What are Lipids?
- Lipids, by definition, are a heterogeneous group of organic compounds. These compounds are characterized by their insolubility in water and solubility in non-polar organic solvents. Therefore, they can be distinctly recognized from many other organic compounds due to this particular trait.
- In the realm of biology, lipids have a broad presence. They naturally occur in a variety of organisms, ranging from plants to animals and even microorganisms. One of the primary roles of lipids in these organisms is to act as cell membrane components. Besides serving this structural function, lipids also play critical roles in energy storage. They are the molecules that organisms rely on for long-term energy needs. Then, there’s the matter of insulation, where lipids provide a protective layer for certain organisms against varying temperatures and other external factors. Hormones, which are vital for regulating various physiological processes in animals, are also derived from lipids.
- Delving deeper into the technical vocabulary associated with lipids, it’s essential to understand their compositional elements. Lipids predominantly consist of fatty acids and their derivatives. Fatty acids, in this context, are hydrocarbon chains that may be saturated or unsaturated. These fatty acids, when combined with alcohols like glycerol through ester bonds, result in what are termed as ‘simple lipids.’ Glycerol, with its three hydroxyl (OH-) groups, is the most commonly found alcohol in lipids. Therefore, depending on the type of alcohol combined with fatty acids, lipids can manifest as fats, oils, or even waxes. While fats and oils result from the esterification of fatty acids with glycerol, waxes result from the esterification with long-chain alcohols.
- Another aspect of lipids that requires emphasis is their state and characteristics. At room temperature, lipids may present themselves as liquids or non-crystalline solids. These substances are typically colorless, odorless, and tasteless, further distinguishing them from many other organic substances.
- Besides these functional roles, lipids also bear nutritional significance. They are essential components of diets due to their high energy value. Additionally, the presence of fat-soluble vitamins and essential fatty acids in natural foodstuffs underscores their dietary importance. Furthermore, lipoproteins, which are combinations of fats and proteins, are vital for the cell membranes and mitochondria of the cell.
- In conclusion, lipids, with their diverse functionalities and structures, are indispensable to life. Whether it’s providing energy, forming cell structures, or participating in regulatory processes, lipids play a central role in biology, making their understanding crucial in the study of life sciences.
Definition of Lipids
Lipids are a group of organic compounds, insoluble in water but soluble in non-polar organic solvents, that serve as energy storage molecules, cell membrane components, and play roles in signaling and insulation.
Properties of Lipids
Physical Properties of Lipids
- Solubility Characteristics:Lipids display specific solubility properties. They are soluble in non-polar solvents. Notably, solvents such as ether, alcohol, chloroform, acetone, and benzene can dissolve lipids efficiently. However, lipids are not soluble in water. This insolubility in water is due to the absence of ionic charges in lipid molecules. Therefore, this property further accentuates the hydrophobic (water-repelling) nature of lipids.
- Sensory Features:Pure fats and oils, which are subcategories of lipids, have some sensory characteristics. They are colorless, odorless, and tasteless. These attributes indicate the purity of the fat or oil, devoid of any contaminants.
- Nature and Behavior:Lipids, by definition, are either hydrophobic or amphiphilic small molecules. Hydrophobic molecules repel water, while amphiphilic molecules possess both water-attracting and repelling properties. Besides, lipids are greasy to touch, emphasizing their distinctive texture.
- Storage within the Body:In the human body, lipids are primarily stored in adipose tissues. These tissues serve as reservoirs for energy and also play a role in insulating and cushioning the body.
- State at Room Temperature:Depending on their molecular structure, lipids can manifest in different states at room temperature. They can be either liquid or non-crystalline solids. This property is mainly determined by the type and number of bonds between their carbon atoms.
- Structural Forms:Lipids can exist in various structural forms, specifically saturated or unsaturated. Saturated lipids possess only single bonds between their carbon atoms, making them more packed and usually solid at room temperature. On the other hand, unsaturated lipids have one or more double bonds, introducing kinks in their structure and often making them liquid at room temperature.
Chemical Properties of Lipids
- Hydrolysis of Triglycerides:Triglycerides, categorized under neutral lipids, undergo a reaction when exposed to water. Through this reaction, they dissociate into two products: carboxylic acid and alcohol. This breakdown signifies the susceptibility of triglycerides to water, leading to the formation of these distinct components.
- Saponification:When triglycerides interact with alkali, such as NaOH or KOH, or undergo hydrolysis in the presence of lipase enzymes, a process termed alkaline hydrolysis, they produce two primary products. These are soap, or fatty acid salts of sodium or potassium, and glycerol. This reaction forms the basis for soap-making in industries.
- Hydrogenation:Unsaturated fatty acids, possessing one or more double bonds, have the ability to react with hydrogen. During this reaction, the double bonds break, turning the unsaturated fatty acid molecules into their saturated counterparts. Therefore, this process is pivotal in the production of saturated fats in the food industry.
- Halogenation:On reacting with halogens, free or combined fatty acids acquire double bonds. Additionally, this reaction results in the decolorization of halogen solutions, indicating the addition of halogens to the fatty acid structure.
- Rancidity:Rancidity is a consequence of the oxidation and hydrolysis of fats and oils. It manifests as a disagreeable odor, marking the degradation of these lipids over time.
- Structural Composition:Lipids predominantly consist of hydrocarbon chains, giving them a heterogeneous nature. While fats and oils are primarily triglycerides, providing concentrated energy storage, phospholipids stand out as essential cell membrane constituents. They form the lipid bilayer, ensuring cellular boundary integrity and enabling selective permeability. Furthermore, specific lipids like cholesterol and steroid hormones boast a four-ring structure, playing roles in membrane fluidity and cellular signaling.
- Functional Importance:Besides their structural roles, lipids offer numerous functions vital to organisms. They provide essential fatty acids that the body cannot synthesize independently. Additionally, they facilitate the absorption of fat-soluble vitamins, ensuring the proper nourishment of organisms.
| Physical Properties | Chemical Properties |
|---|---|
| Soluble in solvents like ether, alcohol, chloroform, acetone, and benzene. | Forms carboxylic acid and alcohol when triglycerides react with water. |
| Insoluble in water. | Produces soap or fatty acid salts and glycerol when hydrolyzed with alkali or enzymes. |
| Lipid molecules do not have ionic charges. | Converts unsaturated fatty acids to saturated by breaking double bonds during hydrogenation. |
| They are colorless, odorless, and tasteless. | Acquires double bonds when reacted with halogens; causes halogen solution decolorization. |
| Hydrophobic or amphiphilic small molecules. | Oxidation and hydrolysis lead to an unpleasant odor (rancidity). |
| Greasy in texture; stored in adipose tissues inside the body. | Comprised mainly of hydrocarbon chains; heterogeneous in nature. |
| Either liquid or non-crystalline solid at room temperature. | Efficient energy storage molecules in the form of fats and oils. |
| Can be saturated (single bonds only) or unsaturated (one or more double bonds). | Key components of cell membranes are phospholipids; they form the lipid bilayer. |
| Cholesterol and steroid hormones have a four-ring structure. | |
| Provide essential fatty acids and aid in the absorption of fat-soluble vitamins. |
Structure of Lipids
Lipids, characterized by their hydrophobic nature, are essential organic molecules that exhibit diverse structural attributes. Composed mainly of the elements Carbon, Hydrogen, and Oxygen, lipids distinguish themselves from other molecules like carbohydrates by having a significantly reduced proportion of water.
