What is cellulose?
- A polysaccharide with the chemical formula (C6H10O5)n, cellulose is an organic molecule that has a linear chain of several hundred to many thousands of (14) connected D-glucose units.
- Green plants, numerous types of algae, and oomycetes all have basic cell walls that contain cellulose as an essential structural element. It is secreted by some bacterial species in biofilms.
- The most prevalent organic polymer on Earth is cellulose. Cotton fiber contains 90% cellulose, wood contains 40%–50%, while dry hemp contains about 57% cellulose.
- Paper and paperboard are the two main products made from cellulose. Lesser amounts are transformed into a wide range of derivative goods, including rayon and cellophane.
- As a renewable fuel source, cellulosic ethanol and other biofuels made from cellulose from energy crops are currently being developed. The major sources of cellulose for industrial application are cotton and wood pulp.
- Termites and ruminants in particular may digest cellulose with the aid of symbiotic microorganisms called Trichonympha that live in their digestive tracts.
- Cellulose, a non-digestible component of insoluble dietary fiber used in human nutrition, acts as a hydrophilic bulking agent for stools and may facilitate defecation.
- Anselme Payen, a French chemist, extracted cellulose from plant matter in 1838 and characterized its chemical composition.
- Hyatt Manufacturing Company created celluloid, the first effective thermoplastic polymer, in 1870 using cellulose. In the 1890s, cellulose was used to produce rayon (sometimes known as “artificial silk”), and cellophane was created in 1912.
- In 1920, Hermann Staudinger identified the cellulose polymer structure. In 1992, Kobayashi and Shoda successfully chemically produced the molecule for the first time (without the use of any enzymes obtained from living organisms).
| (C6H10O5)n | Cellulose |
| Molecular Weight/ Molar Mass | 162.1406 g/mol |
| Density | 1.5 g/cm³ |
| Appears | White powder |
| Melting Point | 260–270 °C |
Properties of cellulose
- Insolubility: Cellulose is insoluble in water and most organic solvents, making it resistant to degradation.
- High tensile strength: Cellulose fibers are incredibly strong and have high tensile strength, making them ideal for providing structural support to plants.
- Biodegradability: Although it is resistant to most forms of degradation, cellulose can be broken down by certain types of bacteria and fungi.
- Hydrophilicity: Despite being insoluble in water, cellulose has hydrophilic properties that allow it to absorb and retain large amounts of water.
- Chemical reactivity: Cellulose can be chemically modified to change its properties or make it more soluble, and can be treated with various chemicals to create new products such as rayon.
- Renewable: Cellulose is a renewable resource, as it is derived from plant materials that can be grown and harvested sustainably.
- Non-toxic: Cellulose is non-toxic and safe for human and animal consumption, and is often used as a dietary fiber supplement.
Structure of cellulose
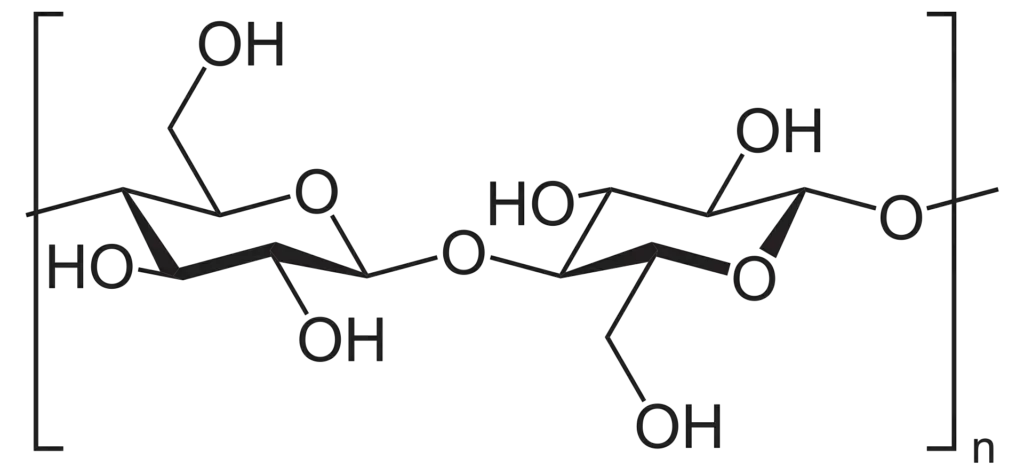
- Cellulose is tasteless, odorless, hydrophilic with a contact angle between 20 and 30 degrees, insoluble in water and the majority of organic solvents, chiral, and biodegradable.
