What is Glycogen?
- Glycogen, a multifaceted branched polysaccharide, stands as the primary glucose storage mechanism in animals, including humans. Composed of glucose units, this polysaccharide is analogous to starch, which serves a similar purpose in plants. However, the structure of glycogen is more intricately branched and denser than that of starch, particularly amylopectin, one of starch’s main components.
- Functioning as a pivotal energy reserve, glycogen is mobilized to produce glucose when the body demands energy. This glucose can then be channeled into various metabolic pathways, such as the glycolytic or pentose phosphate pathway, or be released into the bloodstream to be utilized by other cells. While fungi and certain bacteria also utilize glycogen for glucose storage, its primary significance is observed in the metabolic processes of animals.
- In humans, the liver and skeletal muscles are the primary sites for glycogen synthesis and storage. The liver, which can constitute up to 6% of its fresh weight as glycogen, acts as a glucose reservoir for the body, especially for the central nervous system. The brain, in particular, is a significant consumer of blood glucose, accounting for approximately 60% of its utilization in sedentary, fasting individuals. On the other hand, skeletal muscles store glycogen to cater to their own energy needs. However, the degradation of glycogen in muscles can influence glucose uptake from the blood, thereby modulating glucose availability for other tissues.
- The quantity of glycogen stored within the body is influenced by various factors, including the type of muscle fibers, physical activity, basal metabolic rate, and dietary habits. Interestingly, variations in resting muscle glycogen levels arise from alterations in the number of glycogen particles rather than the enlargement of existing particles.
- Glycogen’s significance is further underscored by its role in maintaining blood glucose levels. In fasting conditions, the body maintains a consistent blood glucose concentration by tapping into the glycogen reserves in the liver and skeletal muscles.
- From a biochemical perspective, glycogen is a member of the polysaccharide carbohydrates, organic molecules primarily composed of carbon, hydrogen, and oxygen. Carbohydrates, as a major biomolecular class, are categorized into simple and complex types based on their digestibility and metabolic impact. While simple carbohydrates offer rapid energy, complex carbohydrates, including glycogen, provide a more sustained energy source and are less likely to induce abrupt changes in blood sugar levels.
- Historically, the discovery of glycogen is attributed to the French physiologist, Claude Bernard, in the mid-19th century. He was the pioneer in isolating this substance from the liver and recognizing its sugar-forming properties. Subsequently, the chemical composition of glycogen was elucidated by the German chemist Friedrich August Kekulé.
- In summary, glycogen is an indispensable glucose storage molecule in animals, playing a crucial role in energy metabolism and glucose homeostasis. Its intricate structure and function underscore its significance in the realm of biochemistry and physiology.

Definition of Glycogen
Glycogen is a branched polysaccharide composed of glucose units and serves as the primary form of energy storage in animals and humans.
Characteristics of Glycogen
Glycogen, often termed “animal starch,” is a pivotal energy reserve in animals, with its counterpart in plants being starch. This complex carbohydrate is responsible for storing surplus glucose in the body. Structurally, glycogen exhibits certain distinctive characteristics that differentiate it from other polysaccharides:
- Molecular Structure: Glycogen’s molecular architecture is reminiscent of the amylopectin component of plant starch. However, a distinguishing feature of glycogen is its more frequent branching, occurring approximately every 8 to 12 glucose units.
