteric molecules. The acidity or basicity of amino acids is determined by the charges on their carboxyl and amino groups.
- The basic amino group in amino acids has a pKa value ranging from 9 to 10, indicating its strength as a base. On the other hand, the acidic carboxyl group has a pKa value close to 2, representing its strength as an acid. The pKa value is a measure of the acid dissociation constant (Ka) and is defined as the negative logarithm base 10 of Ka.
- At physiological pH, amino acids exist as dipolar ions or zwitterions. This occurs when the concentration of protonated groups (positive charge) is equal to that of unprotonated groups (negative charge). The pH at which this balancing of charges occurs is called the isoelectric point (pI). It is important to note that at the isoelectric point, amino acids have a net-zero charge, but they are never considered to have an absolute zero charge. In an aqueous solution, amino acids do not exist in a completely neutral form.
- Understanding the acid-base properties of amino acids is essential in various biochemical processes. The charges on amino acids influence their interactions with other molecules, such as enzymes or receptors, and their behavior within physiological systems. The isoelectric point and the balanced charge at physiological pH play important roles in protein folding, solubility, and other aspects of amino acid function in living organisms.

5. Chemical reactions
Amino acids undergo various chemical reactions involving their functional groups. Here are some important chemical reactions of amino acids:
- Deamination: Deamination involves the transfer of an amino group from an amino acid to another compound, resulting in the formation of a different amino acid. For instance, the transfer of an amino group to alpha-ketoglutarate leads to the formation of glutamate.
- Condensation Reaction: A condensation reaction occurs when two or more amino acids combine, forming a peptide bond and releasing a water molecule. This process links amino acids together, ultimately forming polypeptides and proteins.
- Cysteine Oxidation: Cysteine oxidation involves the oxidation of two cysteine molecules, resulting in the formation of cystine. Disulfide bonds are formed during this reaction, utilizing the high reactivity of the thiol group in cysteine. These disulfide bridges provide stability to proteins and are crucial for their structure and function.
These are just a few examples of the many chemical reactions that amino acids can undergo. These reactions play a significant role in protein synthesis, modification, and structure, influencing the diverse functions that proteins carry out in biological systems.
What are aliphatic amino acids?
Aliphatic amino acids are a group of amino acids characterized by having nonpolar, hydrophobic side chains. These amino acids are not soluble in water and tend to reside in the interior of proteins. The aliphatic amino acids include alanine (Ala), glycine (Gly), isoleucine (Ile), leucine (Leu), methionine (Met), and valine (Val). The side chains of these amino acids are composed of carbon and hydrogen atoms, except for methionine, which contains a sulfur atom in its side chain. The length of the side chains varies, with alanine having the shortest and valine having the longest.
Aliphatic amino acids play important roles in protein structure and function. They contribute to the stability of proteins by participating in hydrophobic interactions with other hydrophobic amino acids. They are also involved in protein folding processes. For instance, methionine is often found at the beginning of protein chains, where it aids in initiating protein folding.
Beyond their structural significance, aliphatic amino acids have various biological functions. For example, alanine serves as an energy source for cells, while methionine is involved in protein synthesis and the production of other molecules.
In summary, aliphatic amino acids possess hydrophobic properties and contribute to protein stability, folding, and function. Their presence in proteins is essential for maintaining proper structure and facilitating vital biological processes.
| Amino acid | Side chain | Number of carbon atoms | Hydrophobicity |
|---|---|---|---|
| Alanine | -CH3 | 1 | High |
| Glycine | -H | 1 | Low |
| Isoleucine | -CH(CH3)CH3 | 3 | High |
| Leucine | -CH2CH(CH3)2 | 4 | High |
| Methionine | -CH2CH2S(CH3) | 5 | High |
| Valine | -CH(CH3)2 | 3 | High |
How to calculate pi of amino acid?
The isoelectric point (pI) of an amino acid can be calculated using the pKa values of its functional groups, specifically the amino group (-NH2) and the carboxyl group (-COOH). The pI is the pH at which the amino acid exists as a zwitterion, meaning it has no net charge.
Here’s a general approach to calculate the pI of an amino acid:
- Determine the pKa values of the amino and carboxyl groups. The pKa of the amino group is typically around 9-10, while the pKa of the carboxyl group is around 2.
- Find the average of the pKa values. Add the pKa of the amino group and the pKa of the carboxyl group, and then divide the sum by 2.