- Pulse tests conducted by Dauenhauer et al. (2016) demonstrated that it melts at 467 °C. It can be chemically broken down into its glucose units by treating it with concentrated mineral acids at high temperature .
- The D-glucose units that condense into (14)-glycosidic bonds to form cellulose. This connection motif is unlike to the (14)-glycosidic bonds seen in starch and glycogen. Cellulose is a linear polymer chain.
- As a result of the equatorial conformation of the glucose residues, the molecule assumes an extended and rather rigid rod-like conformation, unlike starch, which undergoes coiling and branching.
- Several hydroxyl groups on the glucose of one chain make hydrogen bonds with oxygen atoms on the same or a neighboring chain, securing the chains side by side and generating microfibrils with a high tensile strength.
- This imparts tensile strength to cell walls in which cellulose microfibrils are interwoven with polysaccharide matrix. The great tensile strength of plant stems and tree wood is also attributable to the arrangement of cellulose fibers within the lignin matrix.
- The mechanical role of cellulose fibers in the wood matrix, which is responsible for its great structural resistance, can be compared to that of reinforcement bars in concrete, with lignin serving as the “glue” between the cellulose fibers, assuming the role of the hardened cement paste.
- Growth and expansion of plant cells are connected with the mechanical characteristics of cellulose in the main plant cell wall. Live fluorescent microscopy techniques are promising for investigating the role of cellulose in expanding plant cells.
- The D-glucose units that condense into (14)-glycosidic bonds to form cellulose. This connection motif is unlike to the (14)-glycosidic bonds seen in starch and glycogen. Cellulose is a linear polymer chain.
- As a result of the equatorial conformation of the glucose residues, the molecule assumes an extended and rather rigid rod-like conformation, unlike starch, which undergoes coiling and branching.
- Several hydroxyl groups on the glucose of one chain make hydrogen bonds with oxygen atoms on the same or a neighboring chain, securing the chains side by side and generating microfibrils with a high tensile strength.
- This imparts tensile strength to cell walls in which cellulose microfibrils are interwoven with polysaccharide matrix. The great tensile strength of plant stems and tree wood is also attributable to the arrangement of cellulose fibers within the lignin matrix.
- The mechanical role of cellulose fibers in the wood matrix, which is responsible for its great structural resistance, can be compared to that of reinforcement bars in concrete, with lignin serving as the “glue” between the cellulose fibers, assuming the role of the hardened cement paste.
- Growth and expansion of plant cells are connected with the mechanical characteristics of cellulose in the main plant cell wall. Live fluorescent microscopy techniques are promising for investigating the role of cellulose in expanding plant cells.
- There are several varieties of cellulose. This distinction is based on the position of hydrogen bonds between and within the strands. Cellulose I found in nature has the structures I and I.
- The cellulose generated by bacteria and algae is rich in I, whereas the cellulose of higher plants is predominantly composed of I. Cellulose is cellulose II in regenerated cellulose fibers.
- The irreversibility of the conversion of cellulose I to cellulose II suggests that cellulose I is metastable and cellulose II is stable. It is feasible to manufacture cellulose III and cellulose IV using a variety of chemical treatments.
- Many features of cellulose are determined by its chain length or degree of polymerization, that is, the number of glucose units that comprise a single polymer molecule. In contrast to long-chain cellulose, cellodextrins are typically soluble in water and organic solvents.
- cellulose has the chemical formula (C6H10O5)n, where n indicates the degree of polymerization and the number of glucose groups.
- Typically, plant-derived cellulose is found in a mixture of hemicellulose, lignin, pectin, and other compounds, whereas bacterial cellulose is very pure, has a significantly higher water content, and a greater tensile strength due to longer chain lengths.