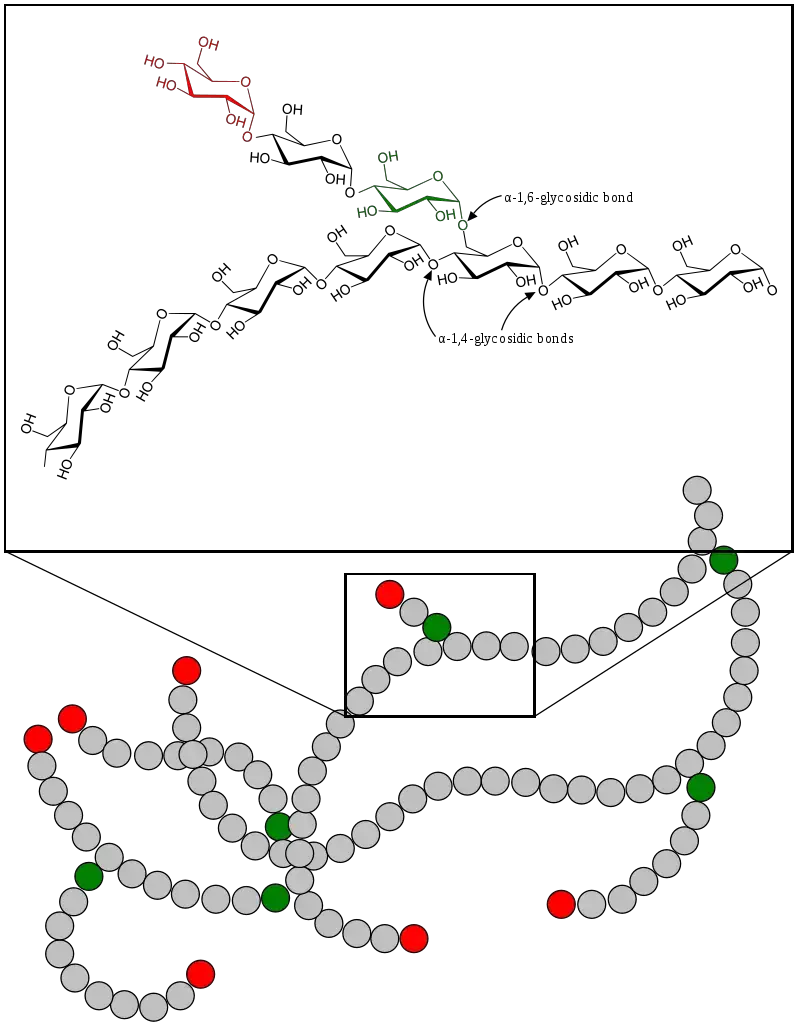
- Glycosidic Bonds: The primary chains of glycogen are constituted by glucose units interconnected through α(1→4) glycosidic linkages. The branching points in the molecule are facilitated by α(1→6) glycosidic bonds. These α-glycosidic connections give rise to open helical polymers, contrasting with the nearly straight strands formed by β-glycosidic bonds, as observed in structural molecules like cellulose.
- Microscopic Appearance: When observed microscopically, glycogen exhibits a unique asterisk or star-like morphology, distinguishing it from other cellular components.
- Granular Form: Within the cellular cytosol, glycogen is present as granules. These granules vary in diameter, typically ranging between 10 to 40 nm.
- Glycogenin Core: Central to each glycogen granule is an enzyme known as glycogenin. This enzyme not only catalyzes the transformation of glucose into glycogen but also acts as a primer, initiating the synthesis of the glycogen molecule.
In essence, glycogen’s characteristics, from its intricate branching to its unique enzymatic core, underscore its specialized role in glucose storage and energy metabolism within animal cells.
Structure of Glycogen
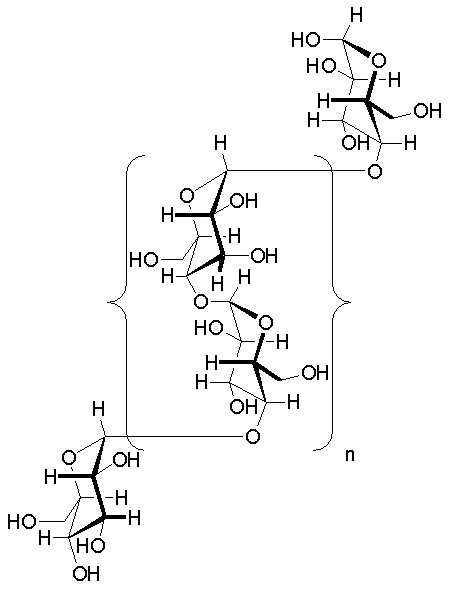
- Glycogen, a complex branched biopolymer, is primarily composed of glucose units. Its intricate structure is characterized by linear chains of glucose residues connected by α-1,4 glycosidic bonds. Approximately every ten glucose units, a branching occurs through α-1,6 glycosidic linkages. This branching pattern imparts a helical configuration to the polymer, a structural feature facilitated by the α-glycosidic bonds.