- If the side chain of the amino acid contains any additional functional groups with acidic or basic properties, consider their pKa values as well.
- The resulting average pKa value is the approximate pI of the amino acid.
It’s important to note that this method provides an estimation and the actual pI may vary due to factors such as temperature and pH conditions.
Additionally, there are online databases and software tools available that can calculate the pI of specific amino acids based on more precise algorithms and incorporating additional factors.
Deficiency of Amino acids
A deficiency in amino acids can have detrimental effects on the body, as they are essential for various physiological processes. Here are some potential pathological disorders associated with amino acid deficiencies:
- Edema: A lack of certain amino acids can contribute to fluid imbalance and lead to the accumulation of fluid in tissues, resulting in edema.
- Anemia: Amino acids, such as iron-containing heme proteins, are necessary for the production of red blood cells. Deficiencies in amino acids involved in this process can lead to various types of anemia.
- Insomnia: Certain amino acids, such as tryptophan, play a role in the synthesis of neurotransmitters that regulate sleep. Deficiencies in these amino acids may disrupt sleep patterns and contribute to insomnia.
- Diarrhea: Amino acid imbalances or deficiencies can affect the proper functioning of the digestive system and lead to diarrhea.
- Depression: Amino acids, particularly those involved in neurotransmitter synthesis, are crucial for maintaining proper mental health. Deficiencies in these amino acids can contribute to depression and mood disorders.
- Hypoglycemia: Amino acids are involved in glucose metabolism and the regulation of blood sugar levels. Deficiencies in amino acids can disrupt this process, leading to hypoglycemia (low blood sugar).
- Loss of Appetite: Amino acid imbalances can impact appetite regulation, potentially leading to a loss of appetite and malnutrition.
- Fat Deposit in the Liver: Certain amino acids are involved in lipid metabolism and the prevention of fatty liver disease. Deficiencies in these amino acids can contribute to the accumulation of fat in the liver.
- Skin and Hair Related Problems: Amino acids are vital for the synthesis of proteins that support healthy skin and hair. Deficiencies can lead to skin issues, such as dryness or reduced elasticity, and hair problems, including thinning or brittle hair.
- Headache, Weakness, Irritability, and Fatigue: Amino acids are involved in energy production and the functioning of the nervous system. Deficiencies can result in symptoms such as headaches, weakness, irritability, and fatigue.
Maintaining a balanced diet that includes all essential amino acids is crucial for preventing amino acid deficiencies and promoting overall health and well-being.
Functions of Amino acids
Amino acids play diverse and essential roles in the body’s metabolism and functioning. Here are some key functions performed by specific amino acids:
- Cysteine: The thiol side chain of cysteine is involved in forming disulfide bonds, which are crucial for protein formation and stability.
- Threonine: Threonine is important for signal transduction and immune system enhancement.
- Phenylalanine: Phenylalanine serves as a precursor for the synthesis of tyrosine, dopamine, norepinephrine, epinephrine, and melanin. Deficiency in phenylalanine metabolism causes phenylketonuria.
- Tryptophan: Tryptophan is involved in anchoring cell membranes, and it serves as a precursor for neurotransmitters (serotonin), hormones (melatonin), and the vitamin niacin.
- Alanine: Alanine plays a crucial role in the glucose-alanine cycle, transferring pyruvate and glutamate from muscle to the liver. Imbalances in this cycle can lead to increased ALT levels and type II diabetes.
- Valine: Valine is essential for the self-renewal of hematopoietic stem cells, which give rise to other blood cells.
- Leucine: Leucine is a ketogenic amino acid that produces acetyl CoA and acetoacetate. These molecules serve as precursors for ketone bodies and myelin, important for brain development in early childhood.
- Isoleucine: Isoleucine assists in wound healing, detoxification of nitrogenous wastes, immune function stimulation, and hormone secretion.
- Methionine: Methionine is a precursor for compounds such as cysteine, taurine, S-adenosyl methionine, and glutathione. Improper conversion can lead to the accumulation of homocysteine, causing atherosclerosis.
- Histidine: Histidine is a precursor for histamine, which plays a role in inflammation. Deficiency in the enzyme histidine ammonia-lyase leads to a rare metabolic disorder called histidinemia.
- Lysine: Lysine is involved in protein stability, histone modification in epigenetic regulation, connective tissue structural proteins, calcium homeostasis, and fatty acid metabolism. Interruption in lysine catabolism results in hyperlysinaemia.
