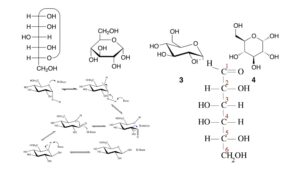
What is Stereoisomerism?
- Stereoisomerism, also known as spatial isomerism, is a type of isomerism that arises when molecules have the same molecular formula and sequence of bonded atoms, but differ in the three-dimensional arrangement of their atoms in space. While structural isomers differ in the connectivity or order of bonds, stereoisomers have identical connectivity but exhibit distinct spatial orientations.
- Isomers, in general, are compounds with the same molecular formula but different chemical structures. When the atoms forming these isomers are bonded together in fundamentally different ways, they are referred to as constitutional isomers. For instance, consider the hydrocarbons with the molecular formula C4H8. Most of the isomers of C4H8 are constitutional isomers, meaning their atoms are connected in diverse patterns. Each of these isomers has a unique IUPAC name, reflecting its distinct structure.
- It is important to note that the molecular formula provides some information about the structural characteristics of the isomers. In the case of C4H8, since it has two fewer hydrogens than butane (C4H10), all isomers with this formula must contain either a ring or a double bond. One possible isomer is CH3CH=CHCH3, which is named 2-butene according to IUPAC rules. However, upon closer examination, this molecule reveals two potential structures, known as cis and trans isomers.
- The cis and trans isomers of 2-butene have the same bonding patterns, differing only in the relative configuration or arrangement of the two methyl groups (along with the associated hydrogen atoms) around the double bond. In the cis isomer, the methyl groups are located on the same side, while in the trans isomer, they are positioned on opposite sides. Isomers that vary solely in the spatial orientation of their constituent atoms are classified as stereoisomers.
- To distinguish between stereoisomers, an additional nomenclature prefix is added to the IUPAC name to indicate their spatial arrangement. For example, in the case of 2-butene, the cis and trans prefixes are used, derived from Latin words meaning “on this side” and “across,” respectively. These prefixes provide crucial information about the spatial relationship between the atoms in the molecule.
- Stereoisomers can be isolated as distinct compounds with characteristic and unique properties. Even though they share the same molecular formula and structural connectivity, their different spatial arrangements lead to differences in physical and chemical properties. These disparities in properties often have significant implications in fields such as pharmaceuticals, where slight changes in stereochemistry can dramatically affect a compound’s biological activity and interactions with receptors.
- Overall, stereoisomerism highlights the importance of considering three-dimensional spatial arrangements in addition to molecular connectivity. It underscores the idea that small changes in the relative orientations of atoms within a molecule can result in distinct compounds with diverse properties and behaviors.
Types of Stereoisomerism
Stereoisomerism, a form of isomerism based on the three-dimensional arrangement of atoms within molecules, can be classified into two main subtypes: conformational isomerism and configurational isomerism. Configurational isomerism, in turn, can be further divided into two subtypes: geometric isomerism (also known as cis-trans isomerism) and optical isomerism (enantiomerism).
- Conformational Isomerism: Conformational isomerism arises due to the rotation of single bonds in a molecule, leading to different spatial arrangements called conformations. These conformations are rapidly interconvertible at room temperature. Conformational isomers are not considered separate stereoisomers since they can be easily interconverted without breaking any bonds. Instead, they represent different conformations of the same molecule. Conformational isomerism is commonly observed in molecules with flexible structures, such as cyclohexane, where the chair and boat conformations are examples of different conformers.
- Configurational Isomerism: Configurational isomerism involves stereoisomers that cannot be interconverted without breaking or forming chemical bonds. Configurational isomers have distinct spatial arrangements and exhibit different chemical and physical properties.
- a) Geometric Isomerism (Cis-Trans Isomerism): Geometric isomerism, also known as cis-trans isomerism or E-Z isomerism, occurs in compounds with restricted rotation around a double bond or in cyclic compounds with substituents. It is characterized by the relative positions of atoms or groups on either side of the double bond or in a cyclic structure. In cis isomers, the similar substituents are on the same side of the molecule, whereas in trans isomers, they are on opposite sides. This type of isomerism is commonly observed in alkenes and cyclic compounds. For example, cis-2-butene and trans-2-butene are geometric isomers.