- The granular nature of glycogen is evident in the cytoplasm, where it forms granules ranging from 10-40nm in diameter. Central to each of these granules is the protein glycogenin, which plays a pivotal role in the synthesis of glycogen. This protein serves as the foundational core around which the glucose units aggregate.
- Drawing parallels with plant-based glucose storage, glycogen is analogous to starch. However, while both are glucose polymers, they differ in their branching patterns and compactness. Glycogen exhibits a more frequent and extensive branching, making it denser than starch, particularly its component, amylopectin.
- Delving deeper into its structure, a glycogen molecule can be visualized as a spherical assembly of glucose trees, encompassing approximately 12 layers. These layers are centered on a glycogenin protein and consist of three distinct glucose chains: A, B, and C. The C-chain, singular in nature, is directly attached to the glycogenin and originates from the self-glucosylation of the protein. Branching out from the C-chain are the B-chains, which further give rise to both B- and terminal A-chains. The A-chains, being terminal, lack any branching.
- The storage form of glycogen in cells, particularly in muscles, liver, and fat cells, is hydrated. This means that for every part of glycogen, there are three to four parts of water, along with an associated 0.45 millimoles of potassium per gram of glycogen. This hydrated storage is crucial, as glucose, in its raw form, can disrupt osmotic pressure, potentially damaging or even killing cells. Glycogen, being non-osmotic, offers a safe storage solution, ensuring that glucose is available without compromising cellular osmotic balance.
- In essence, the structure of glycogen is a testament to its function as an efficient and compact energy storage molecule. Its branched architecture, coupled with its association with the protein glycogenin, ensures rapid mobilization of glucose to meet the energy demands of the cell.
Glycogen Metabolism
Glycogen metabolism is a meticulously orchestrated process that governs the storage and release of glucose in response to the body’s energy demands. This metabolic pathway encompasses two primary processes: glycogenesis (glycogen synthesis) and glycogenolysis (glycogen degradation).
Glycogenesis:
- Energy Requirement: The synthesis of glycogen necessitates energy, which is furnished by uridine triphosphate (UTP).
- Initial Phosphorylation: Enzymes, hexokinases or glucokinase, instigate the process by phosphorylating free glucose to yield glucose-6-phosphate. This molecule is subsequently transformed into glucose-1-phosphate via the action of phosphoglucomutase.
- Activation of Glucose: UTP-glucose-1-phosphate uridylyltransferase facilitates the activation of glucose, leading to the formation of UDP-glucose.
- Initiation of Glycogen Synthesis: The protein glycogenin plays a pivotal role in de novo glycogen synthesis by catalyzing the attachment of UDP-glucose to itself. This protein possesses a tyrosine residue that acts as an anchor for glucose attachment.
- Chain Elongation: Glycogen synthase extends this initial glucose chain by incorporating additional glucose molecules through α-1,4 glycosidic linkages.
- Branching: The enzyme amylo-(1,4 to 1,6)-transglucosidase, or glycogen branching enzyme, introduces branches within the glycogen molecule by transferring glucose fragments and establishing α-1,6 glycosidic linkages.
Glycogenolysis:
- Release of Glucose: Glycogen phosphorylase is instrumental in cleaving glucose from glycogen, producing glucose-1-phosphate.
- Conversion to Glucose-6-Phosphate: The enzyme phosphoglucomutase facilitates the transformation of glucose-1-phosphate to glucose-6-phosphate.
- Debranching Mechanism: Glycogen debranching enzyme (GDE) is essential for removing glucose residues near branch points. GDE transfers the terminal glucose residues from one branch to another and subsequently cleaves the α-1,6-linked glucose at the branch point, releasing free glucose.
- Fate of Glucose-6-Phosphate: In tissues like the liver, intestine, and kidney, glucose-6-phosphate can be dephosphorylated to glucose by glucose-6-phosphatase and then released into the bloodstream. However, in muscle cells, which lack this enzyme, glucose-6-phosphate is channeled into the glycolytic pathway, serving as an energy source. Alternatively, glucose-6-phosphate can also enter the pentose phosphate pathway, yielding NADPH and five-carbon sugars.