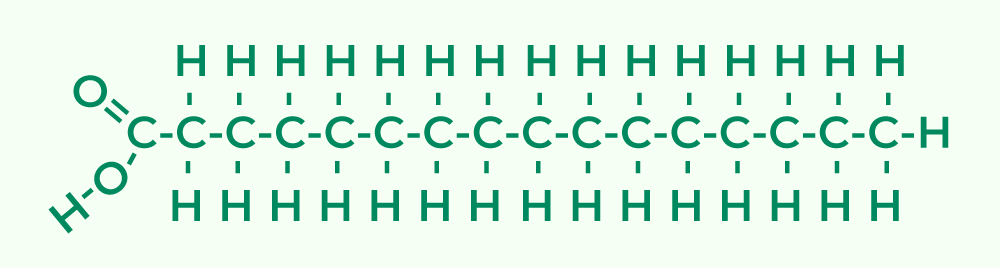
- Basic Constituents of Lipids:Unlike polysaccharides and proteins, lipids are not structured as polymers. Therefore, they don’t possess repeating monomeric units. Instead, the foundational blocks of lipids are two distinct molecules: glycerol and fatty acids.
- Glycerol: A central component in many lipids, glycerol consists of three carbon atoms. Each of these carbons is attached to a hydroxyl group, with the remaining bonds occupied by hydrogen atoms.
- Fatty Acids: These are long hydrocarbon chains terminated by a carboxyl group (COOH). The hydrocarbon part of fatty acids, often represented by the letter ‘R’, can vary in length and in the degree of saturation.
- Saturation in Fatty Acids:The saturation of a fatty acid pertains to the type and number of bonds present within its hydrocarbon chain.
- Saturated Fatty Acids: If every potential bond within a fatty acid is occupied by a hydrogen atom, leaving no carbon-carbon double bonds (C=C), the fatty acid is termed as saturated.
- Unsaturated Fatty Acids: In contrast, unsaturated fatty acids do possess one or more C=C bonds. Based on this, they can be further classified into:
- Monounsaturated Fatty Acids: Containing a single C=C bond.
- Polyunsaturated Fatty Acids: Characterized by the presence of multiple C=C bonds.
- Formation and Structure of Triglycerides:A notable category of lipids is triglycerides. In the structure of triglycerides, the glycerol molecule functions as the backbone. To this backbone, three fatty acid molecules are attached via ester bonds, a type of covalent bond. The resulting molecule is hydrophobic, due to the hydrocarbon chains of the fatty acids.
- Amphipathic Nature of Phospholipids:Beyond triglycerides, phospholipids represent another significant class of lipids. These molecules are built similarly, with a glycerol backbone. However, in phospholipids, one of the fatty acid chains is replaced by a hydrophilic phosphate group. Consequently, while the fatty acid chains repel water, the phosphate group attracts it, rendering the molecule amphipathic.
Classification of lipids
Lipids, given their diverse nature and crucial role in various biological processes, have been extensively studied and classified. Their classification primarily arises from their structural and functional characteristics. Herein is a detailed breakdown of the lipid classification:
- Simple Lipids: Simple lipids are essentially esters of fatty acids combined with alcohols. These can be primarily broken down into two main types:
- a. Fats and Oils (Triacylglycerols): These are esters of fatty acids combined with glycerol. The distinguishing feature between fats and oils is their state at room temperature: fats are solid, whereas oils are liquid. These serve a significant function in the body, being the primary concentrated storage form of energy. Beyond energy storage, they play a role in cellular structures and various biochemical processes.
- b. Waxes: Unlike fats and oils that esterify with glycerol, waxes are esters of fatty acids combined with alcohols other than glycerol. These alcohols might be either aliphatic or alicyclic, with cetyl alcohol being a common constituent. Waxes find applications in products like candles, lubricants, cosmetics, ointments, and polishes.
- Complex (or Compound) Lipids: Moving beyond simple esters, complex lipids are esters of fatty acids with alcohols that contain additional groups. These additional components could be phosphate, nitrogenous bases, carbohydrates, proteins, among others. Complex lipids are subdivided as:
- a. Phospholipids: These lipids are characterized by the presence of phosphoric acid, often accompanied by a nitrogenous base, in addition to the usual alcohol and fatty acids.
- i. Glycerophospholipids: Here, the alcohol component is glycerol. Examples include lecithin and cephalin.
- ii. Sphingophospholipids: These contain sphingosine as their alcohol. Sphingomyelin is a classic representative of this category.
- b. Glycolipids: These lipids comprise a fatty acid, a carbohydrate, and a nitrogenous base. They lack glycerol and phosphate. Given that sphingosine is the alcohol component, they are sometimes referred to as glycosphingolipids. Cerebrosides and gangliosides are examples of glycolipids.
- c. Lipoproteins: These are macromolecular assemblies of lipids in conjunction with proteins.
- d. Other Complex Lipids: This category includes sulfolipids, aminolipids, and lipopolysaccharides, to name a few.
- a. Phospholipids: These lipids are characterized by the presence of phosphoric acid, often accompanied by a nitrogenous base, in addition to the usual alcohol and fatty acids.
- Derived Lipids: Derived lipids are the products resulting from the hydrolysis of both simple and complex lipids. However, they still retain the characteristic features of lipids. They encompass a broad range of compounds including, but not limited to, glycerol, various alcohols, fatty acids, mono- and diacylglycerols, lipid-soluble vitamins, steroid hormones, hydrocarbons, and ketone bodies.
- Miscellaneous Lipids: This category captures an array of compounds that exhibit lipid-like properties. Examples in this category include carotenoids, squalene, hydrocarbons like pentacosane found in beeswax, and terpenes.
Lastly, a special mention goes to Neutral Lipids. These lipids are uncharged and encompass mono-, di-, and triacylglycerols, cholesterol, and cholesteryl esters.
What are Fatty acids?
Fatty acids are fundamental organic molecules, classified under the category of carboxylic acids. Characterized by their long chains, fatty acids typically contain between 4 to 36 carbon atoms. Delving into their structure reveals additional layers of complexity, with certain key features and classifications to consider.
- Basic Structure:At their core, fatty acids are carboxylic acids appended with a hydrocarbon side chain. This hydrocarbon chain can vary in length and in bond types, making it a distinguishing feature of different fatty acids. It is this structure that primarily classifies fatty acids as the simplest form of lipids.
- Saturation in Fatty Acids:A pivotal feature of fatty acids is the nature of the bonds between the carbon atoms in their hydrocarbon chains. These bonds can be either single or double, leading to:
- Saturated Fatty Acids: When all the carbon-carbon bonds are single, a fatty acid is considered saturated.
- Unsaturated Fatty Acids: Conversely, the presence of one or more carbon-carbon double bonds designates a fatty acid as unsaturated.
- Occurrence and Associations:Naturally occurring fatty acids predominantly have unbranched chains. Their association with other molecules gives rise to various lipid classes. For instance:
- Triglycerides: Here, fatty acids remain linked to an alcohol, specifically glycerol, through an ester linkage.
- Phospholipids: Another class where fatty acids play a role.
- Cholesteryl Esters: Yet another class featuring fatty acids.
- Function and Storage:One cannot discuss fatty acids without emphasizing their functional significance. Primarily, they serve as an energy reserve. When stored, fatty acids are linked to glycerol through an ester bond to form triglycerides, which effectively serve as the body’s fat reserves.
Nomenclature of fatty acids
The naming or nomenclature of fatty acids, akin to other scientific entities, follows specific rules and guidelines to ensure uniformity and clarity in communication. To comprehend the naming conventions of fatty acids, it is imperative to understand the foundational principles that govern these conventions.
- Base Naming by Hydrocarbon Derivation:
- Saturated Fatty Acids: The systematic names of these fatty acids derive from the hydrocarbon they originate from and end with the suffix “-anoic.” For instance, a fatty acid derived from the hydrocarbon ‘octane’ would be termed “octanoic acid.”
- Unsaturated Fatty Acids: Contrary to their saturated counterparts, unsaturated fatty acids conclude with the suffix “-enoic.” An example being “octadecenoic acid.”
- Common Names:Besides the systematic names, fatty acids also possess common names. These names are often more prevalent and are typically utilized for everyday reference. For instance, while the systematic naming might refer to a particular fatty acid with a specific term, the common name might be simpler and more easily recognized within the community.