- Cellulose is composed of fibrils that have both crystalline and amorphous areas. Mechanical treatment of cellulose pulp, frequently aided by chemical oxidation or enzymatic treatment, produces semi-flexible cellulose nanofibrils generally 200 nm to 1 m in length, depending on the treatment intensity.
- Cellulose pulp may also be treated with strong acid to hydrolyze the amorphous fibril regions, yielding short rigid cellulose nanocrystals a few 100 nm in length.
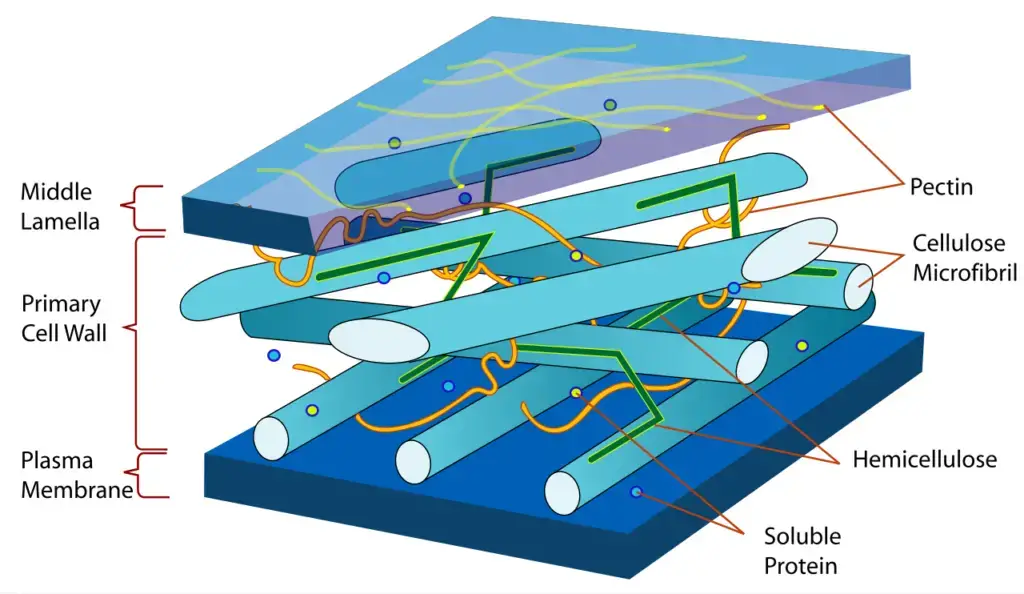
Commercial applications of cellulose
- Paper products: Cellulose is the predominant component of paper, paperboard, and card stock. Cellulose is utilized in a variety of forms as insulation in transformers, cables, and other electrical equipment.
- Fibers: Cellulose is the primary component of textile fibers. Cotton and synthetics (nylons) account for around 40% of the market by volume. Other plant fibers (jute, sisal, hemp) account for around 20% of the market. Rayon, cellophane, and other “regenerated cellulose fibers” constitute only 5% of the total.
- Consumables: Microcrystalline cellulose (E460i) and powdered cellulose (E460ii) are employed as inactive fillers in medicine tablets, while a wide range of soluble cellulose derivatives, E numbers E461 to E469, are utilized as emulsifiers, thickeners, and stabilizers in processed foods. For instance, cellulose powder is utilized in processed cheese to minimize internal caking. Cellulose is a natural component of some foods and an additive in processed foods, contributing an indigestible component used for texture and bulk, which may aid un defecation.
- Building material: As an alternative to plastics and resins, the hydroxyl bonding of cellulose in water generates a sprayable, moldable building material. It is possible to make the recyclable material water- and fire-resistant. It is strong enough to be used as a building material. Cellulose insulation derived from recycled paper is gaining popularity as an environmentally preferable building insulation material. As a fire retardant, boric acid can be used.
- Miscellaneous: Cellulose can be turned into cellophane, which is a thin, translucent film. Until the mid-1930s, it was the foundation material for the celluloid used for photographic and motion picture films. Cellulose is utilized in the production of water-soluble adhesives and binders, such as methyl cellulose and carboxymethyl cellulose, which are utilized in wallpaper paste. Sponge materials that are hydrophilic and extremely absorbent are produced from cellulose. Cellulose is used to produce nitrocellulose (cellulose nitrate), which is utilized in smokeless gunpowder.
