Isomerism Definition
Isomerism refers to the phenomenon where more than one compound has identical chemical formulas, but different chemical structures.
Chemical compounds with identical chemical formulae, but differ in the properties and arrangement of the atoms within the molecule are known as isomers. Thus, compounds with isomerism are referred to as isomers.
The term “isomer” is derived from the Greek words “isos” and “meros” that mean “equal parts”. The word was coined by Swedish scientist Jacob Berzelius in the year 1830.
Types of Isomerism
There are two kinds of isomerism that can further be classified into various subtypes. These two kinds are
- Structural Isomerism
- Stereoisomerism.
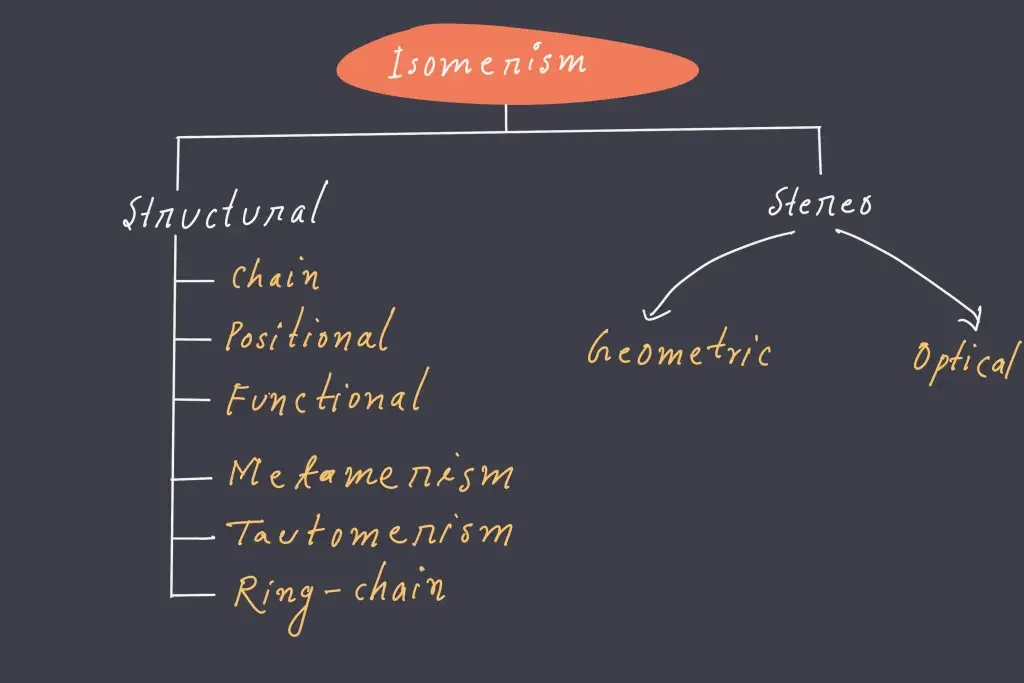
Structural Isomerism
Structural isomerism is often known as constitutional isomerism. Functional groups and molecules that contain the atoms of these isomers are connected in various ways. Different structural isomers are given distinct IUPAC names due to the fact that they may or might not have the same functional group.
Types of structural isomerism
The various types of structural isomerism are covered in this section.
1. Chain Isomerism
- It’s sometimes referred to as skeletal isomerism.
- The isomers’ components display different branches.
- Commonly chain isomers differ in the branching of carbon.
- A good example of chain isomerism could be seen in the compound C5H12 in the following illustration.
