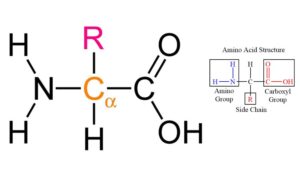
What Is a Peptide Bond?
- A peptide bond is a specific type of covalent bond that plays a crucial role in the formation of peptides and proteins. Peptides themselves are short chains of amino acids, typically ranging from two to fifty amino acids in length. They can be classified based on their size; those consisting of ten or fewer amino acids are termed oligopeptides, while longer chains are referred to as polypeptides. When a polypeptide reaches approximately 100 amino acids, it is classified as a protein.
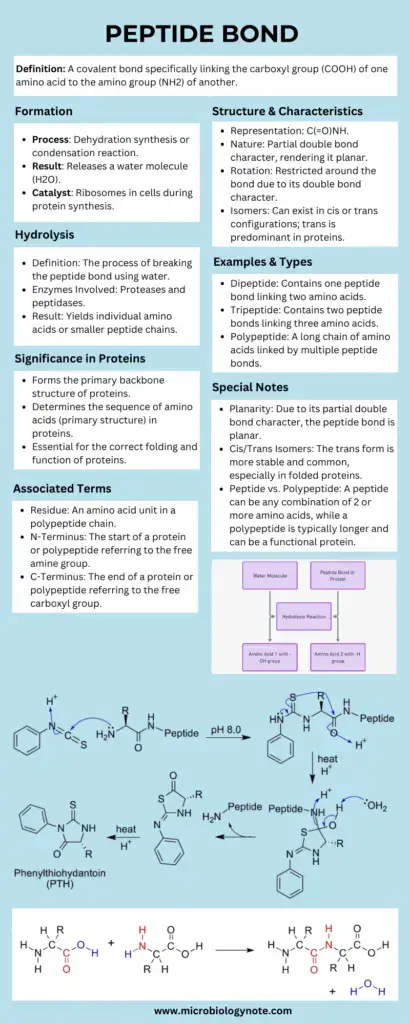
- The formation of a peptide bond occurs through a biochemical reaction between the α-carboxyl group of one amino acid and the α-amino group of another. This process, known as a condensation reaction, involves the release of a water molecule, hence categorizing it as a dehydration reaction. The bond that forms is not only integral to the structure of peptides but is also pivotal in the larger context of protein synthesis.
- In addition to the standard peptide bond, there are variations known as isopeptide bonds. These bonds can occur between the carboxyl group of one amino acid and an amino group of another amino acid located at positions other than the alpha position. This distinction is important for understanding the diversity and functionality of peptides in biological systems.
- The peptide bond itself exhibits characteristics that enhance its stability. A partial double bond exists between the carbon and nitrogen atoms of the amide bond, contributing to the overall stabilization of the structure. The nitrogen atom involved donates its lone pair of electrons to the carbonyl group, resulting in a resonance effect. This resonance allows for electron delocalization across multiple atoms, further stabilizing the bond. However, this stabilization comes with a constraint; the presence of the partial double bond limits the rotational freedom around the C-N bond. As a result, the peptide bond maintains a planar configuration, while the adjacent single bonds exhibit a higher degree of rotational motion.
- The implications of the peptide bond’s properties extend into the realm of protein structure and function. Due to its rigidity and planarity, the peptide bond influences the overall conformation of protein chains, dictating their three-dimensional shapes and, consequently, their biological functions. The structural constraints imposed by the peptide bond are essential for the proper folding and stability of proteins, highlighting the bond’s significance in the context of molecular biology.
Definition of Peptide Bond
A peptide bond is a covalent bond formed between the α-carboxyl group of one amino acid and the α-amino group of another, resulting in the release of a water molecule. This bond links amino acids together in a peptide or protein chain and is characterized by partial double bond characteristics that restrict rotation, contributing to the stability and structure of proteins.
Characteristics of Peptide Bond
Peptide bonds, the foundational linkages in proteins, exhibit a range of distinctive characteristics that underscore their significance in biological systems. These bonds are pivotal in connecting amino acids, the building blocks of proteins. Herein, we elucidate the salient characteristics of peptide bonds, drawing insights from the provided contents:
- Formation through Dehydration Synthesis: Peptide bonds are established when two amino acids are linked through a process known as dehydration synthesis. This reaction involves the removal of a water molecule, facilitating the bond formation between the amino and carboxyl groups of the participating amino acids.
- Partial Double Bond Character: One of the hallmark features of peptide bonds is their partial double bond character, attributed to resonance. This characteristic imparts rigidity to the bond, making it resistant to rotation. As a consequence, peptide bonds are kinetically stable, necessitating high activation energy for their cleavage.