Applications of Amino Acids
Amino acids find a wide range of applications in various industries. Here are some notable applications:
- Animal Feed Additives: Amino acids like lysine, methionine, threonine, and tryptophan are added to animal feed. These additives are chelated with metal cations to enhance mineral absorption, promoting better animal health.
- Artificial Sweetener: Aspartame, derived from aspartic acid and phenylalanine, is a widely used artificial sweetener in food and beverages.
- Flavor Enhancer: Glutamic acid, in the form of monosodium glutamate (MSG), is a popular flavor enhancer used in the food industry to improve the taste of various dishes.
- Mineral Absorption Supplements: Amino acids are utilized as supplements to enhance mineral absorption in the human body, particularly for individuals with mineral deficiencies.
- Pharmaceutical and Cosmetic Manufacturing: Amino acids are used in the production of drugs and cosmetic products due to their various properties and benefits for skin and hair health.
- Fertilizer Development: Amino acids are incorporated into fertilizers to improve mineral absorption in plants, preventing deficiencies and promoting healthy growth without compromising overall productivity.
- Biodegradable Polymers: Amino acids are utilized in the manufacturing of biodegradable polymers. These polymers are used to develop eco-friendly packaging materials, drug delivery carriers, and prosthetic implants.
The diverse applications of amino acids demonstrate their importance in different industries, ranging from agriculture and animal husbandry to food, pharmaceuticals, and materials science. Their unique properties make them valuable components in various products and processes.
FAQ
What are amino acids?
Amino acids are organic compounds that serve as the building blocks of proteins. They contain an amino group (-NH2), a carboxyl group (-COOH), and a side chain (R group) attached to a central carbon atom.
How many amino acids are there?
There are 20 standard amino acids that are commonly found in proteins. These are known as proteinogenic amino acids. However, there are also other non-standard and modified amino acids that have specific roles in certain biological processes.
What are essential amino acids?
Essential amino acids are amino acids that cannot be synthesized by the human body and must be obtained through the diet. There are nine essential amino acids: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
What are non-essential amino acids?
Non-essential amino acids are amino acids that can be synthesized by the human body from other sources. Although they are still important for various biological functions, they do not need to be obtained from the diet. Examples of non-essential amino acids include alanine, glutamine, glycine, and proline.
What is the role of amino acids in the body?
Amino acids have numerous roles in the body, including protein synthesis, enzyme production, hormone regulation, immune function, neurotransmitter synthesis, and energy production. They are essential for growth, repair, and maintenance of tissues and organs.
Can amino acids be used as dietary supplements?
Yes, amino acids are available as dietary supplements. They are often used by athletes and bodybuilders to support muscle growth, recovery, and overall performance. However, it is important to use supplements under the guidance of a healthcare professional to ensure proper dosage and safety.
Are amino acids only found in animal-based foods?
No, amino acids are found in both animal-based and plant-based foods. Animal sources such as meat, fish, eggs, and dairy products are considered complete proteins as they contain all essential amino acids. Plant sources like legumes, grains, nuts, and seeds can provide amino acids as well, but some plant proteins may lack certain essential amino acids.
Can amino acid deficiencies occur?
Yes, deficiencies in specific amino acids can occur, especially if the diet is imbalanced or lacks variety. Amino acid deficiencies can lead to various health problems, including impaired growth, muscle wasting, immune dysfunction, and neurological disorders.
Can amino acids have side effects?
While amino acids are generally safe when consumed through food, high doses of individual amino acid supplements may have side effects. Excessive intake of certain amino acids can disrupt the balance of other amino acids or interfere with metabolic processes. It is important to follow recommended dosages and consult a healthcare professional before taking amino acid supplements.
Can amino acids be obtained from vegetarian or vegan diets?
Yes, it is possible to obtain all essential amino acids from vegetarian or vegan diets by combining different plant-based protein sources. Consuming a variety of legumes, grains, nuts, seeds, and vegetables can provide adequate amounts of essential amino acids. It is important to plan meals carefully to ensure a balanced intake of amino acids for vegetarians and vegans.
Which part of an amino acid is always acidic?
The carboxyl group (-COOH) of an amino acid is always acidic. This group can release a proton (H+) in an aqueous solution, resulting in the formation of a negatively charged carboxylate ion (-COO-). The acidic nature of the carboxyl group is responsible for the acid-base properties of amino acids.
Which two functional groups are always found in amino acids?
