What is Cis and Trans Isomers?
- Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a fundamental concept in chemistry that revolves around the spatial arrangement of atoms within molecules. It is a type of stereoisomerism, wherein molecules have the same chemical formula but differ in the orientation of their functional groups in three-dimensional space.
- The terms “cis” and “trans” originate from Latin, with “cis” meaning “this side of” and “trans” meaning “the other side of.” In the context of chemistry, these terms are used to describe the relative positions of functional groups or substituents within a molecule. Specifically, “cis” indicates that the functional groups are on the same side of a plane or axis, while “trans” signifies that they are on opposing sides or transverse to each other.
- Cis–trans isomers can be observed in a variety of compounds, both organic and inorganic. In organic chemistry, these isomers commonly arise in molecules with double bonds that cannot freely rotate or in ring structures where bond rotation is restricted or prevented. In such cases, the spatial arrangement of substituents around the double bond or within the ring leads to the formation of cis and trans isomers.
- It is important to note that cis–trans notation does not always correspond to E–Z isomerism, which is an absolute stereochemical description. E–Z isomerism provides a detailed specification of the spatial arrangement of substituents around a double bond, whereas cis–trans isomerism refers to the relative positions of functional groups without precise stereochemical information.
- In the field of coordination chemistry, cis and trans isomers can also be found in coordination complexes. These complexes consist of a central metal ion surrounded by ligands, and the arrangement of these ligands can give rise to cis or trans isomers depending on their positions relative to each other.
- It is worth mentioning that the term “geometric isomerism” is considered an obsolete synonym of “cis–trans isomerism” by the International Union of Pure and Applied Chemistry (IUPAC). This emphasizes the preference for using the more precise and specific term “cis–trans isomerism” to describe this type of spatial isomerism.
- In summary, cis–trans isomerism is a form of stereoisomerism that involves the spatial arrangement of atoms or functional groups within molecules. The cis isomer refers to the arrangement where functional groups are on the same side, while the trans isomer describes the arrangement where functional groups are on opposing sides. This concept applies to both organic and inorganic compounds, where the rotation of bonds is restricted or prevented, leading to distinct geometric isomers with different properties and behaviors.
What causes cis and trans isomerism?
Cis and trans isomerism arise due to the restricted rotation around a double bond or in a ring structure. This restricted rotation locks the atoms or groups into specific spatial arrangements, leading to different isomeric forms. Let’s explore an example to understand the cause of cis and trans isomerism.
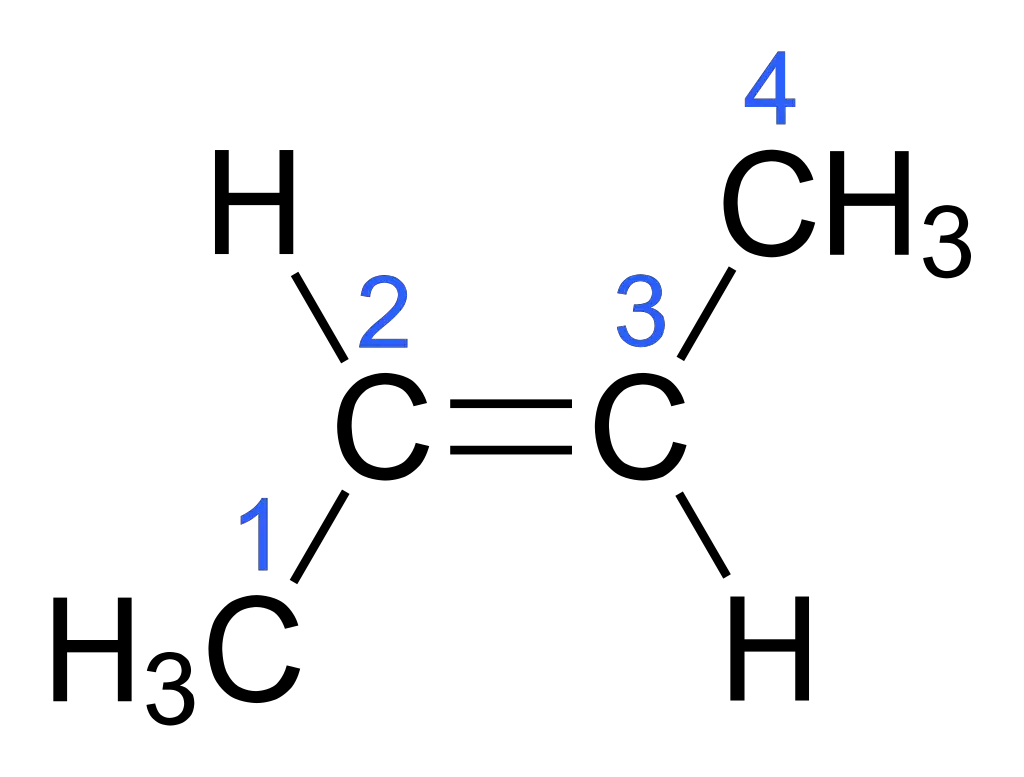
Consider the compound but-2-ene, which has a double bond between two carbon atoms. In but-2-ene, the carbon atoms are each bonded to two different groups, represented here as R1, R2, R3, and R4.