- Stability and Resistance: Peptide bonds are robust and are not easily disrupted by factors such as heating or high salt concentrations. Their stability is such that only prolonged exposure to strong acids or bases at elevated temperatures can break them. Additionally, certain specialized enzymes, notably digestive enzymes, can cleave these bonds.
- Planarity and Configuration: Peptide bonds are inherently planar, meaning the C=O and N-H bonds coexist on the same plane. This planarity restricts rotation around the peptide bond. Furthermore, the peptide group can adopt either a cis or trans configuration, with the trans configuration being favored due to reduced steric hindrance from adjacent amino acid side chains. This stereochemical aspect plays a pivotal role in stabilizing protein structures.
- Polarity and Hydrogen Bonding: The peptide bond is characterized by polar entities, specifically the partially positive hydrogen atoms of amino groups and the partially negative oxygen atoms of carboxyl groups. This inherent polarity facilitates the formation of hydrogen bonds between different regions of the peptide, further stabilizing the structure.
- Enzymatic Cleavage: While peptide bonds are inherently stable, they are not impervious to enzymatic action. Specific enzymes, particularly those involved in digestion, can target and cleave these bonds, underscoring the dynamic nature of biological systems.
Structure of Peptide Bond
The structure of a peptide bond is fundamental to understanding protein architecture and function. It possesses distinct characteristics that influence its stability, geometry, and the overall conformation of peptides and proteins.
- Planarity: The peptide bond is inherently planar due to the rigid nature of the bond itself. This planarity results from the partial double bond character of the bond formed between the carbon atom of the carboxyl group and the nitrogen atom of the amide group. The electron sharing creates a resonance structure, which restricts rotation around the bond and contributes to the overall stability of the peptide.
- Trans Configuration: In addition to being planar, peptide bonds adopt a trans configuration. In this arrangement, the hydrogen atom of the amide group is positioned opposite the oxygen atom of the carboxyl group. This trans orientation minimizes steric hindrance between adjacent side chains of amino acids, promoting a more favorable spatial arrangement.
- Rigid Character: The rigidity of the peptide bond arises from its partial double bond character, which restricts the rotation of the bond. This rigidity is crucial as it plays a significant role in defining the three-dimensional structure of proteins. The constrained rotation ensures that the amino acids are held in a specific alignment, contributing to the secondary and tertiary structures of polypeptides.
- Coplanarity of Atoms: The atoms involved in the peptide bond—carbon (C), nitrogen (N), hydrogen (H), and oxygen (O)—are coplanar. This coplanarity encompasses not only the atoms directly involved in the bond but also includes the hydrogen atom from the amide group and the oxygen from the carboxyl group. The alignment of these atoms in a single plane facilitates the formation of secondary structures, such as alpha helices and beta sheets.
- Historical Insights: The fundamental understanding of peptide bond structure was advanced by scientists Linus Pauling and Robert Corey. Their work revealed the rigid and planar nature of peptide bonds, which has been instrumental in elucidating the structural properties of proteins.
Peptide Bond Formation or Synthesis – How are Peptide bonds formed?
Peptide bond formation is a vital biochemical process, primarily occurring through a dehydration synthesis reaction. This mechanism plays a crucial role in the synthesis of proteins by linking amino acids together.
- Initiation of the Reaction: The process begins with two amino acids. Each amino acid comprises a carboxyl group (—COOH) and an amino group (—NH₂).
- Approach of Functional Groups: The carboxyl group of one amino acid approaches the amino group of another. This proximity is essential for the subsequent chemical interaction that leads to peptide bond formation.
- Removal of Atoms: During the reaction, one hydrogen atom (H) is removed from the amino group of the second amino acid, and one hydroxyl group (—OH) is removed from the carboxyl group of the first amino acid. This removal is crucial as it facilitates the formation of the bond.
- Water Molecule Release: The loss of these atoms results in the release of a water molecule (H₂O), which is characteristic of dehydration synthesis reactions. The elimination of water signifies that the reaction is endergonic, indicating that it requires an input of energy.
- Formation of the Peptide Bond: The final outcome of this interaction is the establishment of a covalent bond known as a peptide bond (—C(=O)NH—) between the two amino acids. This bond specifically forms between the carbon atom of the carboxyl group of the first amino acid and the nitrogen atom of the amino group of the second amino acid.
- Resulting Structure: As a result of this bond formation, a dipeptide molecule is created, consisting of two amino acids linked together. This dipeptide can further participate in additional peptide bond formations, leading to the creation of longer polypeptide chains, which ultimately fold into functional proteins.
- Energy Requirements: The peptide bond formation process is energetically demanding, necessitating the involvement of adenosine triphosphate (ATP) as an energy source in living organisms. This energy is essential for driving the reaction forward and ensuring the efficient synthesis of proteins.