- Numbering of Carbon Atoms:
- From the Carboxyl End: Numbering begins from the carboxyl carbon, which is assigned the number 1. The adjacent carbons are sequentially numbered as 2, 3, 4, and so on, progressing down the chain.From the Methyl End: An alternative approach is to start numbering from the terminal end, which contains the methyl group. This carbon is termed the omega (ω) carbon. Subsequent carbons, moving inwards from the methyl end, are designated as omega-1, omega-2, omega-3, and so forth.
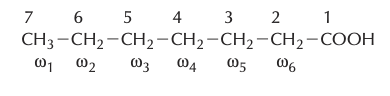
Length of hydrocarbon chain of fatty acids
The structural diversity of fatty acids largely stems from variations in their hydrocarbon chains. One of the pivotal aspects that dictate the physical and chemical properties of fatty acids is the length of their hydrocarbon chain. Therefore, understanding this facet is paramount for a comprehensive grasp of fatty acid behavior and functionality.
- Criteria for Classification:Fatty acids are primarily differentiated based on the number of carbon atoms within their hydrocarbon chains. As per this criterion, fatty acids can be meticulously categorized into three distinct groups:
- Short-Chain Fatty Acids (SCFAs):These fatty acids are characterized by hydrocarbon chains that contain fewer than 6 carbon atoms. Due to their relatively shorter length, SCFAs have specific physical properties, such as a lower melting point, which differentiates them from their longer-chained counterparts.
- Medium-Chain Fatty Acids (MCFAs):As the name suggests, MCFAs strike a middle ground in terms of chain length. Specifically, these fatty acids possess hydrocarbon chains with a length ranging from 8 to 14 carbon atoms. This intermediate length imparts to MCFAs certain characteristics that make them distinct from both SCFAs and LCFAs.
- Long-Chain Fatty Acids (LCFAs):These fatty acids have hydrocarbon chains that span between 16 to 24 carbon atoms. Due to their extended length, LCFAs typically have higher melting points and exhibit distinct solubility behaviors when compared to the shorter chain variants.
Shorthand representation of fatty acids
In the realm of biochemistry, the intricacies of fatty acid structures necessitate a more streamlined method of representation. Therefore, instead of utilizing full-length structural depictions, biochemists have devised a concise shorthand notation system for fatty acids. This system is not only efficient but also provides essential information about the fatty acid in question.
- Basics of the Notation:The primary principle of the shorthand notation is sequential representation. The total count of carbon atoms in the fatty acid chain is presented first. Following this, the number of double bonds is indicated. Subsequently, the position of these double bonds, commencing from the carboxyl end, is detailed.
- Examples:
- Saturated Fatty Acid: For instance, palmitic acid, a saturated fatty acid with no double bonds, is succinctly represented as 16:0.
- Monounsaturated Fatty Acid: Oleic acid, which possesses one double bond, is denoted as 18:1;9. This implies that there’s a double bond between the 9th and 10th carbon atoms.
- Polyunsaturated Fatty Acid: Arachidonic acid, having four double bonds, is described as 20:4;5,8,11,14, specifying the positions of all its double bonds.
- Positional Notations:
- Carboxyl-End Position: The notation like “Δ9” signifies the location of the double bond between the 9th and 10th carbon atoms, counting from the carboxyl end.
- Methyl-End Position: On the other hand, a notation like “ω9” delineates the position of the double bond, counting from the methyl end or the opposite side of the carboxyl end.
- Notable Series:Naturally occurring unsaturated fatty acids can be categorized into distinct series based on the position of their initial double bond. These include the ω9, ω6, and ω3 series. For illustration:
- ω9 Series: The exemplar is Oleic acid represented as 18:1;9.
- ω6 Series: This series includes Linoleic acid (18:2;9,12) and Arachidonic acid (20:4;5,8,11,14).
- ω3 Series: Linolenic acid falls under this category with the notation 18:3;9,12,15.
Even and odd carbon fatty acids
- Even Carbon Fatty Acids:
- Prevalence: A significant majority of fatty acids found in natural lipids fall under the category of even carbon fatty acids. They usually range from 14 carbon atoms to 20 carbon atoms.
- Biosynthesis: The dominance of even-carbon fatty acids is not random but rooted in the very process of their formation. Specifically, the biosynthesis of fatty acids predominantly transpires through the sequential addition of 2 carbon units. This method naturally lends itself to the production of even-numbered chains.
- Examples: Among these even-carbon fatty acids, palmitic acid, which has 16 carbon atoms, and stearic acid, with 18 carbon atoms, are notably prevalent.
- Odd Carbon Fatty Acids:
- Rarity: Contrasting with their even-carbon counterparts, odd-carbon fatty acids are less commonly found in nature.
- Examples: However, they do exist, and some of the known types include propionic acid, which contains 3 carbon atoms, and valeric acid, which has 5 carbon atoms.
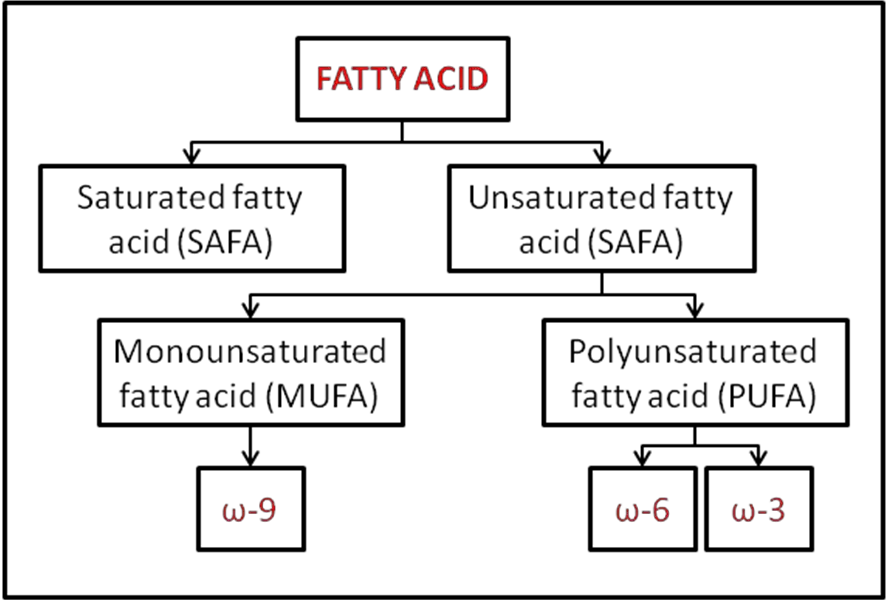
Saturated and unsaturated fatty acids
- Saturated Fatty Acids:
- Basic Structure: Saturated fatty acids possess unbranched linear chains of CH2 groups. These chains are exclusively linked together by carbon-carbon single bonds, ending with a terminal carboxylic acid group.
- Saturation Explained: The term ‘saturated’ signifies that each carbon atom within the molecule is bonded to the maximum number of hydrogen atoms possible. This is reflected in the general formula CnH2n+1COOH.
- Physical Properties: Because of their structure, saturated fatty acids have a higher melting point. Therefore, they predominantly remain solid at room temperatures.
- Sources: While a significant portion of saturated fatty acids comes from animal sources, such as butter, meat, and whole milk, there are plant-derived examples too, such as coconut and peanut oil.
- Unsaturated Fatty Acids:
- Complex Structure: Unlike their saturated counterparts, unsaturated fatty acids feature one or more carbon-carbon double bonds in their bent hydrocarbon chains, culminating in a terminal carboxylic acid group.
- Unsaturation Elaborated: The term ‘unsaturated’ indicates a deficiency in the number of hydrogen atoms bonded to carbon atoms, due to the presence of double bonds.
- Importance of Conformations: The existence of double bonds gives rise to cis and trans conformations. Notably, the cis conformation is more prevalent in the human body.
- Physical Properties: The presence of double bonds reduces the melting point of unsaturated fatty acids, causing them to be liquid at room temperatures.
- Sources and Types: Commonly found in vegetable oils and fish oils, unsaturated fatty acids can further be differentiated. Those containing a single double bond are termed monounsaturated, while those with multiple double bonds are called polyunsaturated fatty acids (PUFA).