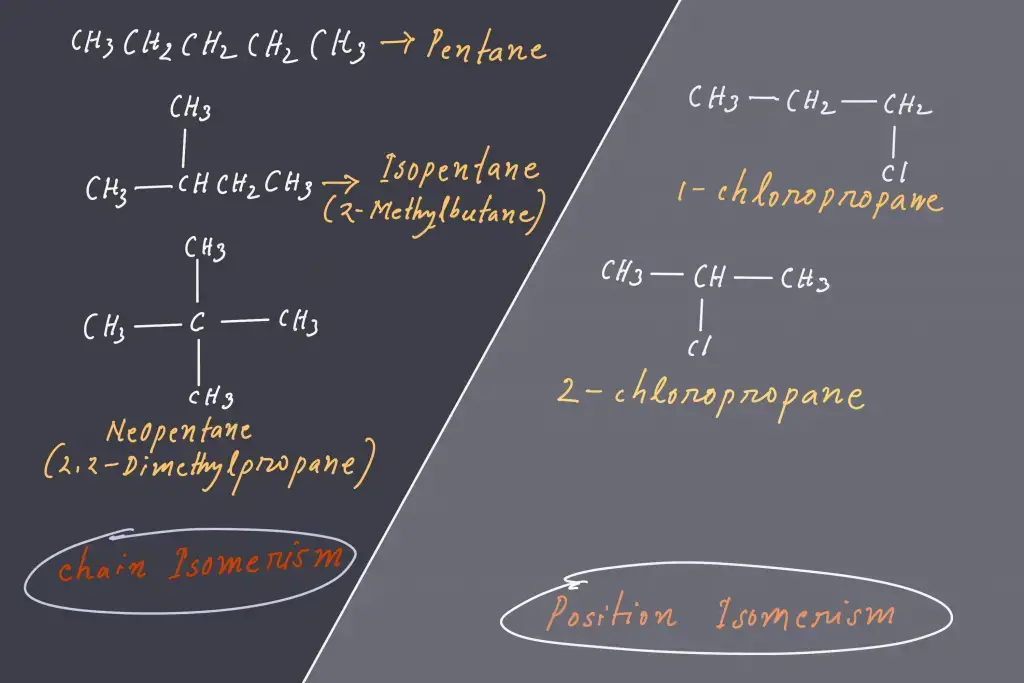
2. Position Isomerism
- In the case of positional isomerism, sometimes referred to as position isomerism, isomers share the same functional groups, but at different locations within the carbon chain.
- One example is the compound with the molecular formula C6H4Br2 There exist three different isomers: 1,2-dibromobenzene 1,3-dibromobenzene, and 1,4-dibromobenzene. The three isomers differ with respect to the positions of bromine atoms in the circular structure.
- Another alternative is the compound with the molecular formula C3H8O and of which there are two isomers, 1-propanol or the n-propyl alcohol, and 2-propanol or isopropyl Alcohol. The isomers differ with respect to the location of the hydroxyl group along the carbon chain.
3. Functional Isomerism
- It’s sometimes referred to as functional group isomerism.
- The name implies it’s referring to compounds with similar chemical formulas but with different functional groups associated with them.
- One example is the compound that has the molecular formula C2H6O and of which there are two different isomers dimethyl ether and alcohol or ethanol and ethyl alcohol, which have two functional groups, including an O-group, an ether group and a hydroxyl groups or OH group.