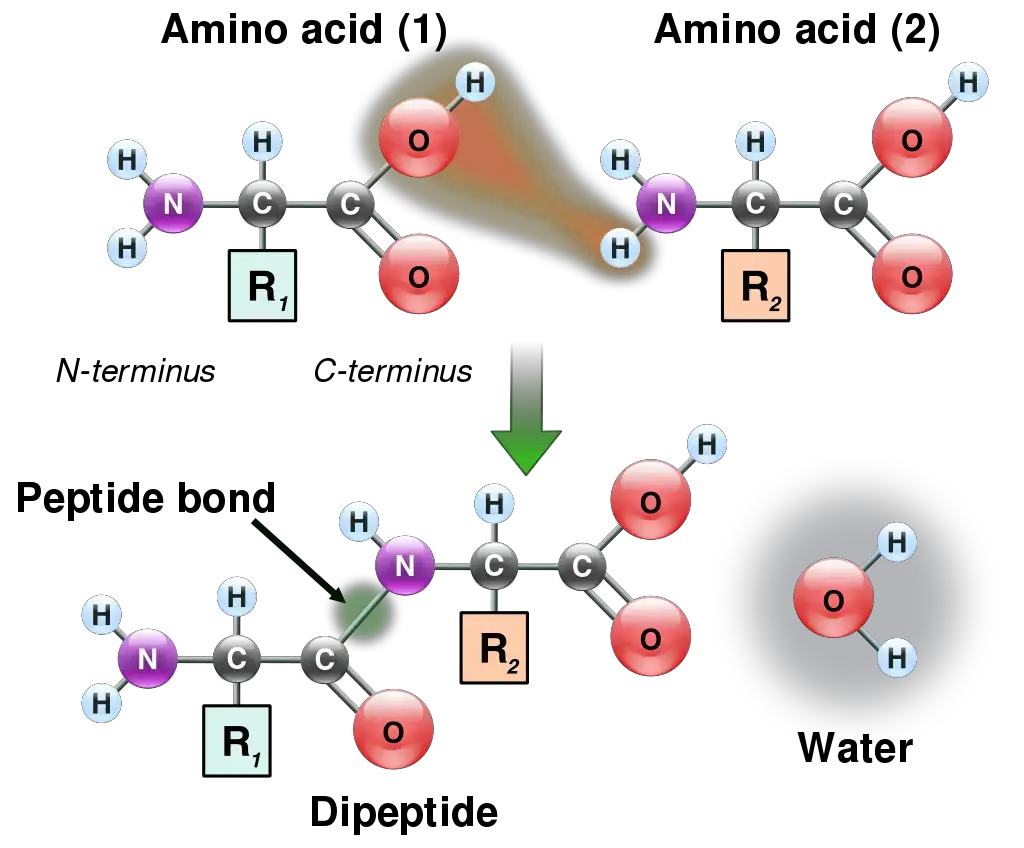
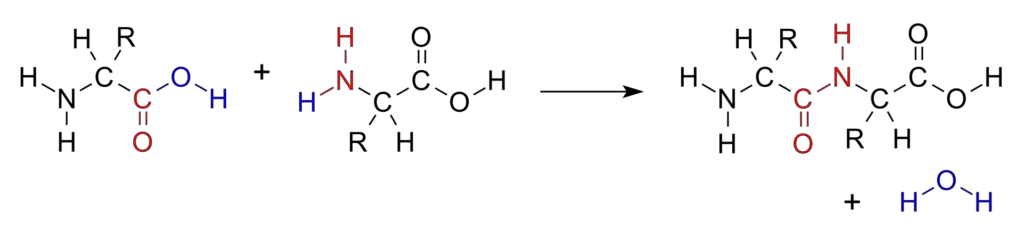
Degradation of Peptide Bond
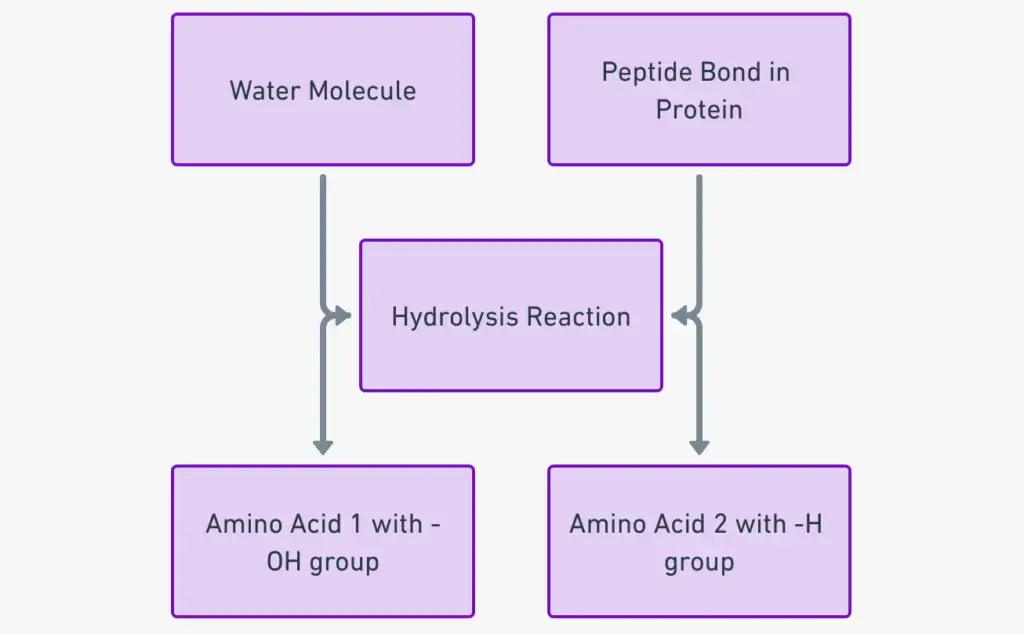
Peptide bond degradation is a crucial biochemical process characterized by hydrolysis, wherein water molecules facilitate the breakdown of peptide bonds. This mechanism is essential for various biological functions, including digestion and the regulation of peptide levels within cells.