- b) Optical Isomerism (Enantiomerism): Optical isomerism, also referred to as enantiomerism, arises when molecules have a chiral center, a carbon atom bonded to four different substituents. Enantiomers are non-superimposable mirror images of each other and exhibit the property of chirality. They have identical physical properties, such as boiling point and solubility, but differ in their interaction with plane-polarized light, rotating it in either a clockwise (dextrorotatory) or counterclockwise (levorotatory) direction. Enantiomers often have different biological activities due to their distinct interactions with chiral receptors in living systems. A well-known example of optical isomerism is seen in the enantiomers of the drug ibuprofen.
These subtypes of stereoisomerism demonstrate the various ways in which the three-dimensional arrangement of atoms can lead to distinct isomeric forms. Conformational isomerism accounts for different conformations within a single molecule, while configurational isomerism encompasses geometric isomerism, which involves the relative positions of substituents around a double bond or in a cyclic structure, and optical isomerism, which arises from non-superimposable mirror image forms known as enantiomers. Understanding these types of stereoisomerism is essential in fields such as chemistry, biochemistry, and pharmacology, as it allows for a comprehensive analysis of the diverse behaviors and properties exhibited by isomeric compounds.
Enantiomers
- Enantiomers, also referred to as optical isomers, are a type of stereoisomer that exhibit a unique relationship with each other—they are non-superposable mirror images. Imagine your hands as a macroscopic analogy: they have the same overall structure, but they cannot be placed on top of each other perfectly. Enantiomers possess this property of chirality, where every stereogenic center in one enantiomer has the opposite configuration in the other.
- When it comes to physical properties, enantiomers share many similarities. They have the same melting point, boiling point, solubility, and chemical reactivity. However, there is one crucial distinction: enantiomers differ in the direction in which they rotate plane-polarized light and how they interact with other optical isomers of compounds. This phenomenon is known as optical activity.
- Enantiomers can exhibit optical activity in two forms: D-(+) and L-(−). These labels refer to the rotation of polarized light. D-(+) enantiomers rotate polarized light in a clockwise direction, while L-(−) enantiomers rotate it counterclockwise. This naming convention is based on the Latin words dexter (right) and laevus (left), respectively.
- The unique spatial arrangement of enantiomers plays a significant role in their biological effects. Due to the presence of chiral receptors in living systems, enantiomers can interact with biological targets differently. This means that two enantiomers of a compound may have distinct biological activities. For example, one enantiomer of a drug might exhibit therapeutic effects, while its mirror image enantiomer could be inactive or even possess detrimental effects.
- The separation of enantiomers is a crucial task in pharmaceutical and chemical industries. Since enantiomers have identical physical properties, separating them requires the use of a chiral agent or a chiral chromatographic column that can differentiate between the enantiomers based on their interactions. This separation process is essential to ensure the production of pure enantiomers for research, drug development, and therapeutic applications.
- It is worth noting that in nature, only one enantiomer of most chiral biological compounds is typically found. For instance, in living organisms, amino acids are mainly found in one enantiomeric form (except for glycine, which is achiral). This phenomenon, known as homochirality, remains a fascinating area of study in the field of astrobiology, as it raises questions about the origins of life and the prevalence of chiral asymmetry in the universe.
- In conclusion, enantiomers are mirror image stereoisomers that cannot be superposed onto each other. While they share many physical properties, their interaction with polarized light and other chiral compounds can vary. The distinct spatial arrangements of enantiomers can lead to significant differences in their biological activities. Understanding enantiomers and their separation techniques is crucial in fields such as pharmaceuticals, where the therapeutic effects and safety of drugs can be dependent on their chirality.
What is Diastereomers?
