What is Triosephosphate isomerase?
- Triosephosphate isomerase (TPI or TIM) is an essential enzyme that plays a crucial role in glycolysis, the metabolic pathway responsible for breaking down glucose and producing energy. It facilitates the reversible conversion of two triose phosphate isomers: dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate.
- Found in a wide range of organisms, TPI is present in animals, including mammals and insects, as well as in fungi, plants, and bacteria. However, certain bacteria that do not engage in glycolysis, such as ureaplasmas, lack the presence of TPI.
- TPI deficiency in humans leads to a progressive and severe neurological disorder known as triose phosphate isomerase deficiency. This condition is characterized by chronic hemolytic anemia, a condition in which red blood cells are prematurely destroyed. While there are multiple mutations associated with this disease, the most common involves the substitution of glutamic acid at position 104 with aspartic acid.
- One remarkable aspect of triosephosphate isomerase is its exceptional efficiency as an enzyme. It accelerates the reaction between dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate billions of times faster than it would occur naturally in solution. This level of efficiency has earned it the term “catalytically perfect.” The enzyme’s activity is limited solely by the rate at which the substrate can enter and exit its active site, highlighting the remarkable efficiency of TPI.
- Overall, triosephosphate isomerase is a vital enzyme involved in glycolysis, contributing to the efficient production of energy. Its widespread presence in various organisms emphasizes its fundamental role in cellular metabolism. The study of TPI deficiency and its associated neurological disorder provides further insight into the importance of this enzyme in maintaining human health and well-being.
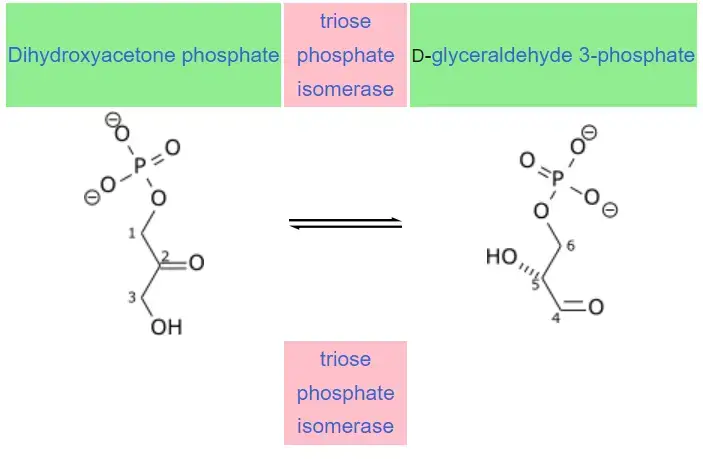
Mechanism of Triosephosphate isomerase
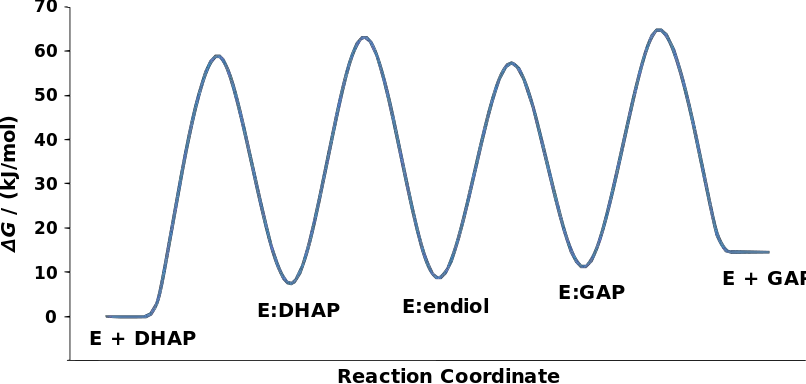
- The mechanism of triosephosphate isomerase (TPI) involves several steps and the formation of an intermediate called enediol. Experimental studies have determined the relative free energy of each ground state and transition state involved in the mechanism.
- The structure of TPI is designed to facilitate the conversion between dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GAP). The process begins with the nucleophilic glutamate 165 residue of TPI deprotonating the substrate, leading to the formation of an enediol intermediate. Simultaneously, the electrophilic histidine 95 residue donates a proton to contribute to the formation of the enediol intermediate.