- Hydrolysis Process: The degradation of peptide bonds occurs through hydrolysis, which requires the presence of water. During this reaction, the amide bond between amino acids is cleaved, resulting in the formation of individual amino acids or smaller peptides.
- Stability of the Peptide Bond: The degradation reaction is inherently slow due to the stabilization provided by the partial double bond characteristics of the peptide bond. The carbon-nitrogen bond exhibits a partial double bond nature, which imparts significant stability to the peptide structure.
- Attack by Hydroxide Ions: In the presence of water, hydroxide ions (OH⁻) attack the carbon atom of the peptide bond. This interaction is pivotal as it initiates the cleavage of the bond.
- Formation of Amino and Carboxyl Groups: Following the attack on the carbon atom, the remaining hydrogen ion from the water molecule subsequently attacks the nitrogen atom. This results in the generation of a free amino group from the original peptide structure.
- Cleavage Outcome: As a result of these interactions, the peptide molecule is cleaved into two distinct units: one containing a carboxyl group and another containing an amino group. This process highlights the bifurcation of the peptide into its constituent amino acids.
- Energy Release: The degradation of the peptide bond is classified as an exergonic reaction, which releases approximately 8-16 kJ/mol of energy. This energy release is characteristic of reactions that result in a more stable product.
- Role of Enzymes: Due to the slow nature of spontaneous peptide bond hydrolysis, these reactions are often catalyzed by proteolytic enzymes such as proteases and peptidases. These enzymes enhance the rate of hydrolysis, facilitating the breakdown of proteins in various physiological contexts.
- Significance in Biological Processes: Peptide bond hydrolysis serves as the primary step in all protein hydrolysis reactions. One of the most common methods of protein degradation is through acid-catalyzed hydrolysis, which is fundamental in various biological processes, including digestion.
- Toxin Removal and Synthetic Reactions: Furthermore, peptide bond hydrolysis is essential in the removal of accumulated peptides and proteins that could lead to toxicity within cells. In synthetic reactions, hydrolysis allows for the cleavage of amino acids from one peptide for incorporation into another, aiding in the synthesis of new peptides.
- Digestive Role: In living organisms, peptide bond hydrolysis is integral to the digestion of proteins. This process allows for the breakdown of dietary proteins into absorbable units, namely amino acids.
Different Types of Peptide Bond
Peptide bonds are fundamental in forming various types of peptides and proteins. The classification of peptides based on the number of amino acids they contain reveals distinct forms, each with unique characteristics and biological significance.
- Dipeptide: A dipeptide consists of two amino acid units linked by a single peptide bond. This simple structure serves as a basic building block for more complex peptides and proteins. Dipeptides are often formed during protein digestion or biosynthesis and can influence biological activities.
- Tripeptide: A tripeptide is composed of three amino acid units connected by two peptide bonds. This configuration allows for a slight increase in complexity and functionality compared to dipeptides. Tripeptides can also play critical roles in various biochemical processes, including signaling and metabolism.
- Tetrapeptide: A tetrapeptide contains four amino acid units linked together through three peptide bonds. The addition of another amino acid unit enhances the diversity of potential functional groups and conformations, contributing to the complexity of peptide interactions in biological systems.
- Oligopeptide: Oligopeptides are short chains comprising up to ten amino acid units. This category encompasses dipeptides, tripeptides, tetrapeptides, and others up to the specified limit. Oligopeptides often exhibit significant biological activity and can serve as hormones or signaling molecules.
- Polypeptide: Polypeptides are larger chains consisting of more than ten amino acids, typically ranging up to 100 residues. The length and sequence of amino acids in a polypeptide chain influence its structural configuration and functional properties. Polypeptides may fold into specific three-dimensional shapes, leading to various functional roles in biological systems.