In summary, glycogen metabolism is a dynamic interplay of enzymatic reactions that ensure a steady supply of glucose, catering to the body’s energy requirements. Whether it’s the synthesis of glycogen in times of glucose abundance or its breakdown during energy deficits, the body efficiently manages its glucose reserves through these metabolic pathways.
Exercise and Glycogen Depletion
Endurance exercises pose a significant challenge to the body’s energy reserves, particularly the glycogen stored in muscles. As athletes engage in prolonged physical activities, there is a potential risk of glycogen depletion. This phenomenon refers to the substantial reduction of glycogen levels within muscle tissues, leading to pronounced fatigue and a marked decrease in physical performance.
The ramifications of glycogen depletion are not trivial. Athletes may experience profound fatigue, making even basic movements arduous. However, there are strategies to counteract or delay the onset of this depletion:
- Carbohydrate Consumption During Exercise: One effective approach to mitigate glycogen depletion is the intake of high glycemic index carbohydrates during the exercise. Carbohydrates with a high glycemic index are rapidly converted into blood glucose. By replenishing glucose levels during physical activity, the body can partially offset the glucose expended, thereby conserving muscle glycogen.
- Muscle Conditioning: Specialized training regimens can be designed to train muscles to rely more on fatty acids as an energy substrate. By enhancing the muscles’ ability to oxidize fatty acids, the rate of glycogen breakdown can be reduced, allowing athletes to sustain their performance for more extended periods.
- Carbohydrate Loading: Another strategy employed by athletes, especially before major events, is carbohydrate loading. This involves the consumption of copious amounts of carbohydrates in the days leading up to the event. The rationale behind this is to maximize glycogen storage capacity in muscles, providing a more substantial energy reservoir for the upcoming physical exertion.
In conclusion, while glycogen depletion poses a challenge for endurance athletes, understanding its implications and employing strategies to counteract its effects can significantly enhance performance. Through a combination of dietary interventions and specialized training, athletes can optimize their energy utilization and push the boundaries of their endurance.
Examples of Glycogen Storage Diseases
Glycogen storage diseases (GSDs) are a group of inherited metabolic disorders that arise from enzyme deficiencies affecting the synthesis or degradation of glycogen. These diseases can be broadly categorized based on the primary tissue affected: liver or muscle. The clinical manifestations and severity of these diseases vary, but they often lead to significant morbidity if not appropriately managed.
- Pompe Disease:
- Genetic Origin: This disease results from mutations in the GAA gene, responsible for encoding lysosomal acid α-glucosidase, commonly known as acid maltase.
- Pathophysiology: Acid maltase plays a pivotal role in glycogen degradation. Mutations in the GAA gene lead to an accumulation of glycogen within cells, predominantly affecting skeletal and cardiac muscles.
- Clinical Variants: Pompe Disease manifests in three primary forms:
- Infantile Form: The most severe variant, it often results in mortality within the first two years of life if untreated.
- Juvenile Form: Intermediate in severity.
- Adult Form: The mildest variant.
- McArdle Disease:
- Genetic Origin: This condition arises from mutations in the PYGM gene, which encodes the enzyme myophosphorylase. This enzyme variant, specific to muscles, is essential for glycogen breakdown.
- Clinical Presentation: Symptoms typically encompass muscle pain and fatigue. While initial manifestations may appear during childhood, definitive diagnosis might be delayed until adulthood. Proper management is crucial as the disease can pose life-threatening risks.
- Andersen Disease:
- Genetic Origin: Andersen Disease is attributed to mutations in the GBE1 gene, responsible for encoding the glycogen branching enzyme.
- Pathophysiology: This enzyme is vital for the proper structuring of glycogen molecules. Its deficiency leads to aberrant glycogen accumulation, primarily affecting the liver and muscles.