- Pharmaceuticals: Cellulose derivatives, such as microcrystalline cellulose (MCC), retain water, act as a stabilizer and thickening agent, and reinforce medicine tablets.
What are cellulases?
- Enzymes known as “cellulases” are responsible for the hydrolysis of cellulose’s β-1,4 bonds. Cellulose breakdown is carried out by these enzymes.
- Although cellulose is chemically homogeneous, it exists in crystalline and amorphous forms, and no one enzyme can hydrolyze it. Due to its insoluble, crystalline, and heterogeneous character, is a resistant and difficult substrate for enzymatic hydrolysis.
- Given the structure of cellulose, it is almost impossible for an enzyme to bind cellulose to its substrate site, making it hard for a single enzyme to hydrolyze cellulose.
- Along with its interaction with other polymers, this enables cellulose-containing materials to tolerate extreme circumstances, making them durable and resistant to degradation, thereby fulfilling their structural and protective roles.
- Cellulose can only be completely digested by a combination of enzymes operating simultaneously and interacting with one another. Hence, real cellulolytic organisms develop a system of numerous enzymes.
- These multiple-enzyme systems facilitate the hydrolysis of cellulose through synergistic action. For the full hydrolysis of this polymeric substrate into its monomeric unit, at least three distinct enzyme activities are required:
- Endoglucanase exercise
- Exoglucanase exercise (also called cellodextrinase or cellobiohydrolase)
- β – glucosidases activity
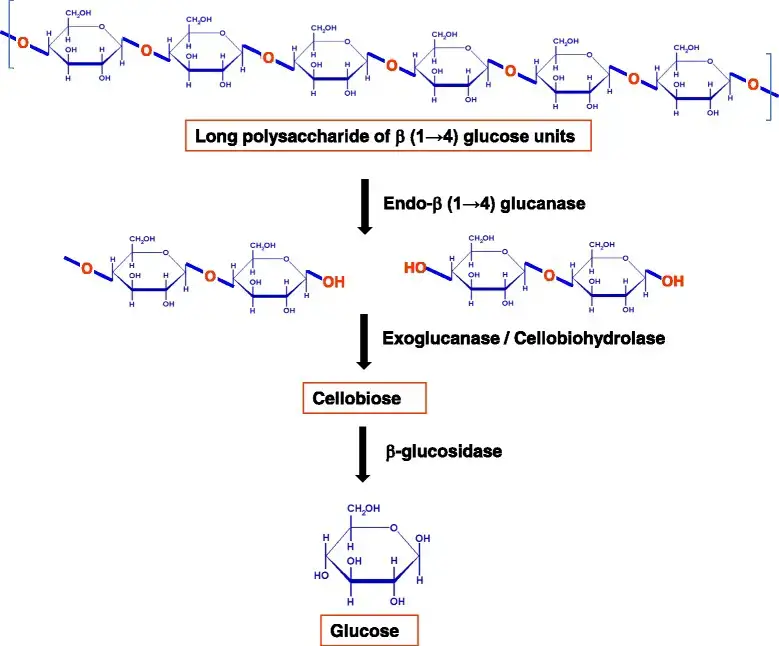
- Only through the cooperation of these actions are enzymes able to destabilize the structure at the solid–liquid boundary, so rendering the individual fiber accessible to hydrolysis.
- Endoglucanases make random internal cuts within the amorphous portion of the cellulose molecule, hence producing new chain ends.
- Exoglucanases produce glucose, cellobiose, and/or cellooligosaccharides by acting progressively on the reducing and/or non-reducing ends. These soluble cellodextrins and cellobiose are subsequently converted to glucose by β-glucosidases.
- Endoglucanases have an accessible active site because they can attach to the inside of long cellulose fibers. In contrast, exocellulases have an active site in a tunnel, which is compatible with their processive nature and the sequential release of cellobiose from the end of the cellulose chain. The three types of enzymes hydrolyze cellulose in a coordinated manner.