The two functional groups that are always found in amino acids are the amino group (-NH2) and the carboxyl group (-COOH). These two groups are attached to a central carbon atom called the alpha carbon (-C-). The amino group acts as a base, capable of accepting a proton (H+) to form a positively charged amino group (-NH3+). The carboxyl group, on the other hand, acts as an acid, capable of donating a proton to form a negatively charged carboxylate group (-COO-). The presence of both the amino and carboxyl groups is what gives amino acids their unique properties and enables them to participate in peptide bond formation and protein synthesis.
What type of bonds link individual amino acids together?
Individual amino acids are linked together through peptide bonds. A peptide bond forms through a condensation reaction, also known as a dehydration reaction, between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. During this process, a water molecule is released, and the carbon of the carboxyl group becomes covalently bonded to the nitrogen of the amino group, forming the peptide bond. This bond is crucial for the formation of polypeptides and proteins, as it connects the amino acids in a linear chain and determines the primary structure of the protein.
Which type of mutation results in abnormal amino acid sequence?
A type of mutation known as a missense mutation can result in an abnormal amino acid sequence. In a missense mutation, a single nucleotide change in the DNA sequence leads to the substitution of one amino acid for another during protein synthesis. This substitution can alter the structure and function of the resulting protein. The specific amino acid change introduced by the missense mutation may disrupt the protein’s normal folding, interactions, enzymatic activity, or stability, leading to a protein with abnormal properties. Missense mutations can have various effects, ranging from mild to severe, depending on the location of the mutation within the protein and the specific amino acid substitution that occurs.
a bunch of amino acids attached together is called a
A bunch of amino acids attached together is called a polypeptide. A polypeptide is a linear chain of amino acids that are linked together by peptide bonds. The length of a polypeptide can vary, ranging from just a few amino acids to hundreds or even thousands of amino acids. Polypeptides are the precursor molecules to proteins. They undergo further folding and modifications to form the three-dimensional structure of a functional protein, which performs specific biological functions in the body.
How many codons code for amino acids?
There are 64 possible codons, of which 61 code for amino acids. The genetic code is a set of rules that determines how the sequence of nucleotides in DNA or RNA is translated into the sequence of amino acids in a protein. Each codon consists of three nucleotides, and there are 4 different nucleotides (A, C, G, and U) that can occupy each position in a codon.
Out of the 64 possible codons, three of them are stop codons (UAA, UAG, and UGA) that signal the end of protein synthesis. The remaining 61 codons correspond to the 20 different amino acids that are used to build proteins. However, some amino acids are represented by multiple codons. For example, the amino acid leucine is coded by six different codons (CUU, CUC, CUA, CUG, UUA, and UUG), while others, like methionine and tryptophan, are coded by only one codon each.
It’s worth noting that the genetic code is nearly universal, with few exceptions across different organisms, allowing for the accurate translation of genetic information into proteins.
How many codons equal one amino acid?
Each amino acid is coded by one or more codons. There are 20 standard amino acids used in protein synthesis. However, the number of codons that code for a specific amino acid can vary. Some amino acids are represented by a single codon, while others are encoded by multiple codons. For example, methionine and tryptophan are each coded by a single codon (AUG and UGG, respectively). On the other hand, amino acids like leucine, serine, and arginine have multiple codons that specify them. Leucine, for instance, is coded by six different codons (CUU, CUC, CUA, CUG, UUA, and UUG). The precise number of codons per amino acid depends on the specific genetic code being considered.
Which pair of statements best describes an essential amino acid?1 It is an amino acid that contains peptide bonds. An example is proline.2 It is an amino acid that contains nitrogen. An example is aspartic acid.3 It is an amino acid that cannot be made by the body. It must be obtained from eating certain foods.4 It is an amino acid that can be produced by the body. Vitamin supplements maintain healthy levels.
The correct pair of statements that best describes an essential amino acid is:
It is an amino acid that cannot be made by the body. It must be obtained from eating certain foods.
Essential amino acids are those that the body cannot synthesize on its own and must be obtained from the diet. They are necessary for proper functioning and growth. Different foods contain essential amino acids, and a balanced diet is required to ensure an adequate intake of all essential amino acids.
What makes amino acids unique from one another?
Amino acids are differentiated from one another primarily by their side chains, also known as R groups. The side chain is a variable component of the amino acid structure that distinguishes one amino acid from another. The side chain can vary in size, shape, charge, and chemical properties, which imparts distinct characteristics to each amino acid.