- Macropeptides: Macropeptides are defined as chains containing more than 100 amino acids. This category includes many proteins and enzymes, which are critical for cellular functions. The extensive length of macropeptides allows for intricate folding patterns and complex interactions, enabling diverse biochemical functions.
Resonance Structure of Peptide Bond
The peptide bond, a fundamental linkage in the realm of proteins and peptides, exhibits a unique structural characteristic that contributes to its stability: the phenomenon of resonance. This resonance is not merely a theoretical construct but has profound implications for the bond’s physical and chemical properties.
At the heart of the peptide bond’s configuration is the amide group, which is inherently planar and rigid. The stability conferred upon the peptide bond arises from the resonance of the amide. This resonance is a consequence of the interplay of electrons between the carbonyl group’s double bond (C=O) and the C–N bond. Such electron sharing or delocalization between bonds is emblematic of resonance structures.
This resonance effect imparts a partial double bond character to the C-N bond, while simultaneously bestowing a partial single bond character to the C=O bond. The implications of this are profound for the bond lengths within the peptide structure. Typically, a single bond between two atoms is longer than a double bond between the same pair. However, due to the resonance effect, the C–N bond in a peptide bond is shorter than what one would anticipate for a conventional C–N single bond. Conversely, the C=O bond, because of its partial single bond character, is longer than a standard C=O double bond.
In essence, the resonance structure of the peptide bond is a testament to the intricate dance of electrons that governs the bond’s stability and configuration. This nuanced electron sharing ensures that peptide bonds, the backbone of proteins, maintain their structural integrity and play their pivotal role in the biochemistry of life.
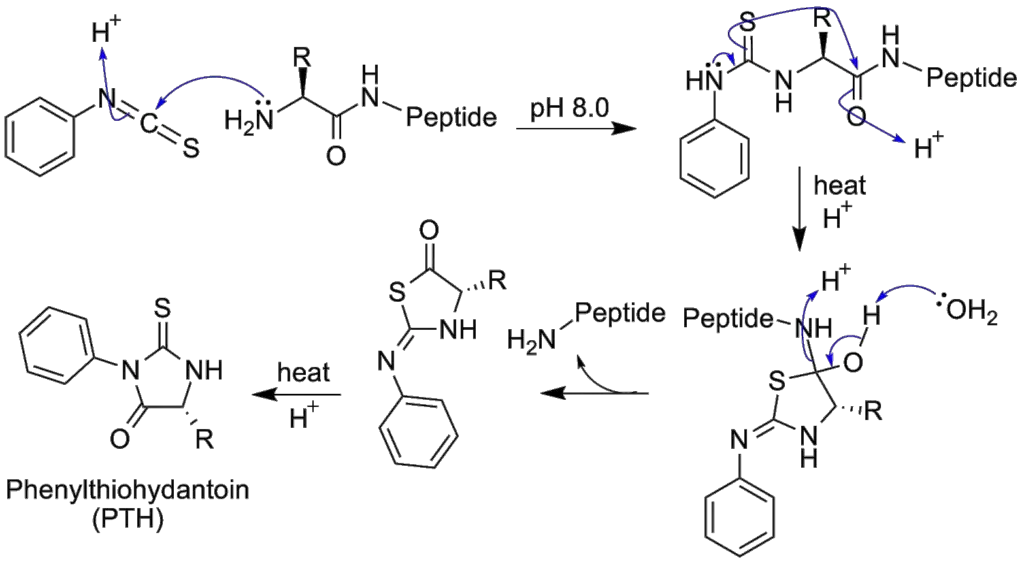
What is Cis/trans isomers of the peptide group?
The peptide group, a fundamental component of proteins, exhibits unique structural characteristics due to the significant delocalization of the lone pair of electrons on its nitrogen atom. This delocalization imparts a partial double-bond character to the peptide group, rendering it planar. Consequently, the peptide group can exist in two distinct geometric isomers: cis and trans.
The planarity of the peptide group arises from the partial double bond, allowing it to adopt either the cis or trans configuration. In an unfolded protein state, peptide groups can freely isomerize between these configurations. However, once the protein assumes its folded state, typically only one isomer predominates at each position, with exceptions being rare. The trans isomer is predominantly favored in most peptide bonds, with a striking ratio of approximately 1000:1 in favor of trans over cis. An intriguing exception is observed in X-Pro peptide groups, where the ratio is closer to 30:1. This deviation can be attributed to the symmetrical nature of proline’s Cα and Cδ atoms, rendering the energies of cis and trans isomers nearly equivalent.