- Once the enediolate is formed, it collapses, and a proton is abstracted from the protonated glutamate 165, resulting in the formation of the final product, GAP. The reverse reaction follows a similar mechanism, where the enediol is formed, but the enediolate collapses from the oxygen at position C2.
- It is important to note that TPI operates at a diffusion-limited rate, meaning that the rate of the reaction is determined by the speed at which the substrate can enter and exit the enzyme’s active site. In terms of thermodynamics, the formation of DHAP is favored over the production of GAP by a ratio of 20:1. However, in glycolysis, the subsequent steps of metabolism utilize GAP, which helps drive the reaction towards its production.
- TPI can be inhibited by various ions, including sulfate, phosphate, and arsenate, which bind to the enzyme’s active site. Additionally, inhibitors such as 2-phosphoglycolate, a transition state analog, and D-glycerol-1-phosphate, a substrate analog, can also hinder the activity of TPI.
Structure of Triosephosphate isomerase
- Triosephosphate isomerase (TPI) exhibits a specific structural organization that contributes to its function. It exists as a dimer composed of identical subunits, with each subunit consisting of approximately 250 amino acid residues. The three-dimensional structure of a subunit reveals eight α-helices forming the outer surface and eight parallel β-strands forming the inner core. This structural motif is commonly known as an αβ-barrel or TIM-barrel and represents one of the most prevalent protein folds observed in nature. The ribbon backbone of each subunit is color-coded from N-terminus to C-terminus, ranging from blue to red in the illustration.
- The active site of TPI is situated at the center of the barrel. Within this active site, a glutamic acid residue and a histidine residue play critical roles in the catalytic mechanism. The sequence surrounding these active site residues is conserved among all known triose phosphate isomerases.
- In addition to the catalytic residues, the structure of TPI includes a ten- or eleven-amino acid loop (residues 166 to 176) that stabilizes the enediol intermediate. This loop closes and forms a hydrogen bond with the phosphate group of the substrate, contributing to the stabilization of the enediol intermediate and other transition states in the reaction pathway.
- The TPI loop serves not only to make the reaction feasible in terms of kinetics but also to sequester the reactive enediol intermediate, preventing its decomposition into methylglyoxal and inorganic phosphate. The hydrogen bond formed between the enzyme and the phosphate group of the substrate renders the decomposition of the enediol intermediate stereoelectronically unfavorable. Since methylglyoxal is a toxin, its formation is inefficient in the presence of TPI. If formed, methylglyoxal is removed through the glyoxalase system.
- Furthermore, studies suggest that a lysine residue located near the active site (position 12) plays a crucial role in TPI function. This lysine residue, protonated under physiological pH conditions, likely helps neutralize the negative charge of the phosphate group. Substituting this lysine residue with a neutral amino acid abolishes TPI function, while variants with different positively charged amino acids retain some level of activity.
- The precise structure of triosephosphate isomerase, including the αβ-barrel fold and the active site residues, allows for efficient catalysis of the isomerization reaction. The presence of the stabilizing loop and the strategic positioning of key residues contribute to the overall functionality and specificity of TPI in facilitating the interconversion of triose phosphates during glycolysis.
Triosephosphate isomerase in glycolysis
Triosephosphate isomerase (TPI) plays a crucial role in the glycolysis pathway, which is the central metabolic pathway responsible for the breakdown of glucose to produce energy. TPI catalyzes the reversible isomerization of two triose phosphate molecules: dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GAP).
In glycolysis, glucose is initially converted into two molecules of glyceraldehyde 3-phosphate through a series of enzymatic reactions. TPI acts at the second step of glycolysis, where it interconverts DHAP and GAP. DHAP, an unstable intermediate, is converted into GAP, which is a more stable molecule and can continue to undergo further metabolic reactions.
The conversion of DHAP to GAP by TPI is crucial for several reasons:
- Energy Production: The isomerization of DHAP to GAP generates high-energy phosphate bonds in the form of 1,3-bisphosphoglycerate. These bonds store energy that can be harvested later in the glycolytic pathway to produce ATP, the cell’s main energy currency.
- Equilibrium Shift: The conversion of DHAP to GAP helps maintain the thermodynamic equilibrium of the glycolytic pathway. Although the conversion is thermodynamically favorable, the continuous utilization of GAP in downstream reactions helps drive the reaction forward, ensuring the efficient utilization of glucose for energy production.