In the cis isomer of but-2-ene, the two R1 and R2 groups are on the same side of the double bond. This arrangement is fixed and cannot be rotated without breaking the double bond.
R1 R3
| |
C = C
| |
R2 R4
In the trans isomer of but-2-ene, the R1 and R2 groups are on opposite sides of the double bond.
R1 R3 | | C = C | | R4 R2
The restricted rotation around the double bond in but-2-ene causes the two isomeric forms, cis and trans, to exist. The spatial arrangement of the R groups in the isomers leads to differences in physical and chemical properties.
In the cis isomer, the two similar groups (R1 and R2) are on the same side, resulting in different steric interactions and intermolecular forces compared to the trans isomer, where the similar groups are on opposite sides. This difference in arrangement affects the overall shape and properties of the molecule.
Therefore, cis and trans isomerism occurs when the rotation around a double bond or in a ring structure is restricted, locking the atoms or groups into specific spatial arrangements.
Differences in Properties
The differences in properties between cis and trans isomers can be attributed to various factors, including the spatial arrangement of atoms and the dipole moments of the molecules. These differences often result in contrasting physical and chemical characteristics. Here are some key variations in properties observed between cis and trans isomers:

- Boiling Point: The boiling points of cis and trans isomers can differ due to variations in intermolecular forces. For example, in pent-2-ene, the cis isomer has a slightly higher boiling point (37°C) compared to the trans isomer (36°C) due to the relatively low bond polarity.
- Dipole Moments: Cis and trans isomers may exhibit different dipole moments based on the spatial orientation of their functional groups. In 1,2-dichloroethylene, the cis isomer has a higher boiling point (60.3°C) compared to the trans isomer (47.5°C) because the cis isomer experiences a net dipole moment resulting from the asymmetry of the C-Cl bonds, while the dipole moments of the trans isomer cancel each other out.
- Reactivity: Cis and trans isomers can display different reactivities. For instance, butenedioic acid exists as cis (maleic acid) and trans (fumaric acid) isomers. Maleic acid is more reactive than fumaric acid due to the presence of the cis arrangement, which allows for intramolecular hydrogen bonding and facilitates reactions.
- Melting Point: The melting points of cis and trans isomers can also differ significantly. Elaidic acid, a trans isomer, is solid at room temperature with a higher melting point (43°C), whereas oleic acid, a cis isomer, is a liquid with a lower melting point (13.4°C).
- Solubility: Trans isomers often have lower solubility in solvents that are inert in nature compared to their cis counterparts. This difference is attributed to the spatial arrangement of atoms and the resulting interactions with the solvent molecules.
- Density: Trans isomers generally exhibit lower densities compared to cis isomers. This variation can be attributed to the cancellation of individual bond dipole moments in trans isomers due to their opposite spatial orientations.
- Stability: Cis isomers of acyclic systems tend to be less stable than their trans counterparts. This instability arises from steric interactions between the substituents, which can hinder the optimal arrangement of atoms.