The dihedral angle, represented by ω, associated with the peptide group is defined by the sequence of atoms Cα–C’–N–Cα. For the cis isomer, ω is 0°, indicating a synperiplanar conformation. In contrast, the trans isomer exhibits an antiperiplanar conformation with ω being 180°. Isomerization between these forms involves rotation around the C’–N bond. This process, although feasible, is slow, with a typical time frame of around 20 seconds at ambient conditions. The transition state, characterized by ω values of ±90°, necessitates the disruption of the partial double bond, leading to a substantial activation energy of approximately 80 kJ/mol. However, certain factors, such as a hydrophobic environment or hydrogen bond donation to the nitrogen atom in an X-Pro peptide group, can reduce this activation energy. These mechanisms have been observed in peptidyl prolyl isomerases (PPIases), enzymes that facilitate the cis-trans isomerization of X-Pro peptide bonds.
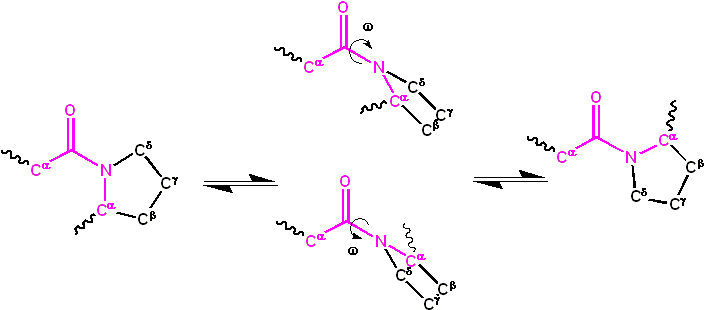
The kinetics of protein folding and cis-trans isomerization are markedly different. While protein folding typically occurs within 10-100 milliseconds, isomerization can take 10-100 seconds. The presence of non-native isomers can significantly impede the protein folding process. Some non-native isomers can either slow down or entirely halt folding until the native configuration is achieved. However, the impact of non-native isomers on folding is not uniform across all peptide groups. Some might have negligible effects, while others can be more disruptive.
In conclusion, the cis/trans isomerism of the peptide group plays a pivotal role in protein structure and function. Understanding this dynamic is crucial for insights into protein folding, stability, and interactions, which are fundamental to numerous biological processes and potential therapeutic interventions.
What is polypeptide?
A polypeptide is a linear chain of amino acids linked together by peptide bonds. The term “polypeptide” is derived from the Greek words “poly,” meaning many, and “peptos,” meaning digested, reflecting the fact that polypeptides are formed by the polymerization of amino acids.
Key Points:
- Formation: Polypeptides are formed during the process of protein synthesis, where individual amino acids are connected in a specific sequence as dictated by the genetic code. This sequence is determined by the order of codons in a segment of mRNA, which is translated by ribosomes.
- Length: Polypeptides can vary in length, ranging from just a few amino acids long to several thousand. Typically, shorter chains are referred to as peptides, while longer chains are termed proteins, especially if they have a well-defined structure and function.
- Structure: The sequence of amino acids in a polypeptide determines its primary structure. This sequence influences the higher levels of protein structure, including the secondary (e.g., alpha helices and beta sheets), tertiary (three-dimensional folding), and quaternary (assembly of multiple polypeptide chains) structures.
- Function: Once a polypeptide folds into its functional three-dimensional shape, it can serve a wide range of roles in the cell, from catalyzing biochemical reactions as enzymes, to providing structural support, to facilitating communication between cells.
- Diversity: The 20 standard amino acids can be combined in countless sequences, allowing for an immense diversity of polypeptides with unique properties and functions.
Importance of Peptide Bond
The peptide bond is a fundamental chemical linkage that plays a pivotal role in the structure and function of proteins. Its significance in biological systems can be understood through the following aspects:
- Primary Structure of Proteins: Peptide bonds covalently link amino acids in a specific sequence, forming the primary structure of proteins. This sequence determines the protein’s identity and dictates its higher-order structures and functions.
- Stability and Rigidity: The peptide bond’s planar and rigid nature, coupled with its partial double bond character, provides stability to the protein’s structure. This rigidity is essential for maintaining the protein’s shape and ensuring its proper functioning.
- Protein Folding: The orientation and spatial arrangement of peptide bonds influence the way a protein folds, leading to the formation of secondary structures like alpha-helices and beta-sheets. These structures are crucial for the protein’s overall three-dimensional conformation.
- Enzymatic Reactions: The formation and breaking of peptide bonds are catalyzed by specific enzymes. Ribosomes facilitate peptide bond formation during protein synthesis, while proteolytic enzymes, like proteases, catalyze the hydrolysis of peptide bonds during protein degradation.
- Information Storage: The sequence of amino acids, linked by peptide bonds, encodes genetic information. This sequence is a direct translation of the genetic code from mRNA, ensuring the accurate expression of genes into functional proteins.