- Substrate Channeling: TPI contributes to the efficient channeling of intermediates within the glycolytic pathway. By converting DHAP to GAP, TPI ensures that the glycolytic intermediates are directed toward the production of pyruvate and subsequent ATP generation, rather than being shunted into alternative metabolic pathways.
Overall, triosephosphate isomerase plays a vital role in glycolysis by facilitating the interconversion of DHAP and GAP. Its activity ensures the efficient production of ATP and the proper flow of intermediates through the glycolytic pathway, contributing to cellular energy production and metabolic homeostasis.
Triosephosphate isomerase inhibitor
Triosephosphate isomerase (TPI) inhibitors are substances that interfere with the activity of the TPI enzyme, thereby disrupting the isomerization of triose phosphates and affecting glycolysis. Inhibition of TPI can have significant implications for cellular metabolism and energy production. Here are a few examples of TPI inhibitors:
- Phosphate analogs: Compounds that mimic the structure of phosphate, such as inorganic phosphate analogs, can competitively inhibit TPI by binding to its active site and preventing the proper binding and catalysis of the substrate triose phosphates.
- Sulfate analogs: Similar to phosphate analogs, sulfate analogs can competitively inhibit TPI by binding to the active site and interfering with substrate binding and catalysis.
- Arsenate: Arsenate ions can bind to the active site of TPI and inhibit its activity. Arsenate acts as a structural analog of phosphate, but it forms a more stable complex with TPI, thereby preventing its normal function.
- Transition state analogs: Compounds that mimic the transition state of the TPI-catalyzed reaction can bind tightly to the enzyme’s active site and inhibit its activity. These analogs stabilize the transition state, preventing its progression to the desired product and effectively blocking the enzymatic reaction.
- Substrate analogs: Molecules that resemble the structure of the substrate triose phosphates can competitively inhibit TPI by binding to the active site but not undergoing the necessary isomerization reaction. These analogs effectively block the active site, preventing the binding of the actual substrate and inhibiting TPI activity.
It’s important to note that TPI inhibitors can have significant consequences for cellular metabolism and energy production, as glycolysis is a central pathway for energy generation. Inhibition of TPI can disrupt the flow of intermediates, hinder ATP production, and affect various cellular processes reliant on glycolysis.
Triosephosphate isomerase function
The function of triosephosphate isomerase (TPI) is to catalyze the reversible isomerization of two triose phosphate molecules: dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (GAP). This enzymatic reaction is a critical step in the glycolysis pathway, which is the central metabolic pathway responsible for the breakdown of glucose to generate energy.
TPI facilitates the conversion of DHAP to GAP by rearranging the positions of functional groups within the molecule. DHAP is an unstable intermediate, while GAP is a more stable molecule that can proceed through subsequent metabolic reactions.
The specific function of TPI can be summarized as follows:
- Isomerization: TPI catalyzes the rearrangement of the carbonyl and hydroxyl groups in DHAP, leading to the formation of GAP. This isomerization reaction is essential for the efficient progression of glycolysis and the subsequent production of energy-rich molecules.
- Energy Production: The isomerization of DHAP to GAP contributes to the generation of high-energy phosphate bonds in the form of 1,3-bisphosphoglycerate. These bonds store energy that can be later harnessed through subsequent enzymatic reactions to produce ATP, the primary energy currency of the cell.
- Equilibrium Maintenance: TPI helps maintain the thermodynamic equilibrium of the glycolytic pathway. The interconversion of DHAP and GAP ensures that the pathway progresses in a favorable direction, with GAP being utilized for further metabolic reactions. This equilibrium maintenance is crucial for the efficient utilization of glucose and the regulation of energy production.
- Substrate Channeling: TPI plays a role in directing the flow of intermediates within the glycolytic pathway. By converting DHAP to GAP, TPI ensures that the intermediates are channeled toward the production of pyruvate and subsequent ATP generation, rather than being diverted to alternative metabolic pathways.
Overall, the function of triosephosphate isomerase is to catalyze the isomerization of DHAP and GAP, enabling the efficient breakdown of glucose and the production of ATP through the glycolysis pathway. Its activity ensures the proper flow of intermediates and the regulation of energy production, making TPI a critical enzyme in cellular metabolism.