Overall, the distinct properties of cis and trans isomers highlight the significance of spatial arrangement and dipole moments in determining their behavior. These differences play a crucial role in various aspects of chemistry, including physical properties, reactivity, and stability, making cis-trans isomerism an important concept to consider in the study of molecules and their behavior.
| Molecule | Polarity | Melting Point | Boiling Point | Solubility in Neutral Solvent |
| Cis | Polar | Lower | Higher | Higher |
| Trans | Non-Polar | Higher | Lower | Lower |
Examples of Cis-Trans Isomers
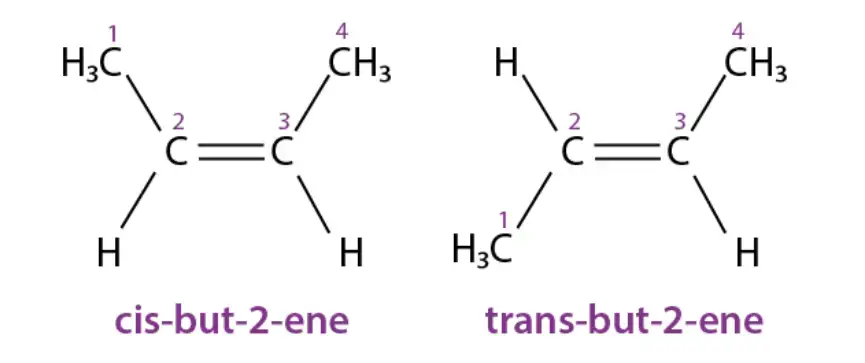
Cis-trans isomerism can be found in various organic and inorganic compounds. Here are some examples of cis-trans isomers:
- Organic Compounds: But-2-ene: This organic compound exhibits cis-trans isomerism. The cis isomer of but-2-ene has both methyl groups on the same side of the double bond, while the trans isomer has them on opposite sides.
- Inorganic Compounds: Diazenes and Diphosphenes: Many diazenes (compounds containing N=N bonds) and diphosphenes (compounds containing P=P bonds) display cis-trans isomerism. The position of the substituents on the nitrogen or phosphorus atoms determines the cis or trans configuration.
Coordination Complexes: Coordination compounds with square planar or octahedral geometries can also exhibit cis-trans isomerism based on the arrangement of ligands. For example, consider the coordination compound Pt(NH3)2Cl2. The cis isomer has the two ammonia (NH3) ligands on one side and the two chloride (Cl) ligands on the other side, while the trans isomer has the ligands positioned on opposite sides.

These examples demonstrate the occurrence of cis-trans isomerism in both organic and inorganic compounds. The spatial arrangement of atoms and ligands in these isomers leads to distinct properties and behavior. Exploring such examples helps in understanding the concept of cis-trans isomerism and its significance in chemical compounds.
Identifying Cis and Trans Isomers
To distinguish between cis and trans isomers, it is important to examine the functional groups and hydrogen atoms on the bond. Follow these steps to identify cis and trans isomers:
- Draw or Envision a Line: Draw a line down the length of the double bond in question. This line represents the plane of the bond.
- Analyze the Spatial Arrangement: For cis and trans isomers, “same side” and “other side” refer to the positions of the functional groups relative to the plane of the bond.
- Compare Functional Group Positions:
- Cis Isomers: In cis isomers, the functional groups are on the same side of the double bond. They are located on the same side of the plane of the bond.
- Trans Isomers: In trans isomers, the functional groups are on opposite sides of the double bond. They are positioned on different sides of the plane of the bond.
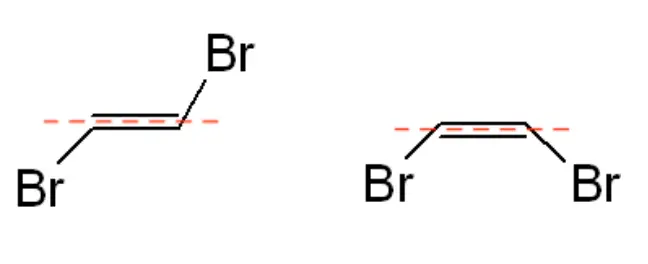
- Visualize the Isomers: Envision the molecule based on the positions of the functional groups. The leftmost molecule in the example given has the halogen groups (bromine) on opposite sides of the dashed line, indicating a trans isomer. In contrast, the example on the right shows the halogen groups on the same side of the double bond, indicating a cis isomer.
By following these steps and considering the spatial arrangement of functional groups relative to the plane of the bond, it is possible to identify cis and trans isomers. This understanding is essential for recognizing the different structural configurations and properties of these isomers in chemical compounds.
Difference Between Between Cis and Trans Isomers
Cis and trans isomers differ in several key aspects, including the arrangement of atoms, polarity, melting points, boiling points, solubility, and acid strength. Here are the main differences between cis and trans isomers:
- Arrangement of Atoms:
- Cis Isomers: Cis isomers have the same side groups positioned on the same side of a double bond.