The side chain can be simple, such as a single hydrogen atom in the case of glycine, or more complex with functional groups like hydroxyl, amino, carboxyl, methyl, aromatic rings, sulfur, and others. These side chains contribute to the amino acid’s unique chemical properties, such as polarity, hydrophobicity, acidity, basicity, and reactivity.
The differences in the side chains give amino acids their diverse characteristics, including their ability to interact with other molecules, participate in protein folding, enzymatic activity, and contribute to the overall structure and function of proteins.
The variations in side chains allow for a wide range of interactions and molecular recognition in biological systems. These unique properties of the side chains play a crucial role in determining the behavior, specificity, and functionality of amino acids and the proteins they form.
What is limiting amino acid?
A limiting amino acid refers to an essential amino acid that is present in the lowest quantity relative to the requirements for protein synthesis. When a diet lacks an adequate amount of a specific essential amino acid, that amino acid becomes the limiting factor in protein synthesis.
The availability of the limiting amino acid can restrict the rate of protein synthesis and limit the overall efficiency of protein production. Even if all other essential amino acids are present in sufficient amounts, protein synthesis will be hindered by the inadequate supply of the limiting amino acid.
The concept of limiting amino acids is often discussed in the context of formulating balanced diets, particularly for animals or individuals with specific nutritional needs. It involves identifying the amino acid(s) that are most deficient relative to the requirements for protein synthesis and ensuring that their intake is increased or supplemented.
By addressing the deficiency of the limiting amino acid(s), one can optimize protein synthesis and support proper growth, development, and overall health. It is important to note that the specific amino acid(s) that function as limiting factors can vary depending on the species or individual’s physiological requirements.
What is the amino acid pool?
The amino acid pool refers to the collective and readily available supply of amino acids in the body. It is a dynamic reservoir of amino acids that can be used for protein synthesis, energy production, and various metabolic processes.
The amino acid pool is maintained through a balance between dietary intake and the breakdown of proteins within the body. When dietary protein is consumed, the amino acids from the digested proteins are absorbed into the bloodstream and contribute to the amino acid pool. Additionally, the body can also break down its own proteins (such as those from muscle tissue) to release amino acids into the pool.
The amino acid pool serves as a crucial resource for protein synthesis, enabling the body to build and repair proteins as needed. It provides the necessary raw materials for the synthesis of enzymes, structural proteins, hormones, antibodies, and other vital molecules.
The amino acid pool also plays a role in energy metabolism. During periods of fasting or intense physical activity, amino acids can be used as an energy source through processes like gluconeogenesis, where amino acids are converted into glucose.
Maintaining a balanced amino acid pool is essential for overall health and proper functioning of various physiological processes. It requires an adequate intake of dietary protein and a healthy balance between protein breakdown and synthesis within the body.
what two functional groups are found in amino acids
The two functional groups that are found in amino acids are the amino group (-NH2) and the carboxyl group (-COOH). These functional groups are attached to the central carbon atom, known as the alpha carbon (-C-), of the amino acid structure.
The amino group is composed of a nitrogen atom bonded to two hydrogen atoms. It acts as a base, capable of accepting a proton (H+), and can become positively charged (NH3+) when protonated.
The carboxyl group consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-OH). It acts as an acid, capable of donating a proton, and can become negatively charged (-COO-) when deprotonated.
The combination of these two functional groups in amino acids makes them amphoteric, meaning they can act as both acids and bases depending on the pH conditions. This characteristic is essential for the role of amino acids in biochemical processes, such as protein synthesis and acid-base balance in the body.
how many nucleotides are needed to specify three amino acids
To specify three amino acids, a minimum of 9 nucleotides would be needed. Each amino acid is encoded by a codon, which consists of three nucleotides. Therefore, three amino acids would require three codons, and each codon consists of three nucleotides.
It’s important to note that while 9 nucleotides can specify three amino acids, the specific sequence of the nucleotides within the codons determines which amino acids are encoded. Different combinations of nucleotides can result in different amino acids being specified.
References
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- https://biochemden.com/physio-chemical-properties-of-amino-acids/
- https://www.onlinebiologynotes.com/properties-of-amino-acids-physical-and-chemical/
- https://microbiologynotes.org/amino-acids-physical-chemical-properties-and-peptide-bond/
- https://www.thermofisher.com/in/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/amino-acid-physical-properties.html