Isomerism of fatty acids
Isomerism in the realm of unsaturated fatty acids can be understood by considering two primary types: geometrical isomerism and positional isomerism. Isomerism, in its essence, denotes the phenomenon where molecules possess the same structural elements and chemical formula but differ in the spatial arrangement or position of specific atoms within the molecule.
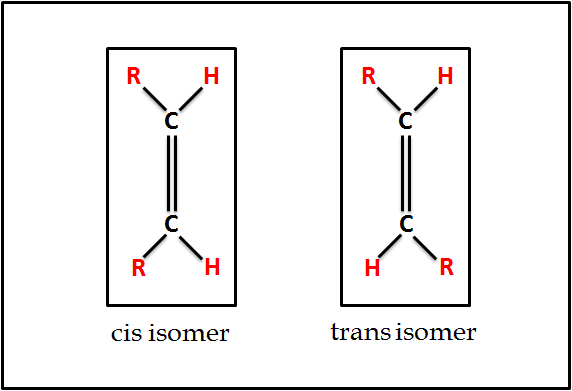
Geometrical Isomers of Fatty Acids
- Considering the structural configuration of carbon atoms in fatty acids, these atoms exhibit a three-dimensional tetrahedral structure. However, a transformation occurs when two of these carbon atoms with a tetrahedral structure unite via a double bond. This results in the double bond assuming a flat or planar structure. Now, when focusing on this double bond, it’s essential to note that rotation around single bonds (C-C) is unrestricted. In contrast, the rotation is restricted in the case of double bonds (C=C). Therefore, hydrogen atoms connected to each of the carbons involved in this double bond might either align on the same side, leading to what is called a cis isomer, or on opposing sides, resulting in a trans isomer.
- One significant observation is the profound impact this cis or trans arrangement has on the fatty acids’ physical attributes, with the melting point standing out as particularly notable. For instance, when comparing the melting points of various cis and trans geometric isomers of fatty acids, notable variations are evident. Such melting point discrepancies impart distinct biochemical and nutritional behaviors to the geometrical isomers of a fatty acid. Notably, trans fatty acids, especially those of industrial origin, have been linked to detrimental health effects in humans, with an accentuated risk for cardiovascular diseases.
Positional Isomers of Fatty Acids
- The concept of positional isomerism revolves around the varying positions that one or multiple double bonds can occupy within a fatty acid’s structure. A classic illustration is the comparison between oleic acid and vaccenic acid. While the former predominantly exists in vegetable oils like olive oil, the latter is more abundant in animal fats. This scenario offers a dual perspective: both fatty acids are geometric isomers, and they also classify as positional isomers due to the distinct positions of their double bonds.
- It’s pivotal to recognize that many naturally occurring fatty acids exhibit positional isomerism. Nonetheless, these isomers typically exist in minute concentrations. While the biological implications of trans geometric isomers are well-understood, the biological ramifications of positional isomers remain relatively under-explored. A notable exception is the conjugated linoleic acid, a geometric and positional isomer of linoleic acid, which has been ascribed with multiple health benefits. However, the scientific consensus on these purported benefits remains inconclusive. Furthermore, similar to geometrical isomerism, fatty acid manipulation through technological means augments the diversity and intricacy of positional isomers.
What are Essential Fatty Acids?
The human body is an intricate system that requires various compounds for its proper functioning. Among these compounds are specific types of fatty acids termed “Essential Fatty Acids” (EFAs). As the name suggests, these fatty acids are crucial for the body. Here is an in-depth examination of EFAs.
- Definition:Essential Fatty Acids (EFAs) are distinctive in that the human body is incapable of synthesizing them on its own. Therefore, it becomes imperative to obtain them through dietary intake. They are characterized by their polyunsaturated nature.
- Primary Types:The two primary EFAs are:
- Linoleic Acid: It has the chemical representation 18:2;9,12, denoting that it has 18 carbon atoms, 2 double bonds, and these double bonds are located between carbons 9-10 and 11-12.
- Linolenic Acid: Represented as 18:3;9,12,15, this acid contains 3 double bonds situated at specific locations on the carbon chain.
- Biochemical Basis for Essentiality:The essence of linoleic and linolenic acids lies in the human body’s absence of specific enzymes. Without these enzymes, the body cannot introduce double bonds beyond the 9th and 10th carbons.
- Functions:EFAs have a plethora of roles in the human system. These include:
- Contributing to the membrane’s structure and its function.
- Assisting in the transportation of cholesterol.
- Playing a role in the formation of lipoproteins.
- Preventing the onset of conditions like fatty liver.
- Facilitating the synthesis of a significant group of compounds known as eicosanoids.
- Deficiency and its Effects:A scarcity of EFAs in the diet can have detrimental effects on the body. The deficiency manifests in conditions known as phrynoderma or “toad skin.” This condition is typified by horny eruptions appearing on various parts of the body, hair loss, and hindered wound healing processes.
- Isomerism in Unsaturated Fatty Acids:It is noteworthy to mention that unsaturated fatty acids can exhibit geometric isomerism. The orientation of groups around the double bond determines this. The cis configuration is when acyl groups or atoms are on the same side of the double bond, while the trans configuration occurs when these groups are on opposite sides. An example includes oleic acid as a cis isomer and elaidic acid as its trans counterpart. Predominantly, naturally occurring unsaturated fatty acids adopt the cis isomeric form, contributing to the packing of the membrane structure efficiently.
Triacylglycerols
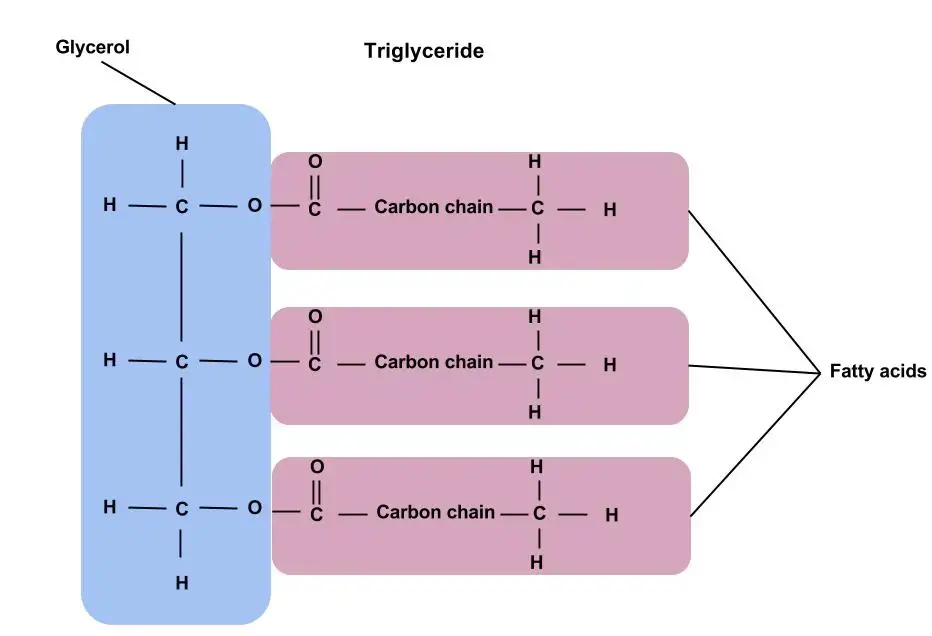
Triacylglycerols, previously known as triglycerides, are fundamentally the esters of glycerol combined with fatty acids. Both plants and animals extensively contain fats and oils, which are, on a chemical level, triacylglycerols. They have a distinct characteristic of being insoluble in water, thanks to their non-polar nature, and they are frequently referred to as neutral fats.
Therefore, when examining the primary function of triacylglycerols, it’s evident that they predominantly serve as the fuel reserves in animals. These reserves, in the form of fats, are incredibly abundant. To illustrate, in average humans, men possess about 20% and women about 25% by weight of these reserves. This stored fat is adequate to sustain the body’s caloric needs for approximately 2 to 3 months.