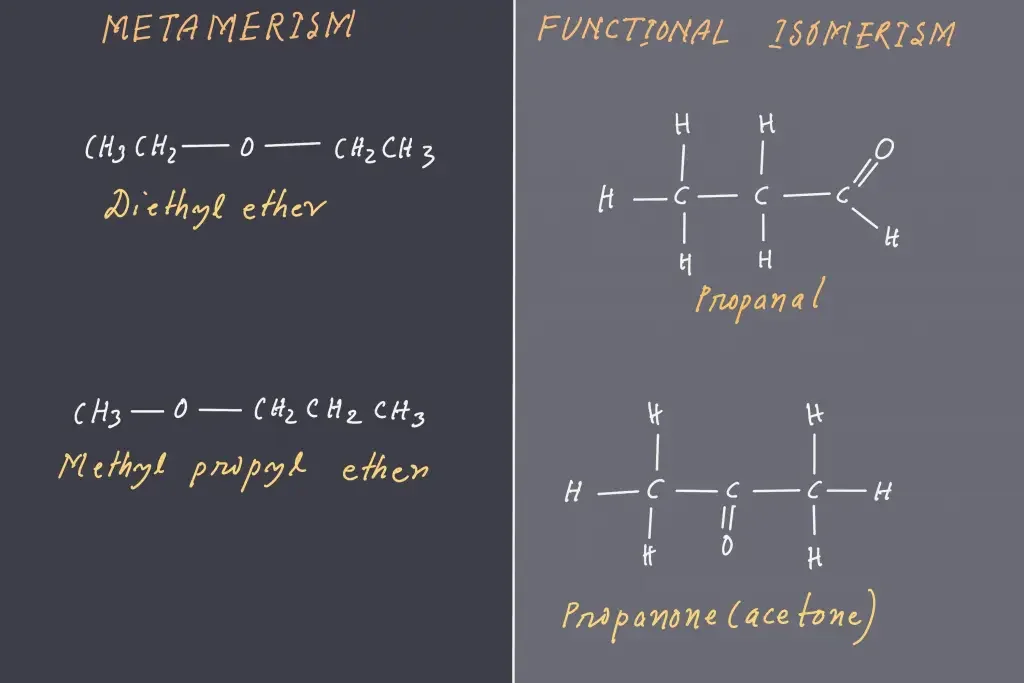
4. Metamerism
- This kind of isomerism is because of the presence of multiple alkyl chains that are on either part of the functional group.
- Rare form of isomerism that is usually restricted to molecules with divalent atoms (such as oxygen or sulphur) and are surrounded by the alkyl groups.
- Example: C4H10O can be represented as methoxyethane (C2H5OC2H5) and methoxypropane (CH3OC3H7).
5. Tautomerism
- A tautomer of a chemical refers to the isomer the compound that differs only in the positions of electrons and protons.
- Typically, the tautomers in the compound are in equilibrium and can easily swap.
- It is caused by the intramolecular transfer of proton.
- One of the most prominent examples of this phenomenon is Keto-enol-tautomerism.
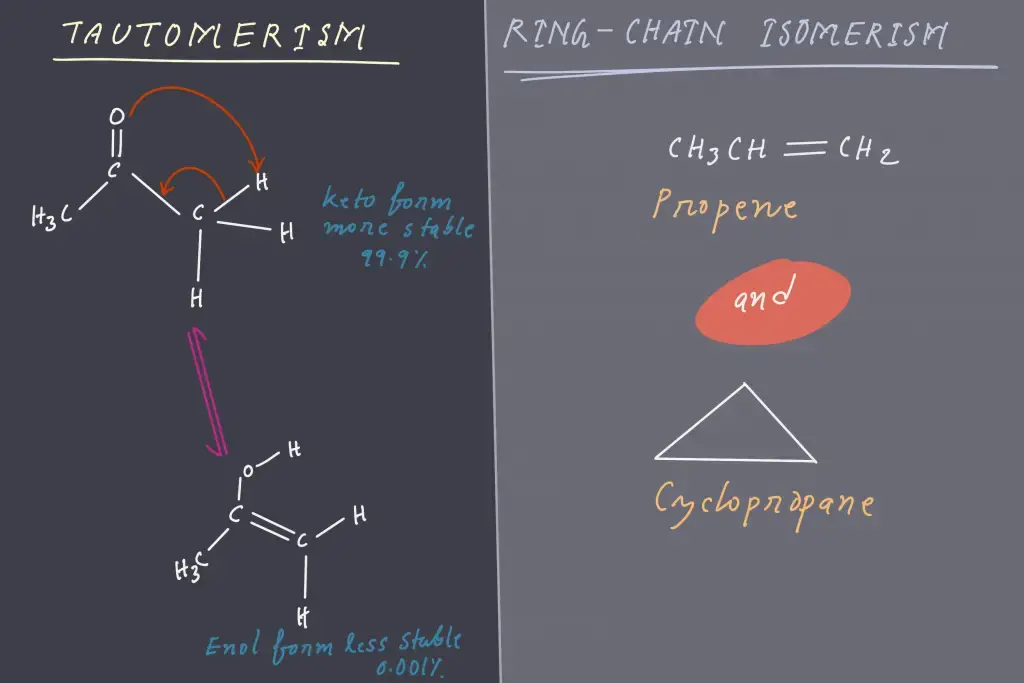
6. Ring-Chain Isomerism
- In ring-chain isomerism one isomer has an open-chain structure , whereas the other one has an rings structure.