- Trans Isomers: Trans isomers have the same side groups positioned on opposite sides of a double bond.
- Polarity:
- Cis Isomers: Cis isomers are generally polar molecules due to the asymmetric distribution of charges caused by the arrangement of atoms.
- Trans Isomers: Trans isomers are usually non-polar molecules because the symmetric arrangement of atoms leads to a cancellation of dipole moments.
- Melting Points:
- Cis Isomers: Cis isomers tend to have relatively lower melting points compared to trans isomers. This is because the molecules in cis isomers are loosely packed, resulting in weaker intermolecular forces.
- Trans Isomers: Trans isomers typically have higher melting points than cis isomers because the molecules are more tightly packed, leading to stronger intermolecular forces.
- Boiling Points:
- Cis Isomers: The boiling points of cis isomers are often higher than those of trans isomers. This is because cis isomers exhibit stronger intermolecular forces of attraction between the atoms.
- Trans Isomers: Trans isomers generally have lower boiling points as there are no strong intermolecular forces due to the symmetric arrangement of atoms.
- Solubility:
- Cis Isomers: Cis isomers are more soluble in inert solvents due to their polarity and the presence of strong attractive forces.
- Trans Isomers: Trans isomers have relatively lower solubility in neutral solvents due to their non-polar nature and weak intermolecular forces.
- Acid Strength:
- Both Forms: Both cis and trans isomers can exhibit higher acid strength compared to other compounds.
- Cis Isomers: Cis isomers tend to readily emit protons, making them more acidic.
- Trans Isomers: Trans isomers have less acidic strength because the protons are not readily emitted.
These differences in properties between cis and trans isomers arise from the spatial arrangement of atoms and the resulting variations in intermolecular forces, polarity, and symmetry. Understanding these distinctions is essential for comprehending the behavior and characteristics of molecules exhibiting cis-trans isomerism.
| Property | Cis Isomers | Trans Isomers |
|---|---|---|
| Arrangement | Same side groups on the same side of a double bond | Same side groups on opposite sides of a double bond |
| Polarity | Generally polar | Usually non-polar |
| Melting Points | Relatively lower melting points | Higher melting points |
| Boiling Points | Higher boiling points due to strong intermolecular forces | Lower boiling points due to weak intermolecular forces |
| Solubility | More soluble in inert solvents | Relatively lower solubility in neutral solvents |
| Acid Strength | Higher acidity | Less acidic strength |
FAQ
What are cis and trans isomers?
Cis and trans isomers are types of stereoisomers that have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of groups around a double bond or in a ring structure.
How can cis and trans isomers be distinguished?
Cis and trans isomers can be distinguished by examining the positions of functional groups relative to the plane of the double bond or ring structure. Cis isomers have groups on the same side, while trans isomers have groups on opposite sides.
What causes cis and trans isomerism?
Cis and trans isomerism arises due to the restricted rotation around a double bond or in a ring structure, which locks the atoms or groups into specific spatial arrangements.
Do cis and trans isomers have different physical properties?
Yes, cis and trans isomers often exhibit different physical properties such as boiling points, melting points, solubility, and polarity due to the variations in intermolecular forces and symmetry.
Are cis and trans isomers optically active?
No, cis and trans isomers are not optically active as they lack chiral centers. They are geometric isomers rather than stereoisomers based on optical activity.
Can cis and trans isomers interconvert?
Cis and trans isomers are stable configurations that do not easily interconvert without breaking the double bond or ring structure. They require an energy input to convert from one isomer to another.
Where can cis and trans isomerism be observed?
Cis and trans isomerism can be observed in various organic compounds containing double bonds, such as alkenes and cyclic compounds, as well as in some inorganic coordination complexes.
Are cis and trans isomers the only types of geometric isomers?
No, cis and trans isomers are a specific type of geometric isomerism based on the arrangement of groups around a double bond. Other forms of geometric isomers, such as E-Z isomers, can exist in compounds with multiple substituents on a double bond.
Can cis and trans isomerism affect chemical reactivity?
Yes, cis and trans isomers can exhibit different chemical reactivities due to the distinct spatial arrangements of groups. The orientation of functional groups can impact how they interact with other molecules or undergo reactions.
Can cis and trans isomers have different biological activities?
Yes, the spatial arrangement of groups in cis and trans isomers can significantly influence their biological activities. Even small changes in molecular structure can lead to different interactions with biological targets, affecting their pharmacological properties.