Besides the function of energy storage, these fats mainly reside in adipose tissue. The adipocytes, cells specialized in storing fat, predominantly exist in the subcutaneous layer and within the abdominal cavity. These cells store fat as globules that are distributed throughout their cytoplasm. Interestingly, triacylglycerols don’t contribute as structural elements of biological membranes.
Then, diving deeper into the structures of acylglycerols, there are three variants: monoacylglycerols, diacylglycerols, and triacylglycerols. These consist of one, two, or three fatty acid molecules esterified to glycerol, respectively. Among them, triacylglycerols hold the most biochemical significance.
There are two categories for triacylglycerols based on their composition:
- Simple triacylglycerols: These contain the same fatty acid residue on all three carbons. An example includes tristearoyl glycerol or tristearin.
- Mixed triacylglycerols: These are more prevalent. They possess two or three varied types of fatty acid residues. Typically, the fatty acid attached to C1 is saturated, C2’s is unsaturated, and C3’s could be either. The naming convention for triacylglycerols depends on the acyl radical’s position on glycerol. For instance, 1,3-palmitoyl 2-linoleoyl glycerol is a type of triacylglycerol.
It’s noteworthy to mention that plant-based triacylglycerols generally contain a higher percentage of unsaturated fatty acids compared to those of animals.
Regarding the stereospecific numbering of glycerol, even though it might appear that carbons 1 and 3 are identical, in a 3D structure, they are distinct. Biochemists utilize a stereospecific numbering system (sn) and attach an ‘sn’ prefix to glycerol. Thus, it’s crucial to understand that C1 and C3 are different. Specific enzymes in cells can differentiate these two carbons. For instance, glycerokinase only phosphorylates sn-3 glycerol, resulting in sn-glycerol 3-phosphate, not sn-1 glycerol.
Structure of Triglycerides
- Basic Composition: At the core, triglycerides are tri-esters, meaning they comprise three ester groups. These are formed when three fatty acid molecules bond covalently to a single glycerol molecule. This covalent bond, specifically termed an ester bond, is what grants the molecule its stability and characteristic properties.
- Chemical Reaction for Formation: The formation of triglycerides can be represented by the following chemical reaction: HOCH2CH(OH)CH2OH+RCO2H+R′CO2H+R″CO2H→RCO2CH2CH(O2CR′)CH2CO2R″+3H2O
This reaction showcases how three distinct fatty acids condense with a glycerol molecule, resulting in the formation of a triglyceride molecule and three water molecules. - Variability in Fatty Acids: The fatty acids contributing to a triglyceride can differ in their chain lengths and may or may not be identical. In most naturally occurring triglycerides, the fatty acid chains predominantly consist of 16, 18, or 20 carbon atoms. Interestingly, the presence of even-numbered carbon atoms in both plants and animals indicates their biosynthetic origin from the two-carbon compound, acetyl CoA. Triglycerides with identical fatty acids are termed homotriglycerides.
- Solubility Characteristics: A significant feature of triglycerides is their solubility profile. Due to the even distribution of charges throughout the molecule, triglycerides cannot form hydrogen bonds with water molecules. Therefore, they remain insoluble in water, a characteristic that influences their behavior in biological systems.
Properties Of Triacylglycerols
- Hydrolysis: One of the primary properties of triacylglycerols is their ability to undergo hydrolysis. This enzymatic process breaks down triacylglycerols in a stepwise manner, eventually leading to the liberation of free fatty acids and glycerol. Lipases, specialized enzymes, catalyze this hydrolysis. Therefore, this hydrolytic activity is pivotal for two main functions: the digestion of fat within the gastrointestinal tract and the mobilization of fat from adipose tissues.
- Saponification: Another distinct property of triacylglycerols is saponification. This refers to the hydrolysis of triacylglycerols by alkali, resulting in the formation of glycerol and soaps. The reaction can be represented as:Triacylglycerol + 3 NaOH → Glycerol + 3 R-COONa (soaps)
- Rancidity: The term ‘rancidity’ describes the degradation of fats and oils, leading to an off-putting taste. Fats, especially those with unsaturated fatty acids, are particularly prone to rancidity. Rancidity manifests when fats and oils are exposed to various external factors such as air, moisture, light, and bacteria. Two primary forms of rancidity exist:
- Hydrolytic Rancidity: Caused by the partial hydrolysis of triacylglycerols due to bacterial enzymes.
- Oxidative Rancidity: Resulting from the oxidation of unsaturated fatty acids, leading to the creation of undesirable products like dicarboxylic acids, aldehydes, and ketones. Consuming rancid fats and oils is inadvisable for human health.
- In Vivo Lipid Peroxidation: Within living cells, lipids can undergo oxidation, producing peroxides and free radicals. These free radicals can potentially inflict damage on tissues, and are believed to be linked to several ailments, including inflammatory diseases, aging, cancer, and atherosclerosis. Fortunately, cells have natural defenses in the form of antioxidants such as vitamin E, urate, and superoxide dismutase. These components actively prevent in vivo lipid peroxidation, safeguarding the cells.
Tests to check purity of fats and oils
Given the escalating concerns over the adulteration of fats and oils, ensuring their purity has become paramount. In this light, various laboratory tests have been designed to assess the purity of these essential dietary components. Here, we will sequentially explain the most prevalent tests.
- Iodine Number: This metric denotes the grams of iodine absorbed by 100g of a fat or oil. The iodine number offers insight into the relative unsaturation of fats, making it directly proportional to the content of unsaturated fatty acids. Therefore, a lower iodine number signifies a lesser degree of unsaturation. For instance, the iodine numbers of some common oils/fats are:
- Coconut oil: 7—10
- Butter: 25—28
- Palm oil: 45—55
- Olive oil: 80—85
- Groundnut oil: 85—100
- Cottonseed oil: 100—110
- Sunflower oil: 125—135
- Linseed oil: 175—200
- Saponification Number: This measure represents the milligrams of KOH required to saponify one gram of fat or oil. It primarily indicates the average molecular size of the fatty acids present. Fats with shorter chain fatty acids tend to have a higher value. Some representative values include:
- Human fat: 195–200
- Butter: 230–240
- Coconut oil: 250–260
- Reichert-Meissl (RM) Number: The RM number reflects the volume (in milliliters) of 0.1 N KOH needed to neutralize the soluble volatile fatty acids distilled from 5g of fat. This test is especially pertinent for evaluating the purity of butter, which inherently contains a considerable amount of volatile fatty acids such as butyric, caproic, and caprylic acids. This stands in stark contrast to other fats and oils that contain minimal volatile fatty acids. For reference, butter typically exhibits an RM number between 25-30, while most other edible oils register values below 1. Hence, this RM number is a sensitive indicator to detect butter adulteration.
- Acid Number: This metric specifies the milligrams of KOH essential to neutralize the free fatty acids in one gram of fat or oil. Ideally, refined oils should be devoid of any free fatty acids. However, decomposed oils, whether due to chemical or bacterial contamination, will produce free fatty acids. Consequently, oils showcasing elevated acid numbers are deemed unfit for human consumption.
Functions of Triglycerides
- Energy Storage: Foremost among the roles of triglycerides is their function as the primary energy storage molecules in the body. These molecules are adept at retaining energy, storing it efficiently for extended periods. When the body requires energy, especially during periods of prolonged fasting or rigorous physical activity, triglycerides stored in fat cells are released into the bloodstream. This release is regulated by specific hormones that ensure a consistent energy supply to meet the body’s demands.
- Thermal Insulation: Besides serving as an energy reservoir, triglycerides have another pivotal function. They form a protective layer beneath the skin, acting as an insulating barrier. This layer aids in maintaining the body’s temperature, ensuring it remains within a narrow and optimal range, irrespective of external environmental conditions. Therefore, they contribute significantly to homeostasis, the body’s mechanism to maintain internal stability.