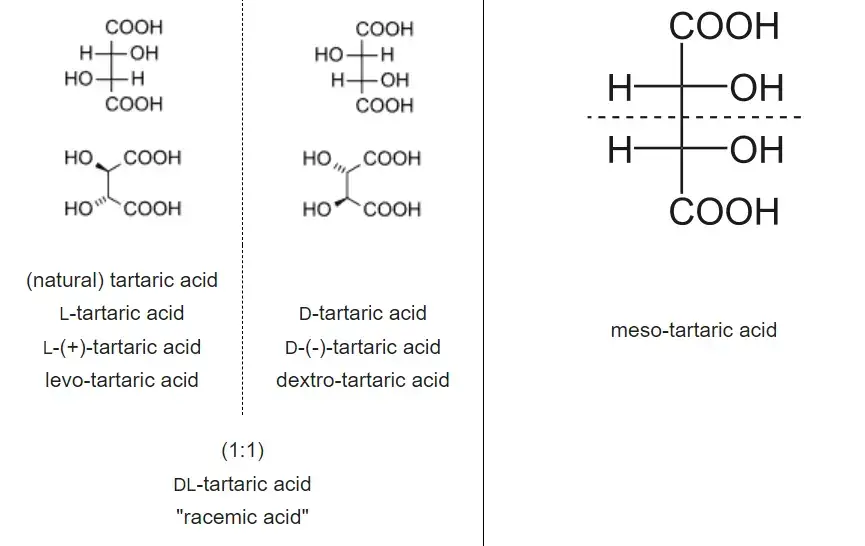
- Diastereomers are a type of stereoisomers that do not exhibit a mirror image relationship. Unlike enantiomers, they are not related through a reflection operation and have distinct three-dimensional arrangements. Diastereomers include various types of isomers, such as meso compounds, cis-trans isomers, E-Z isomers, and non-enantiomeric optical isomers. These diastereomers often have different physical properties.
- One example of diastereomers is found in tartaric acid. Tartaric acid exists in three forms: L-tartaric acid, D-tartaric acid, and meso-tartaric acid. L-tartaric acid and D-tartaric acid are enantiomers, meaning they are mirror images of each other and exhibit opposite optical rotations. On the other hand, meso-tartaric acid is a diastereomer of both L-tartaric acid and D-tartaric acid. Meso-tartaric acid possesses an internal plane of symmetry, making it optically inactive despite having chiral centers.
- Diastereomers, including meso compounds, cis-trans isomers, E-Z isomers, and non-enantiomeric optical isomers, can have significantly different physical properties. This can include differences in melting points, boiling points, solubility, and reactivity. The distinct spatial arrangements of diastereomers contribute to their varied chemical and physical behavior.
- To differentiate between D- and L-isomers in a Fischer projection, the placement of substituents on the penultimate carbon is considered. In D-sugars, the hydrogen is depicted on the left and the hydroxyl group on the right. In contrast, L-sugars are shown with the hydrogen on the right and the hydroxyl group on the left. This labeling convention helps distinguish between the two types of diastereomers.
- Optical rotation is another property that can differentiate between diastereomers. Optical rotation refers to the rotation of the plane of polarization of light. Diastereomers can exhibit different types of optical rotation. Dextrorotatory (d-rotary) compounds rotate the plane of polarization to the right (+), in a clockwise direction. Levorotatory (l-rotary) compounds rotate the plane of polarization to the left (−), in a counterclockwise direction. The dominance of one stereoisomer over the other determines the direction of optical rotation. For example, sucrose and camphor are d-rotary, while cholesterol is l-rotary.
- In summary, diastereomers are stereoisomers that do not possess a mirror image relationship. They encompass various types of isomers, including meso compounds, cis-trans isomers, E-Z isomers, and non-enantiomeric optical isomers. Diastereomers often have distinct physical properties, and their different spatial arrangements contribute to their varied behavior. Differentiation between D- and L-isomers can be achieved through Fischer projections, while optical rotation can determine the direction of rotation of the plane of polarization of light.
Cis–trans and E-Z isomerism
Cis-trans and E-Z isomerism are types of stereoisomerism that arise due to restricted rotation around a double bond. When the substituents on at least one end of a double bond are different, the double bond becomes a stereocenter and can exhibit cis-trans or E-Z isomerism. However, if the two substituents on one end of the double bond are the same, there is no stereoisomerism.