- Clinical Presentation: Symptoms usually manifest within the first few months of life. Affected individuals may exhibit growth retardation, hepatomegaly (enlarged liver), and cirrhosis. The complications associated with Andersen Disease can be severe and life-threatening.
In summary, glycogen storage diseases are a spectrum of disorders stemming from genetic mutations affecting glycogen metabolism. Their clinical presentations and outcomes vary, underscoring the importance of early diagnosis and intervention. Proper understanding and management of these conditions can significantly improve the quality of life for affected individuals.
Functions of Glycogen
Glycogen, a complex polysaccharide, plays a pivotal role in glucose storage and energy metabolism within various tissues of the body. Its functions can be delineated based on its location and the physiological needs of the respective tissues:
- Liver Glycogen:
- Blood Sugar Regulation: Hepatic glycogen serves as a glucose reserve. Hepatocytes release glucose derived from glycogen to maintain normoglycemia, especially during periods of fasting. After an overnight fast, hepatic glycogen can provide approximately 600 kcal, a substantial amount compared to the mere 40 kcal present in body fluids.
- Postprandial State: Following a meal, as blood glucose levels rise, insulin is secreted. This hormone facilitates the uptake of glucose by hepatocytes and stimulates glycogen synthesis. In this state, the liver absorbs more glucose than it releases.
- Glycogenolysis and Gluconeogenesis: As glucose levels decrease post-digestion, insulin secretion diminishes, halting glycogen synthesis. When energy is required, glycogen is degraded to glucose. The hormone glucagon, which acts counter to insulin, promotes glycogenolysis (glycogen breakdown) and gluconeogenesis (glucose synthesis from non-carbohydrate sources).
- Muscle Glycogen:
- Immediate Energy Reserve: Muscle glycogen primarily serves as an immediate energy source during muscle activity. Unlike liver cells, muscle cells cannot release glucose into the bloodstream due to the absence of glucose-6-phosphatase. Thus, the stored glycogen is exclusively for intracellular use.
- Localized Use: Glycogen in muscle cells, primarily in the form of β particles, is utilized locally. This contrasts with liver cells, which can release glucose into the bloodstream to fuel other organs.
- Brain Glycogen:
- Energy During Activity: Located in astrocytes, brain glycogen accumulates during sleep and is mobilized upon waking. This reserve provides a moderate protective buffer against hypoglycemic events.
- Fetal Lung Type II Pulmonary Cells:
- Surfactant Synthesis: Glycogen plays a specialized role in these cells, accumulating around 26 weeks of gestation, subsequently aiding in pulmonary surfactant synthesis.
- Optimal Structure for Metabolic Needs:
- Glycogen’s structure appears to be optimized for metabolic demands. Its design maximizes glucose units available for enzymatic degradation, binding sites for degrading enzymes, and the total number of glucose units stored, while minimizing the overall volume. The balance in branching ensures efficient storage and rapid mobilization. Most organisms, from vertebrates to bacteria, exhibit a consistent branching pattern, with notable exceptions like oysters.
In summary, glycogen’s multifaceted roles across different tissues underscore its significance in energy homeostasis and metabolic regulation. Its intricate structure and strategic storage locations ensure that glucose is readily available to meet the body’s energy demands.
Quiz
What is the primary role of glycogen in the human body?
a) DNA synthesis
b) Protein synthesis
c) Energy storage
d) Lipid transport
Glycogen is primarily stored in which two organs of the human body?
a) Kidneys and Lungs
b) Liver and Heart
c) Liver and Skeletal Muscles
d) Brain and Kidneys
Which enzyme is responsible for the synthesis of glycogen?
a) Glycogen synthase
b) Glycogen phosphorylase
c) Hexokinase
d) Glucokinase
The branching points in the glycogen molecule are formed by which type of glycosidic bond?
a) α-1,2
b) α-1,4
c) α-1,6
d) β-1,4
Which disease is caused by a deficiency in the enzyme lysosomal acid α-glucosidase?
a) McArdle Disease
b) Andersen Disease
c) Pompe Disease
d) Cori Disease
Which enzyme is responsible for the breakdown of glycogen to release glucose-1-phosphate?