- Facilitation of Vitamin Absorption: Fat-soluble vitamins, such as vitamins A, D, E, and K, require a medium for efficient absorption and transport within the body. Triglycerides fulfill this role by aiding in the absorption of these vitamins from the digestive tract and subsequently facilitating their transport to various tissues. Without the presence of triglycerides, the body would struggle to assimilate and utilize these vital nutrients effectively.
- Protection and Cushioning: Although not explicitly mentioned in the provided content, it’s worth noting that triglycerides also serve a protective function. They cushion vital organs, acting as a shock absorber, and thus provide an added layer of protection against potential physical harm.
Phospholipids
Phospholipids, a distinct category within the lipid family, are compound lipids that possess a complex structure, offering them a unique set of functionalities. Detailed below is an organized breakdown of their components, classification, and key roles in biological systems.
- Composition of Phospholipids: Phospholipids are more intricate than simple lipids due to the incorporation of phosphoric acid. Besides phosphoric acid, they also consist of fatty acids, a nitrogenous base, and an alcohol.
- Classification of Phospholipids: Phospholipids are typically classified into two major categories based on the type of alcohol present:
- Glycerophospholipids (or phosphoglycerides): This class of phospholipids features glycerol as the alcohol component.
- Sphingophospholipids (or sphingomyelins): In this type, sphingosine serves as the alcohol.
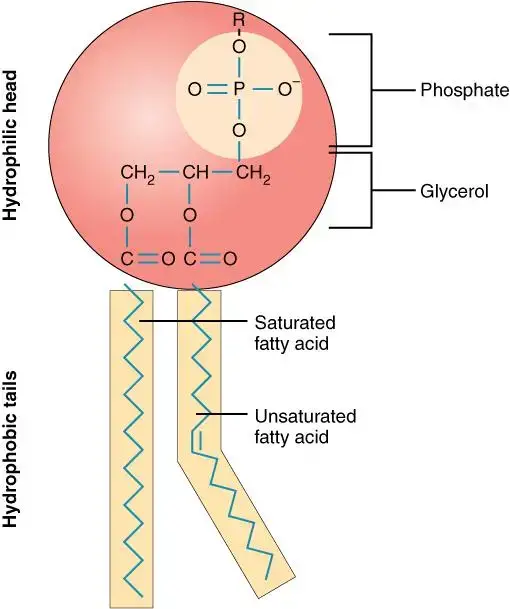
- Molecular Anatomy: At the molecular level, a phospholipid is an organic structure that combines fatty acids, a phosphate group, and a glycerol group. This unique composition enables phospholipids to be the principal components of various cellular membranes.
- The Phospholipid Bilayer: One of the most vital roles of phospholipids is the formation of the phospholipid bilayer, which is crucial to the structural integrity of the cell membrane. This bilayer is responsible for the selective transport of molecules, ensuring that the cell’s internal environment remains regulated.
- Hydrophilic and Hydrophobic Interactions: The phosphate group in a phospholipid forms the hydrophilic (water-attracting) head, while the fatty acids give rise to the hydrophobic (water-repelling) tails. These two regions are conjoined by a glycerol molecule. The interactions between the hydrophobic tails and hydrophilic heads with surrounding molecules render the cell membrane amphipathic. This characteristic, in turn, enables the regulated passage of biomolecules across the membrane.
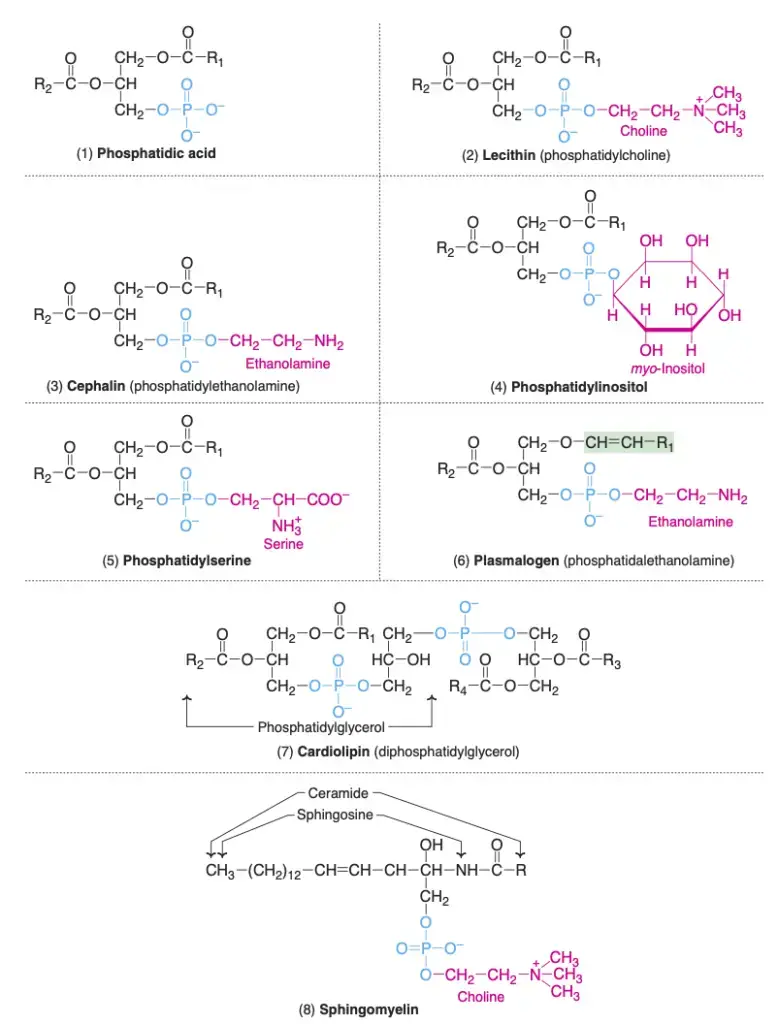
1. Glycerophospholipids
Glycerophospholipids represent a central group of lipids predominantly found in biological membranes. They exhibit a characteristic structure where glycerol 3-phosphate gets esterified at positions C1 and C2 with fatty acids. Notably, the C1 position typically houses a saturated fatty acid, while an unsaturated fatty acid occupies the C2 position.
- Phosphatidic Acid: Regarded as the simplest form of phospholipid, phosphatidic acid does not manifest in significant concentrations within tissues. Essentially, it serves as a stepping stone in the synthesis pathway for both triacylglycerols and other phospholipids. Many other glycerophospholipids with varying nitrogenous bases or other distinct groups can be seen as derivatives of phosphatidic acid.
- Lecithins (Phosphatidylcholine): Lecithins stand out as the predominant group of phospholipids within cell membranes. Chemically, lecithin, derived from the Greek word “lecithos” meaning egg yolk, is a phosphatidic acid variant with choline as its base. Phosphatidylcholines essentially act as storage for the body’s choline supply. Moreover:
- Dipalmitoyl Lecithin plays a pivotal role in the lungs, acting as a surfactant to prevent inner surface adherence due to surface tension. A marked absence of this specific lecithin can lead to respiratory distress syndrome in infants.
- Lysolecithin arises from the removal of a fatty acid from either the C1 or C2 position of lecithin.
- Cephalins (Phosphatidylethanolamine): In cephalins, ethanolamine takes on the role of the nitrogenous base, differentiating it from lecithin.
- Phosphatidylinositol: Here, the steroisomer myo-inositol joins phosphatidic acid to form phosphatidylinositol, a key component of cell membranes. Certain hormones, such as oxytocin and vasopressin, derive their effects through interactions with phosphatidylinositol.
- Phosphatidylserine: This variant of glycerophospholipids contains the amino acid serine, with phosphatidylthreonine also detected in select tissues.
- Plasmalogens: Unique to plasmalogens, a fatty acid attaches through an ether linkage at the C1 position of glycerol. The predominant form is phosphatidalethanolamine, which mirrors phosphatidylethanolamine in structure, save for the presence of an ether linkage in place of an ester. It’s noteworthy that choline, inositol, and serine can substitute ethanolamine, resulting in distinct plasmalogens.