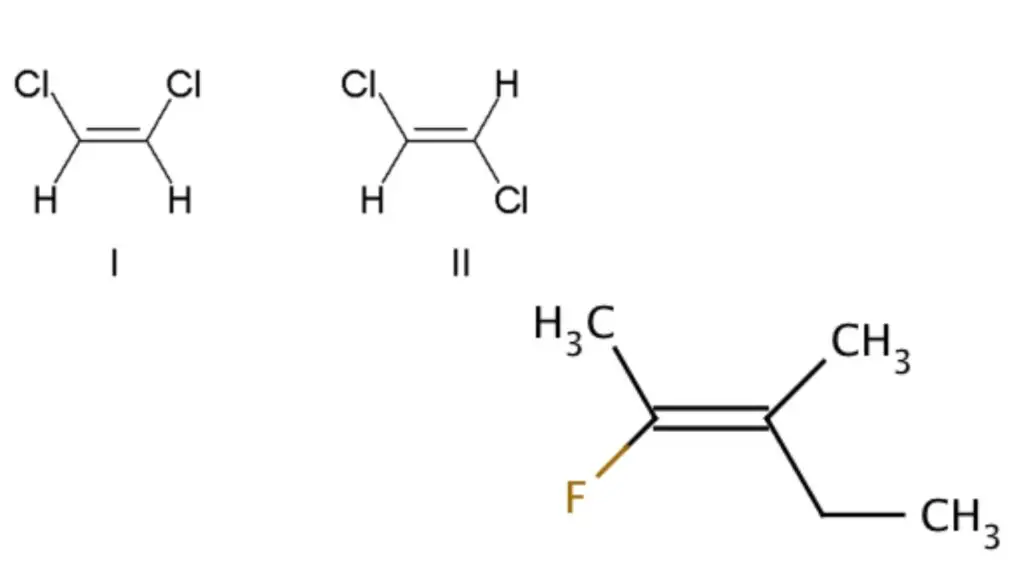
Traditionally, cis-trans isomerism was used to describe the relative positions of substituents on either side of a double bond. The term cis (Latin, “on this side”) was used when the substituents were on the same side of the double bond, and the term trans (Latin, “across”) was used when the substituents were on opposite sides. For example, in 1,2-disubstituted ethenes like dichloroethene (C2H2Cl2), molecule I is cis-1,2-dichloroethene, and molecule II is trans-1,2-dichloroethene.
To provide a more rigorous and unambiguous system, the IUPAC introduced the E-Z system. In this system, priority is assigned to the substituents at each end of the double bond based on their atomic numbers. The highest-priority groups are determined, and if they are on the same side of the bond, it is assigned Z (from the German word “zusammen,” meaning together). If the highest-priority groups are on opposite sides of the bond, it is assigned E (from the German word “entgegen,” meaning opposite). This system eliminates the occasional ambiguity associated with the cis-trans nomenclature.
For example, in the case of the fluoromethylpentene molecule, the proper name can be either trans-2-fluoro-3-methylpent-2-ene (referring to the positions of the alkyl groups across the double bond) or (Z)-2-fluoro-3-methylpent-2-ene (referring to the highest-priority groups being on the same side of the double bond). The fluorine group is the highest-priority group on the left side of the double bond, and the ethyl group is the highest-priority group on the right side.
The terms cis and trans can also be used to describe the relative positions of substituents on a ring. If the substituents are on the same side of the ring, it is called cis, and if they are on opposite sides, it is called trans.
In summary, cis-trans and E-Z isomerism occur in double bonds when the rotation around the bond is restricted. Cis-trans nomenclature traditionally describes the relative positions of substituents on either side of the double bond, while the E-Z system provides a more precise and unambiguous way to designate the configuration of the double bond based on the priority of substituents. These terms can also be used to describe the relative positions of substituents on a ring.
Functions of Stereoisomerism
Stereoisomerism serves several important functions in various fields of science and industry. Here are some key functions of stereoisomerism:
- Biological Activity: Stereoisomerism plays a crucial role in determining the biological activity of drugs, natural compounds, and biomolecules. Enantiomers, which are mirror image stereoisomers, often exhibit different interactions with biological receptors and enzymes. This phenomenon is known as enantioselectivity. The distinct spatial arrangement of enantiomers can lead to differences in their pharmacological, toxicological, and physiological effects. Pharmaceutical companies often produce and study enantiomers separately to understand their specific activities and potential therapeutic applications.
- Chirality and Life: Many biological molecules exhibit chirality, meaning they exist as enantiomers. Chirality is a fundamental characteristic of life, and it influences the behavior and interactions of biomolecules in biological systems. For example, the amino acids that make up proteins are chiral, and their specific arrangement as L-amino acids is essential for protein structure and function. Understanding the chirality of biomolecules helps in unraveling their roles in biological processes and in the design of drugs and therapies.