- Biological Activity: Many biologically active molecules, like hormones and neurotransmitters, are peptides. The specific arrangement of amino acids, connected by peptide bonds, imparts these molecules with their unique functionalities.
- Evolutionary Significance: The universality of the peptide bond in all living organisms suggests its evolutionary importance. It serves as a testament to the shared ancestry of life on Earth.
- Target for Therapeutics: Since peptide bonds are crucial for protein structure and function, they are often targeted in therapeutic interventions. For instance, certain antibiotics work by inhibiting bacterial enzymes responsible for peptide bond formation, thereby disrupting protein synthesis.
Peptide Bond Examples
- Insulin: Insulin is a hormone essential for regulating blood sugar levels. It’s a protein made up of two peptide chains, the A and B chains, linked together by disulfide bonds. The individual amino acids within these chains are connected by peptide bonds.
- Oxytocin: This is a peptide hormone and neuropeptide that plays a role in social bonding, reproduction, and childbirth. It consists of nine amino acids linked together by peptide bonds.
- Hemoglobin: Found in red blood cells, hemoglobin is a protein responsible for transporting oxygen throughout the body. It’s made up of four polypeptide chains (two alpha and two beta chains), and within these chains, amino acids are connected by peptide bonds.
- Enzymes: Almost all enzymes are proteins, and they catalyze various biochemical reactions in the body. For instance, the enzyme pepsin breaks down dietary proteins in the stomach. The structure of pepsin, like other enzymes, is maintained by peptide bonds linking its constituent amino acids.
- Antibodies: These are proteins produced by the immune system to neutralize pathogens like bacteria and viruses. Each antibody is made up of two heavy and two light polypeptide chains, with peptide bonds holding the amino acids of these chains together.
- Collagen: This is the most abundant protein in the human body, providing structural support to connective tissues, skin, and bones. It’s a triple helix structure made up of three polypeptide chains, with peptide bonds connecting the amino acids within each chain.
- Dipeptides and Tripeptides: These are the simplest examples. A dipeptide is formed when two amino acids are linked by a single peptide bond, while a tripeptide is formed from three amino acids linked by two peptide bonds. For instance, carnosine is a dipeptide made up of the amino acids beta-alanine and histidine.
FAQ
What is a peptide bond?
A peptide bond is a covalent bond that forms between the carboxyl group of one amino acid and the amino group of another amino acid.
Are peptide bonds covalent?
Yes, peptide bonds are covalent bonds.
Description with Example:
A covalent bond is formed when two atoms share electrons. In the context of peptide bonds, this covalent linkage occurs between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid.
Example: Consider two amino acids, Glycine and Alanine:
Glycine has the structure: H2N-CH2-COOH Alanine has the structure: H2N-CH(CH3)-COOH
When these two amino acids form a peptide bond, the hydroxyl group (-OH) from the carboxyl group of Glycine and a hydrogen atom from the amino group of Alanine are removed, producing a water molecule (H2O). The remaining parts of the two amino acids then bond covalently, forming the peptide bond:
H2N-CH2-C(=O)NH-CH(CH3)-COOH
In this structure, the C(=O)NH linkage represents the peptide bond. The bond between the carbon (C) of the carboxyl group and the nitrogen (N) of the amino group is the covalent peptide bond.
How are peptide bonds formed?
Peptide bonds are formed through a dehydration synthesis reaction, where a water molecule is released.
A peptide bond links __________.
A peptide bond links the carboxyl group of one amino acid to the amino group of another amino acid.
Where do peptide bonds form?
Peptide bonds form between amino acids during protein synthesis in ribosomes.
Which is released during the formation of a peptide bond?
A water molecule (H2O) is released during the formation of a peptide bond.
What type of bond is a peptide bond?
A peptide bond is a covalent bond.
Where are peptide bonds found?
Peptide bonds are found in proteins, linking amino acids together.
A long chain of amino acids linked by peptide bonds.
This describes a polypeptide or protein.
Do free amino acids have peptide bonds?
No, free amino acids do not have peptide bonds. Peptide bonds form when amino acids are linked together.
What purpose does the peptide bond serve in protein synthesis?
The peptide bond holds amino acids together, forming the primary structure of proteins.
When a peptide bond is created between two amino acids.
This occurs during protein synthesis when the carboxyl group of one amino acid reacts with the amino group of another, releasing a water molecule.
What do peptide bonds hold together?
Peptide bonds hold amino acids together in a protein.
How many peptide bonds are in a tripeptide?
There are two peptide bonds in a tripeptide.
Where are the peptide bonds located in a polypeptide?
Peptide bonds are located between each pair of adjacent amino acids in a polypeptide.
Which describes the function of a peptide bond?
The peptide bond links amino acids together to form the primary structure of proteins.
Are peptide bonds polar?
The peptide bond itself has a partial double bond character, making it relatively nonpolar. However, the surrounding regions can have polar characteristics depending on the amino acids involved.