- Cardiolipin: Initially discovered in heart muscle, cardiolipin comprises two phosphatidic acid molecules tethered by an extra glycerol through phosphate groups. Cardiolipin is an integral part of the inner mitochondrial membrane, and its adequate levels are vital for mitochondrial function. Deficiencies can give rise to ailments like mitochondrial dysfunction, aging, hypothyroidism, and cardioskeletal myopathy (Barth syndrome).
2. Sphingomyelins
- Sphingomyelins, classified under sphingophospholipids, are distinguished from other lipids by their lack of glycerol. Instead, they incorporate a backbone of sphingosine, an amino alcohol, which forms the structural framework of these molecules. The sphingosine backbone is linked to a fatty acid through an amide bond, thus creating ceramide. In sphingomyelins, the ceramide is further linked to phosphorylcholine, a compound comprising a phosphate group and choline.
- These lipids are primarily located in the myelin sheath, the insulating layer that surrounds nerve cells, ensuring rapid and efficient transmission of electrical impulses within the brain and nervous system. Therefore, sphingomyelins are found in considerable concentrations within brain and nervous tissues.
- The functional significance of sphingomyelins extends beyond their structural role in myelin. Ceramide, a central component of these molecules, functions as a second messenger within cellular signaling pathways. It is instrumental in the regulation of apoptosis, which is the programmed death of cells—a critical process for maintaining cellular homeostasis. Furthermore, ceramide regulates other vital cellular activities, including the cell cycle and differentiation, influencing how cells grow and develop.
- A specialized form of ceramide that includes a 30-carbon fatty acid is particularly important in the skin. It contributes to the skin’s barrier function, regulating water permeability and ensuring maintenance of hydration. Consequently, sphingomyelins play a crucial role not only in neural function but also in skin health.
- In summary, sphingomyelins are a unique class of sphingophospholipids that contribute significantly to the structural integrity and functional regulation of neural and integumentary systems. Their presence is critical for both intracellular signaling and the protection of delicate tissues.
Functions of phospholipids
Phospholipids, being complex lipids, play several crucial roles in maintaining and regulating biological systems. Here’s a detailed exploration of their functions:
- Structural Role in Membranes: In conjunction with proteins, phospholipids establish the foundational structure of cellular membranes. Therefore, they are pivotal in regulating the permeability of these membranes, determining which substances can pass in and out of cells.
- Supporting Cellular Respiration: Specific phospholipids like lecithin, cephalin, and cardiolipin, found in mitochondria, are integral for maintaining the proper arrangement of the components in the electron transport chain. This is essential for cellular respiration, a process that generates energy for the cell.
- Facilitating Fat Absorption: Phospholipids are actively involved in the absorption process of fats from the intestine, ensuring efficient nutrient uptake.
- Transport of Lipids: They are indispensable for creating different lipoproteins, playing a key role in the transportation of lipids throughout the body.
- Preventing Fatty Liver: Phospholipids can thwart the accumulation of fat in the liver, which is why they are regarded as lipotropic factors. Their presence helps maintain liver health.
- Precursors for Eicosanoids: Arachidonic acid, an unsaturated fatty acid released from phospholipids, is vital for the synthesis of eicosanoids. This group includes molecules like prostaglandins, prostacyclins, and thromboxanes.
- Assisting in Cholesterol Removal: Phospholipids have a role in reverse cholesterol transport, aiding in the elimination of cholesterol from the body, thus promoting cardiovascular health.
- Acting as Surfactants: Certain phospholipids function as surfactants, which reduce surface tension. A notable example is dipalmitoyl phosphatidylcholine, a crucial surfactant in the lungs. Its deficiency is linked with respiratory distress syndrome in infants.
- Blood Clotting: Cephalins, a significant category of phospholipids, participate in the blood clotting process, ensuring the body’s ability to prevent excessive bleeding.
- Involvement in Hormonal Action: Phosphatidylinositol acts as a precursor for second messengers, specifically inositol triphosphate and diacylglycerol. These messengers are involved in the actions of certain hormones, helping mediate their effects on target cells.
Glycolipids
Glycolipids, commonly referred to as glycosphingolipids, are essential components of the cellular structure, particularly in the cell membrane and neural tissues. A deeper examination of their composition, types, and significance offers a clearer understanding of their role in biological systems.
- Primary Constituents: Glycolipids are notably present in the cell membrane and are especially concentrated in nervous tissues. The brain, in particular, contains significant quantities of glycolipids, emphasizing their role in neural processes.
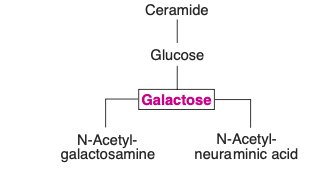
- Cerebrosides: Representing the simplest form of glycolipids, cerebrosides encompass a ceramide, which is a combination of sphingosine and a fatty acid, and one or more sugar molecules. Two primary cerebrosides are galactocerebroside (or galactosylceramide) and glucocerebroside. An important aspect to note about galactosylceramide is its composition that includes the fatty acid known as cerebronic acid. Furthermore, sulfagalactosylceramide emerges as a sulfatide derived from galactosylceramide.
- Gangliosides: Representing a more intricate form of glycosphingolipids, gangliosides are predominantly localized in ganglions. These complex molecules are essentially derivatives of cerebrosides. What differentiates them is the presence of one or more molecules of N-acetylneuraminic acid (NANA), a prominent sialic acid. The structure of NANA can be further explored in carbohydrate chemistry contexts.
- Classification of Gangliosides: The brain houses several significant gangliosides, with GM1, GM2, GD, and GT being the most prominent. In this classification, the letter “G” denotes ganglioside, while the subsequent letters (M, D, and T) symbolize mono-, di-, or tri- sialic acid residues. The subsequent numerical value is indicative of the carbohydrate sequence attached to the ceramide. An important mention is the ganglioside GM2, which notably accumulates in cases of Tay-Sachs disease.
Steroids
Steroids are a class of organic compounds that are characterized by their unique structural core, the cyclic steroid nucleus. This core is formally known as cyclopentanoperhydrophenanthrene (CPPP) and is composed of three six-carbon rings (phenanthrene nucleus) and a five-carbon ring (cyclopentane), which are interconnected.
- Steroid Nucleus Composition: The CPPP consists of a three-ring phenanthrene structure (rings A, B, and C) with a cyclopentane ring (D) joined to it. Within the structure, the carbons are typically saturated, unless the presence of double bonds is indicated. Methyl side chains at the 18th and 19th positions are bound to the 10th and 13th carbons, respectively, displayed as single bonds. A characteristic feature of steroids is the side chain that is commonly found at the carbon 17 position.
- Biological Steroids: The biological system features a multitude of steroids including cholesterol, bile acids, vitamin D, sex hormones, adrenocortical hormones, and plant sterols like sitosterols, as well as certain cardiac glycosides and alkaloids. When a steroid molecule includes one or more hydroxyl groups, it is typically referred to as a sterol, indicative of a solid alcohol form.
- Cholesterol: This sterol is an abundant component found exclusively in animal cells. Cholesterol plays a vital role in cell membrane structure and is a significant constituent of lipoproteins. Structurally, cholesterol has a hydroxyl group at the third carbon, a double bond between the fifth and sixth carbons, and an aliphatic side chain consisting of eight carbons at the seventeenth carbon. Its amphiphilic nature is attributed to the presence of the hydroxyl group, which contributes to its role in determining membrane permeability. Furthermore, cholesterol is involved in forming cholesteryl esters through an esterification reaction with fatty acids at the hydroxyl group.
- Cholesterol Properties: As a substance, cholesterol presents as a yellowish, crystalline solid. It exhibits a characteristic notched appearance under microscopic observation and is insoluble in water while being soluble in various organic solvents. Cholesterol’s qualitative and quantitative analysis can be conducted through reactions such as Salkowski’s test, Liebermann-Burchard reaction, and Zak’s test.