- Endoglucanase first attacks the amorphous sections of cellulose fibers, producing entry points for exoglucanases to enter the crystalline regions of the fiber. They also tend to operate on microcrystalline cellulose, separating the microcrystalline structure from the cellulose strands.
- Ultimately, β-glucosidases divide cellobiose to glucose limiting the build-up of cellobiose which inhibits cellobiohydrolases. The cellulolytic activity of cellulases differs not only in how they attach to the crystalline surface of their insoluble substrate, but also in how they operate on cellulose.
- In fact, all enzymes that act on insoluble substrates contain two binding sites: the “active site,” which is typically located in the enzyme’s catalytic domain, and the “substrate binding site,” which is part of a separately folded and functionally distinct carbohydrate/cellulose binding domain.
- The two domains are separated by a linker peptide that functions as a flexible arm linking the two components.
Factors affecting cellulose degradation
- Temperature: The rate of cellulose degradation increases with temperature, with higher temperatures leading to faster degradation.
- Moisture content: Cellulose degradation requires the presence of water, as it is a hydrolytic process. Higher moisture content can lead to faster degradation.
- pH: The pH of the environment can affect the rate of cellulose degradation, with an optimal pH range of 4.5 to 5.5 for most cellulase enzymes.
- Presence of enzymes: Cellulose degradation is carried out by cellulase enzymes, which can be produced by microorganisms such as bacteria and fungi. The presence of these enzymes can greatly accelerate the degradation process.
- Lignin content: Cellulose degradation can be hindered by the presence of lignin, a complex polymer found in plant cell walls that can form a physical barrier around cellulose fibers.
- Oxygen availability: Cellulose degradation can be affected by the availability of oxygen, as aerobic microorganisms require oxygen to carry out the degradation process.
- Presence of inhibitors: Certain substances, such as heavy metals, can inhibit cellulose degradation by damaging or inhibiting the activity of cellulase enzymes.
- Available minerals: The presence of certain minerals, such as nitrogen, phosphorus, and potassium, can influence the rate of cellulose degradation. These minerals can act as nutrients for microorganisms that produce cellulase enzymes.
- Organic matter: The amount and type of organic matter present can affect cellulose degradation. Organic matter provides a source of carbon and other nutrients for microorganisms, which can accelerate the degradation process.
- Lignin: Lignin is a complex polymer found in plant cell walls that can hinder cellulose degradation by forming a physical barrier around cellulose fibers. The amount and type of lignin present can impact the rate of cellulose degradation.
Microorganisms involved in cellulose degradation/cellulolytic microorganisms
Cellulose degradation is primarily carried out by cellulolytic microorganisms, which are capable of producing cellulase enzymes that break down cellulose into simpler sugars that can be used as a source of energy. Here are some examples of cellulolytic microorganisms:
- Bacteria: Certain species of bacteria, such as Clostridium thermocellum, Cellulomonas fimi, and Bacillus subtilis, are known to produce cellulase enzymes and are capable of degrading cellulose. Examples;
- Clostridium thermocellum: A thermophilic bacterium that can break down cellulose at high temperatures.
- Cellulomonas fimi: A cellulolytic bacterium that can degrade cellulose and hemicellulose.
- Bacillus subtilis: A soil bacterium that produces a variety of cellulases and can degrade cellulose.
- Fungi: Many species of fungi, including Trichoderma reesei, Aspergillus niger, and Phanerochaete chrysosporium, are known to produce cellulase enzymes and are capable of breaking down cellulose. Examples;
- Trichoderma reesei: A filamentous fungus that is widely used for industrial cellulase production.
- Aspergillus niger: A fungus that produces a variety of enzymes, including cellulases, and is used in the production of biofuels.
- Phanerochaete chrysosporium: A white-rot fungus that is capable of breaking down both cellulose and lignin.
- Protozoa: Some species of protozoa, such as termites and ruminants, have specialized digestive systems that contain cellulolytic microorganisms that aid in the breakdown of cellulose. Examples;
- Termites: These insects have a specialized digestive system that contains cellulolytic microorganisms, including bacteria and flagellate protozoa, that help break down cellulose.
- Ruminants: Cows, sheep, and other ruminants have a complex stomach system that contains cellulolytic microorganisms, including bacteria, fungi, and protozoa, that aid in the digestion of plant materials.