- They usually contain a distinct amount in pi bonds.
- An excellent illustration of this kind of isomerism is in C3H6. Propene and Cyclopropane are the isomers that result from it, as seen below.
Stereoisomerism
This isomerism type is found in compounds with the same chemical formula, but different orientations of the atoms that belong to the molecules in three-dimensional space. The compounds with stereoisomerism are commonly called stereoisomers. The phenomenon is further classified into two different subtypes. Each of these subtypes is discussed in this subsection.
1. Geometric Isomerism
Geometric isomerism, also known as cis-trans isomerism, happens when the atoms can’t freely move because of the rigidity of the structure, as:
- Compounds that contain carbon-carbon, carbon nitrogen and nitrogen-nitrogen double bonds, where the rigidity is because of the double bond
- The cyclic nature of compounds, and their rigidity comes from the structure of the ring.
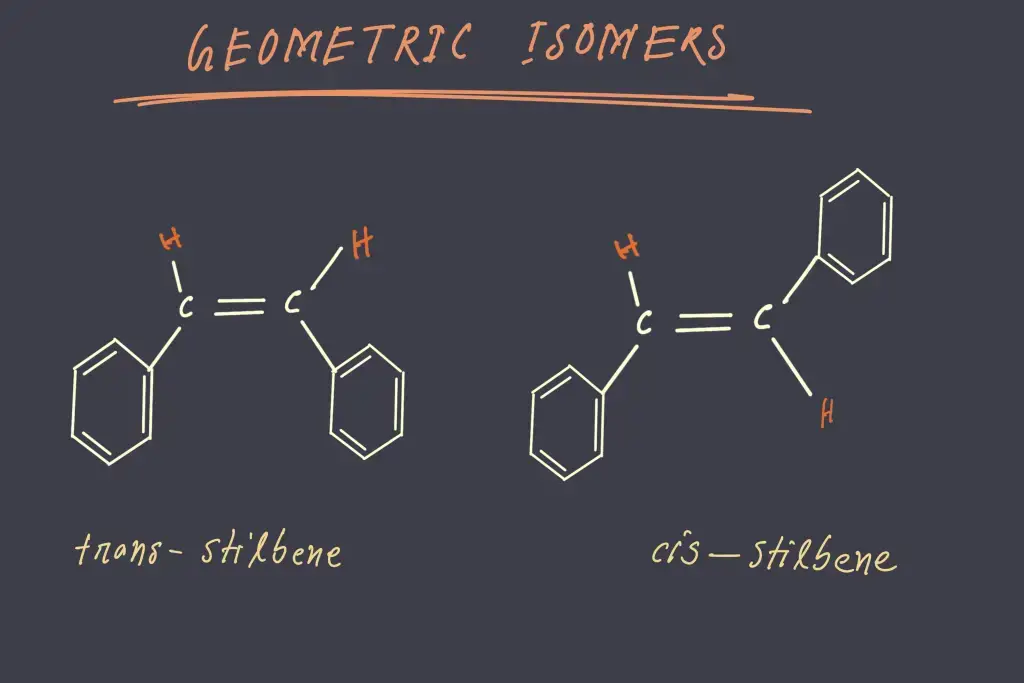
A good illustration of geometrical isomerism caused by the presence of carbon and carbon double bond would be stilbene C14H12 in that there are two different isomers. In one isomer known as the cis isomer, identical groups are located on the same part of the double bond while in the other one, referred to as trans isomer the same groups reside on opposite sides.
It is important to note that the words trans and cis originate from Latin trans, which translates to “across”, and cis is a reference to “on this side of”.