- Cholesterol Functions: Besides contributing to membrane architecture, cholesterol is essential for synthesizing bile acids, hormones, and vitamin D. Its high dielectric constant suggests a role as an insulator in nervous tissues, aiding the transmission of electrical impulses.
- Ergosterol: Unlike cholesterol, ergosterol is found in plant cells and is a key structural component in the cell membranes of yeast and fungi. It serves as a precursor for the synthesis of vitamin D2 (ergocalciferol) upon exposure to light, which involves the opening of ring B in ergosterol’s structure. Other plant sterols include stigmasterol and beta-sitosterol.
In summary, steroids play a multifaceted role in biological systems, contributing to cell structure, hormone synthesis, and vitamin production. Each steroid possesses distinct structural features and functions that are critical to the vitality and functionality of living organisms.
Amphipathic Lipids
Lipids are primarily characterized by their insolubility in water, which stems from their predominant hydrocarbon groups. However, nature showcases several lipids that encompass both hydrophobic and hydrophilic regions, termed as amphipathic lipids. Derived from the Greek words “amphi,” meaning both, and “pathos,” meaning passion, these molecules have dual affinities.
- Nature of Amphipathic Lipids: Amphipathic lipids possess hydrophobic and hydrophilic groups. Notably, among the lipid categories, fatty acids, phospholipids, sphingolipids, bile salts, and to a certain degree, cholesterol, exhibit amphipathic characteristics.
- Structural Composition: A typical amphipathic lipid can be visualized as having a polar or hydrophilic head and a non-polar or hydrophobic tail. In the case of phospholipids, the hydrophilic head consists of a phosphate group bound to compounds like choline, ethanolamine, or inositol. On the other hand, fatty acids encompass a hydrocarbon chain with a carboxyl (COO–) group. This carboxyl group, being polar, has an affinity towards water, making it hydrophilic, while the hydrocarbon chain remains hydrophobic.
- Orientation in an Aqueous Environment: When these amphipathic lipids come into contact with water, a fascinating spatial organization occurs. Their polar groups or “heads” align themselves towards the water, while the non-polar “tails” align in the opposite direction. Therefore, this alignment results in the formation of structures known as micelles.
- Micelles: These are essentially molecular aggregates formed by amphipathic lipids. Critical to the digestion and absorption of lipids, micelle formation is greatly aided by bile salts.
- Membrane Bilayers: In biological contexts, lipids often form a bilayer structure. In such a formation, the polar heads face the external aqueous phase on either side, while the non-polar tails are oriented inwards. This lipid bilayer is foundational to the structure of biological membranes.
- Liposomes and Their Utility: When amphipathic lipids are subjected to sonification in an aqueous medium, liposomes are formed. These structures possess alternating aqueous phases within their lipid bilayers. Intriguingly, in medical science, liposomes combined with tissue-specific antigens serve as drug carriers targeting specific tissues.
- Emulsions: Another manifestation of lipids in an aqueous environment is in the formation of emulsions. In this context, non-polar lipids, like triacylglycerols, when mixed with water, result in larger particles. These particles gain stability with the assistance of emulsifying agents, which are typically amphipathic lipids such as bile salts and phospholipids.
Functions of lipids
- Energy StorageOne of the primary roles of lipids is energy storage. Specifically, triacylglycerols, a type of lipid, act as a concentrated fuel reserve in the body. These reserves, when metabolized, provide the energy necessary for various cellular activities. Besides, in plants, high lipid-containing seeds store energy that facilitates germination and early growth.
- Structural Role in Biological MembranesLipids, such as phospholipids and cholesterol, are instrumental in constructing biological membranes. These membranes regulate permeability, ensuring selective passage of molecules in and out of cells. Therefore, the lipid bilayer in cell membranes serves as the hydrophobic barrier, effectively partitioning the aqueous contents of the cell and its subcellular structures.
- Insulation and ProtectionLipids have protective and insulative properties. They shield internal organs from physical damage and also provide insulation, aiding in the maintenance of body temperature by reducing heat loss. Moreover, in plants, certain lipids protect leaves from excessive heat and desiccation.
- Buoyancy and Aesthetic FunctionsLipids contribute to buoyancy, enabling certain organisms to float. Furthermore, they impart shape and a smooth appearance to the body, playing a cosmetic role in many organisms.
- Hormonal RoleCertain lipids function as hormones. Steroid hormones, which are derivatives of cholesterol, are pivotal in regulating numerous physiological processes, encompassing metabolism, growth, and reproduction.
- Vitamin StorageLipids also store fat-soluble vitamins, namely A, D, E, and K. These vitamins have distinct roles in cellular and metabolic processes.
- Enzymatic ActivationSome lipids are vital for the activation of specific enzymes. For instance, enzymes like glucose-6-phosphatase and β-hydroxybutyric dehydrogenase require specific lipid components for activation.
- WaterproofingIn the plant kingdom, lipids manifest as waxy coatings on leaves, providing waterproofing properties. This is essential for preventing excessive water loss. Similarly, water birds have oils on their feathers, ensuring they remain buoyant and dry.
- Color ImpartationLipids can also influence the coloration of various organisms. The presence of the carotenoid pigment in certain lipids bestows color to numerous fruits and vegetables.
Examples of Lipids
- Triglycerides (Triacylglycerols): These are the primary form of fat storage in animals and plants. A triglyceride molecule consists of three fatty acid chains attached to a glycerol backbone.
- Fatty Acids: These are long hydrocarbon chains with a carboxyl group at one end. Depending on the presence of double bonds, they can be categorized as:
- Saturated Fatty Acids (e.g., palmitic acid and stearic acid) with no double bonds.
- Monounsaturated Fatty Acids (e.g., oleic acid) with one double bond.
- Polyunsaturated Fatty Acids (e.g., linoleic acid and alpha-linolenic acid) with multiple double bonds.
- Phospholipids: Essential components of cell membranes, they consist of a hydrophilic “head” (containing a phosphate group) and two hydrophobic fatty acid “tails”. Examples include phosphatidylcholine and phosphatidylserine.
- Steroids: Characterized by their structure of four fused rings. Examples include:
- Cholesterol: Present in animal cell membranes and a precursor for other steroids.
- Hormones: Such as estrogen, testosterone, and cortisol.
- Glycolipids: Comprised of a lipid bound to a carbohydrate, these molecules are found in the cell membranes of plants and animals.
- Sphingolipids: These lipids contain a backbone of sphingosine and are prominent in the outer leaflet of the plasma membrane. Examples include sphingomyelins and gangliosides.
- Lipoproteins: These are complexes of lipids and proteins that transport lipids through the bloodstream. Examples include LDL (low-density lipoprotein) and HDL (high-density lipoprotein).
- Waxes: These are esters of long-chain fatty acids with long-chain alcohols. Beeswax (used in honeycomb construction) and cutin (found on the surface of plants to prevent water loss) are examples.
- Eicosanoids: These are signaling molecules made from arachidonic acid or other 20-carbon fatty acids. Examples are prostaglandins and leukotrienes.
- Terpenes and Terpenoids: These are diverse lipids primarily found in plants. They can be part of pigments or play roles in plant defense. Examples are limonene (found in citrus) and lycopene (gives tomatoes their red color).
References
- Ahmed S, Shah P, Ahmed O. Biochemistry, Lipids. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525952/
- Valenzuela, R., & Valenzuela, A. (2013). Overview About Lipid Structure. InTech. doi: 10.5772/52306
- https://www.geeksforgeeks.org/lipids/#properties-of-lipids
- https://openoregon.pressbooks.pub/nutritionscience/chapter/5b-lipid-types-structures/
- https://chemistrytalk.org/lipids-structure-function-examples/
- https://collegedunia.com/exams/lipids-definition-structure-types-classification-biology-articleid-1144
- https://conductscience.com/classification-and-biological-functions-of-lipids/
- https://www.onlinebiologynotes.com/classification-of-lipid/
- https://byjus.com/biology/lipids/