- Stereochemistry in Organic Synthesis: Stereoisomerism plays a crucial role in organic synthesis. Chemists often need to control the stereochemistry of reactions to produce specific stereoisomers. This is particularly important in the synthesis of natural products, pharmaceuticals, and agrochemicals, where the stereochemistry can significantly impact the desired properties and activities of the target molecules. Selective formation of stereoisomers requires careful design of reactions and appropriate choice of chiral catalysts or reagents.
- Physical Properties and Materials Science: Stereoisomerism can influence the physical properties of compounds, such as boiling points, melting points, solubility, and optical activity. Different stereoisomers can exhibit variations in these properties due to differences in their molecular arrangement and intermolecular interactions. This property diversity is valuable in materials science and can be utilized to engineer materials with specific characteristics, such as polymers with tailored properties or catalysts with enhanced selectivity.
- Analytical Methods and Separation Techniques: Stereoselective analytical methods are employed to analyze and quantify stereoisomers in complex mixtures. Techniques such as chiral chromatography, chiral capillary electrophoresis, and chiral spectroscopy enable the separation and identification of stereoisomers. These methods are widely used in pharmaceutical, environmental, and forensic analysis, where the determination of stereoisomeric composition is crucial for assessing the quality, safety, and environmental impact of compounds.
- Food and Flavors: Stereoisomerism plays a significant role in the perception of flavors and aromas. Many natural compounds responsible for the taste and smell of food, beverages, and spices exist as stereoisomers. The specific arrangement of stereoisomers can result in different sensory experiences. For example, both enantiomers of carvone are present in nature but have distinct aromas: (+)-carvone smells like spearmint, while (-)-carvone has a scent reminiscent of caraway seeds. Understanding the stereochemistry of flavor compounds helps in the development of food products and the formulation of synthetic flavors.
In summary, stereoisomerism serves important functions in diverse fields such as medicine, chemistry, biology, materials science, and food science. It influences biological activity, enables the synthesis of specific stereoisomers, affects physical properties, aids in analytical methods and separations, and contributes to the perception of flavors and aromas. The ability to control and manipulate stereochemistry is crucial for a wide range of applications and research endeavors.
FAQ
What is stereoisomerism?
Stereoisomerism is a form of isomerism where molecules have the same molecular formula and sequence of bonded atoms but differ in their three-dimensional spatial arrangement.
What is the difference between stereoisomers and structural isomers?
Stereoisomers have the same molecular formula and connectivity of atoms but differ in their spatial arrangement, while structural isomers have the same molecular formula but differ in the connectivity of atoms.
What are enantiomers?
Enantiomers are a type of stereoisomer that are mirror images of each other and cannot be superimposed. They have the same physical properties, except for their interaction with polarized light and other chiral compounds.
How do enantiomers affect biological activity?
Enantiomers can have different biological activities due to their interactions with chiral receptors and enzymes in biological systems. They can exhibit different pharmacological, toxicological, and physiological effects.
What is the difference between cis-trans isomerism and E-Z isomerism?
Cis-trans isomerism refers to the relative positions of substituents on either side of a double bond or a ring. E-Z isomerism, on the other hand, is a more rigorous system based on assigning priority to substituents on each side of the double bond, using atomic number. It provides a more precise description of the spatial arrangement.
What are diastereomers?
Diastereomers are stereoisomers that are not mirror images of each other. They have different physical properties and can include meso compounds, cis-trans isomers, E-Z isomers, and non-enantiomeric optical isomers.
How is stereochemistry important in organic synthesis?
Stereochemistry is crucial in organic synthesis as it allows chemists to control the production of specific stereoisomers. The desired stereochemistry can greatly impact the properties and activities of the synthesized compounds.
How are stereoisomers separated and analyzed?
Chiral separation techniques such as chiral chromatography, chiral capillary electrophoresis, and chiral spectroscopy are used to separate and analyze stereoisomers. These methods exploit the differences in the interactions of stereoisomers with chiral stationary phases or chiral selectors.
Can stereoisomers have different physical properties?
Yes, stereoisomers can have different physical properties such as boiling points, melting points, solubility, and optical activity. These differences arise due to variations in their molecular arrangement and intermolecular interactions.
Where can stereoisomerism be found in everyday life?
Stereoisomerism is prevalent in various aspects of everyday life. It influences the flavors and aromas of food and beverages, the activities of drugs and pharmaceuticals, the properties of materials, and even the perception of natural and synthetic fragrances. Understanding stereoisomerism helps us comprehend and manipulate these aspects for practical purposes.