In the cyclic compounds of carbon, cis trans isomerism is not restricted because of the presence of chiral centres occurs in the structures that contain an even amount of carbon atoms, and is substituted in opposite positions, that is para-substituted. An example is 1,4-dimethylcyclohexane, a cycloalkane, compounds of general formula CnH2n, of which there are two stereoisomers, cis-1,4-dimethylcyclohexane and trans-1,4- dimethylcyclohexane.
This type of stereoisomerism can’t be observed if one of the atoms unable to freely rotate is carrying two groups of identical. Why? In order to switch between trans and cis isomers , the groups that attach to atoms that cannot freely rotate need to be switched. If two groups are similar that switch results in the creation of the identical molecules.
Note: Geometric isomers are an instance of diastereomers and diastereoisomers which, in turn, are stereoisomers that do not mirror images of one another. Other diastereomers include meso compounds as well as the optical non-enantiomers.
2. Optical Isomerism
- Compounds with optical isomerism share similar bonds , but with different configurations of atoms that form reflections that are not superimposable mirror images.
- The optical isomerism can be seen in molecules with at least one or two chirality centers , or centers that chiral, for example, Tetrahedral atoms bearing various ligands. The chiral center could be carbon, phosphorus, nitrogen or sulfur atom.
- Optic isomers do not have an axis of symmetry center or an axis of symmetry. are mirror images of one another, and therefore cannot be superimposed upon each other. They are also known as”enantiomers” derived originated from the Greek”enantios” which means “opposite”, and meros which means “part”.
- Contrary to other isomers two enantiomers possess the same chemical and physical properties, with the exception of two.
- The direction of the plane of polarized light hence the name optical isomerism.
- If the solution of one enantiomer is able to rotate the plane the polarized light in a clockwise manner, the one enantiomer is labeled. (+). On the other hand, a solution from the opposite enantiomer turns the plane of light polarized in a counterclockwise manner by the same angle which is why the enantiomer has been designated (-).
- Though they’re not easily distinguished by any method Two enantiomers are discerned in a chiral atmosphere such as the active site the chiral enzymes.
- For a molecule having two chiral centers The total number of stereoisomers equivalent to 2n.
What is Ionization Isomerism?
The compound that produces different ions in solution, despite having an identical composition, is known as Ionization isomers. This property is referred to as Ionization isomerism. Compounds that give different ions in the solution even though they share the same composition are referred to as isomerism in ionization. Isomergy occurs when the counter Ion in a complex salt can be a ligand, and it can be displaced by a ligand that can later be transformed into the counter Ion.
One illustration of ionisation isomerism could be [Co(NH3)5SO4]Br as well as [Co(NH3)5Br]SO4.
We can make these isomers by ionisation using the following way.
[CoBr(NH3)5]SO4→ [CoBr(NH3)5]2+ + SO42− = Red−Violet
[CoSO4(NH3)5]Br → [CoSO42−(NH3)5]+ + Br− = Red
References
- https://www.biologydiscussion.com/carbohydrates/top-5-classifications-of-isomerism-carbohydrates/41779
- https://www.tuscany-diet.net/2020/03/31/isomerism/
- https://www.ourbiochemistry.com/knowledge-base/isomerism-in-monosaccharides/#:~:text=The%20monosaccharides%20having%20asymmetric%20carbon%20atoms%20exhibit%20isomerism.&text=Figure%2D2%2D%20Glyceraldehyde%20has%20an,it%20does%20not%20have%20isomers.&text=Glyceraldehyde%20has%20a%20single%20asymmetric,two%20stereoisomers%20of%20this%20sugar.
- https://umaine.edu/carbohydrates/carbohydrate-chemistry/monosaccharides/
- https://www.vvc.edu/sites/default/files/files/10.%20Carbohydrates%20for%20colored%20-%20revised%202012.pdf
- https://byjus.com/chemistry/isomerism/
- https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-i/structure-of-organic-molecules/structural-isomers-and-stereoisomers
- https://www.brainkart.com/article/Sugars–Their-Structures-and-Stereochemistry_27626/
- https://www.sydney.edu.au/science/chemistry/~george/isomers.html