How to identify peptide bonds?
Peptide bonds can be identified in a protein structure as the bonds connecting the carboxyl group of one amino acid to the amino group of another.
Where does cleavage of the peptide bond by chymotrypsin occur?
Chymotrypsin cleaves peptide bonds at the carboxyl side of aromatic or large hydrophobic amino acids like tryptophan, tyrosine, phenylalanine, and sometimes methionine.
Which of the following pancreatic enzymes acts on peptide bonds?
Without a specific list of enzymes, I cannot determine which act on peptide bonds. However, enzymes like trypsin and chymotrypsin are pancreatic enzymes that act on peptide bonds.
Why are peptide bonds planar?
Peptide bonds are planar because of the partial double bond character, which restricts rotation around the bond.
A peptide bond forms between which of these groups?
A peptide bond forms between the carboxyl group of one amino acid and the amino group of another amino acid.
Can a peptide bond rotate freely?
No, due to its partial double bond character, a peptide bond cannot rotate freely.
Do free amino acids peptide bonds?
No, free amino acids do not have peptide bonds.
Do proteins have peptide bonds?
Yes, proteins are made up of amino acids linked by peptide bonds.
How are peptide bonds broken?
Peptide bonds are broken through hydrolysis, where a water molecule is added.
How do amino acids form peptide bonds?
Amino acids form peptide bonds through a dehydration synthesis reaction, where a water molecule is released.
Is a peptide bond a hydrogen bond?
No, a peptide bond is a covalent bond.
What are peptide bonds made of?
Peptide bonds are made of the atoms that connect the carboxyl group of one amino acid to the amino group of another amino acid.
What catalyzes peptide bond formation?
Ribosomes, along with transfer RNA (tRNA) molecules, catalyze peptide bond formation during protein synthesis.
What type of bond joins amino acids to make peptides?
Peptide bonds join amino acids to make peptides.
Which bond represents the peptide bond?
The bond between the carboxyl group of one amino acid and the amino group of another represents the peptide bond.
Which of the following polysaccharides contains peptide bonds?
Without a specific list of polysaccharides, I cannot determine which contains peptide bonds. However, typically, polysaccharides do not contain peptide bonds as they are carbohydrates.
Are peptide and amide bonds the same?
Yes, a peptide bond is a type of amide bond that forms between amino acids.
Are peptide bonds charged?
No, peptide bonds themselves are not charged.
Are peptide bonds covalent or ionic?
Peptide bonds are covalent bonds.
Are peptide bonds flexible?
The peptide bond itself is not flexible due to its partial double bond character, but the bonds around it can rotate, allowing flexibility in the protein structure.
Are peptide bonds hydrophobic?
The peptide bond itself is neutral, but the surrounding regions can be hydrophobic or hydrophilic depending on the amino acids involved.
Are peptide bonds intramolecular or intermolecular attraction?
Peptide bonds are intramolecular covalent bonds that connect amino acids within a molecule.
Are peptide bonds only found in proteins?
Primarily, yes. Peptide bonds are most commonly found in proteins, but they can also be found in some peptides and polypeptides.
Are there peptide bonds in tertiary structure?
The tertiary structure of a protein refers to its overall three-dimensional shape. While the tertiary structure is stabilized by various interactions, including hydrogen bonds, ionic bonds, and hydrophobic interactions, the peptide bonds themselves are part of the primary structure, linking amino acids together.
Can proline form peptide bonds?
Yes, proline can form peptide bonds. However, its unique ring structure can introduce kinks or bends in the protein chain.
Do all proteins have peptide bonds?
Yes, all proteins are made up of amino acids linked by peptide bonds.
Do carbohydrates contain peptide bonds?
No, carbohydrates do not contain peptide bonds. Carbohydrates are made up of sugar molecules linked by glycosidic bonds.
- https://biologydictionary.net/peptide-bond/
- https://www.chemistrylearner.com/chemical-bonds/peptide-bond
- https://www.biologyonline.com/dictionary/peptide-bond
- https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/peptide-bond
- https://www.sciencedirect.com/topics/nursing-and-health-professions/peptide-bond
- https://pubs.acs.org/doi/10.1021/jacs.1c02600
- https://www.khanacademy.org/test-prep/mcat/biomolecules/amino-acids-and-proteins1/v/peptide-bond-formation-and-cleavage
- https://www.britannica.com/science/peptide-bond
- https://www.cryst.bbk.ac.uk/PPS95/course/3_geometry/peptide2.html
- https://www2.chem.wisc.edu/deptfiles/genchem/netorial/modules/biomolecules/modules/protein1/prot15.htm
- https://www.nature.com/scitable/topicpage/protein-structure-14122136/
- https://unacademy.com/content/upsc/study-material/chemistry/a-brief-note-on-peptide-bond-formation-or-synthesis/