a) Glucokinase
b) Glycogen synthase
c) Glycogen phosphorylase
d) Hexokinase
Glycogen has a structure most similar to which plant polysaccharide?
a) Cellulose
b) Chitin
c) Amylose
d) Amylopectin
Which hormone stimulates the breakdown of glycogen in the liver?
a) Insulin
b) Glucagon
c) Adrenaline
d) Cortisol
In which cellular location is glycogen primarily found?
a) Mitochondria
b) Nucleus
c) Cytosol
d) Endoplasmic reticulum
Which of the following is NOT a characteristic of glycogen?
a) It is a branched polymer.
b) It serves as a primary energy storage molecule in plants.
c) It contains α-1,4 and α-1,6 glycosidic linkages.
d) It is stored in the liver and muscles.
FAQ
What is glycogen?
Glycogen is a branched polysaccharide that serves as the primary storage form of glucose in animals and humans.
Where is glycogen primarily stored in the body?
Glycogen is primarily stored in the liver and skeletal muscles.
How is glycogen different from starch?
While both glycogen and starch are glucose polymers, glycogen is more extensively branched and compact than starch. Starch is the primary form of glucose storage in plants, whereas glycogen is the primary form in animals.
What is the role of glycogen in energy metabolism?
Glycogen acts as an energy reservoir. When the body requires energy, glycogen is broken down to release glucose, which is then used to produce ATP, the body’s primary energy molecule.
How is glycogen synthesized in the body?
Glycogen synthesis, known as glycogenesis, involves the addition of glucose molecules to a growing glycogen chain, primarily facilitated by the enzyme glycogen synthase.
What happens to glycogen during prolonged exercise?
During prolonged exercise, the body depletes its glycogen stores to produce energy. Once glycogen stores are exhausted, the body experiences fatigue and relies more on fat as an energy source.
What are glycogen storage diseases?
Glycogen storage diseases are a group of inherited metabolic disorders resulting from enzyme deficiencies affecting the synthesis or degradation of glycogen. Examples include Pompe Disease, McArdle Disease, and Andersen Disease.
How does the body regulate glycogen synthesis and breakdown?
The body regulates glycogen metabolism through hormones like insulin (promotes glycogen synthesis) and glucagon (stimulates glycogen breakdown).
Why is glycogen referred to as “animal starch”?
Glycogen is sometimes called “animal starch” because of its structural and functional similarities to starch, the glucose storage molecule in plants.
Can the body convert fats and proteins to glycogen?
While the body primarily uses glucose to make glycogen, in certain situations, it can convert the glycerol portion of fats and some amino acids from proteins into glucose, which can then be stored as glycogen.
References
- Young, F. G. (1957). “Claude Bernard and the Discovery of Glycogen”. British Medical Journal. 1(5033 (Jun. 22, 1957)): 1431–7. doi:10.1136/bmj.1.5033.1431 ://www.bmj.com/content/1/5033/1431 Link
- Berg, J. M., Tymoczko, J. L., & Lubert Stryer. (2002, January 1). Glycogen Metabolism. Retrieved from ://www.ncbi.nlm.nih.gov/books/NBK21190/ Link
- Adeva-Andany, M. M., González-Lucán, M., Donapetry-García, C., Fernández-Fernández, C., & Ameneiros-Rodríguez, E. (2016). Glycogen metabolism in humans. BBA Clinical, 5, 85–100. ://doi.org/10.1016/j.bbacli.2016.02.001 Link
- Physiologic Effects of Insulin. (2019, January 1). Retrieved from ://www.vivo.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin-phys.html Link
- Eicke, S., Seung, D., Egli, B., Devers, E.A., and Streb, S. (2017) “Increasing the carbohydrate storage capacity of plants by engineering a glycogen-like polymer pool in the cytosol.” Metabolic Engineering. 40:23-32.
- Hargreaves, M. and Richter, E.A. (1988) “Regulation of skeletal muscle glycogenolysis during exercise.” Canadian Journal of Sport Sciences. 13(4):197-203.
- Ivy, J.L. (1991). “Muscle glycogen synthesis before and after exercise.” Sports Medicine. 11(1):6-19.