- Archaea: Some species of archaea, such as Methanoculleus marisnigri and Methanoculleus thermophilus, have been found to produce cellulase enzymes and are capable of degrading cellulose. Examples;
- Methanoculleus marisnigri: A methanogenic archaeon that has been found to produce cellulase enzymes and can degrade cellulose.
- Methanoculleus thermophilus: A thermophilic archaeon that can break down cellulose and produce methane.
Cellulolytic microorganisms play an important role in various natural and industrial processes, such as composting, biofuel production, and waste management. Understanding the diversity and activity of these microorganisms can help optimize these processes and improve their efficiency.
Enzymes involved in the degradation of cellulose
The degradation of cellulose is carried out by several enzymes, which act together to break down cellulose into simpler sugars that can be used as a source of energy. Here are some enzymes involved in the degradation of cellulose:
- Endoglucanases: These enzymes break down cellulose by cleaving the internal bonds between glucose units, creating shorter cellulose chains.
- Exoglucanases (also called cellobiohydrolases): These enzymes work on the ends of cellulose chains, releasing cellobiose, a dimer of glucose.
- β-glucosidases: These enzymes break down cellobiose and other short cellulose chains into individual glucose molecules, which can be used as an energy source by microorganisms.
- Lytic polysaccharide monooxygenases (LPMOs): These enzymes are involved in breaking down cellulose and other polysaccharides by oxidizing them, making them more accessible to other enzymes.
- Xylanases: These enzymes break down hemicellulose, a complex carbohydrate that is often associated with cellulose in plant cell walls.
- Cellobiases (also called β-glucosidases): These enzymes break down cellobiose, a dimer of glucose that is formed during the degradation of cellulose, into individual glucose molecules that can be used as an energy source by microorganisms.
- Oxidative cellulases: These enzymes use reactive oxygen species to cleave the bonds in cellulose, making it more accessible to other enzymes.
- Cellobiose phosphorylases: These enzymes break down cellobiose by transferring a phosphate group to the molecule, creating glucose-1-phosphate and glucose.
Mechanism of cellulose decomposition
The degradation of cellulose follows a succession of enzyme processes. Enzymes responsible for cellulose degradation is cellulase. C1 enzyme, β-1,4-glucanase, and β-1,4-glucosidase comprise the cellulase enzyme complex. Complex cellulose is degraded into free glucose molecules by extracellular enzymes through a series of chemical reactions.
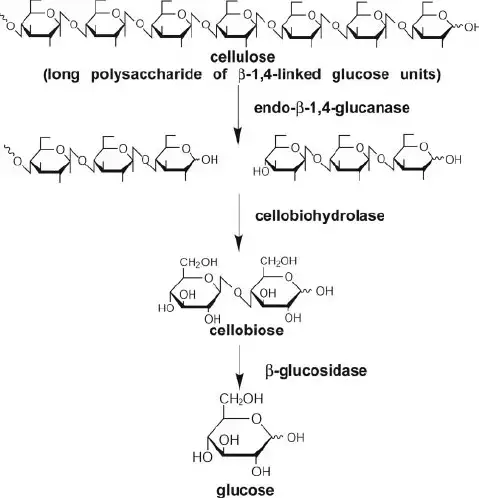
1. Hydrolysis by C1 enzymes
The C1 enzyme breaks down native cellulose polymer into smaller fragments. C1 enzyme is exclusive to bacteria with real cellulolytic activity.
2. Hydrolysis by β-1,4-glucanase enzyme
β-1,4-glucanase hydrolyzes the cellulose fragments into even smaller pieces, including disaccharides, tri-saccharides, etc. Endo-glucanase and Exo-glucanase are the two forms of glucanase. Endo-glucanase breaks fragments randomly in the middle, whereas exo-glucanase releases glucose molecules sequentially from one end of the fragment. By the activity of β-1,4-glucanase, free glucose units, disaccharides, trisaccharides, and various oligosaccharides are generated.
3. Hydrolysis by β-1,4-glucosidase enzyme
β-1,4-glucosidase breaks down di, tri, and oligosaccharides into glucose molecules.
4. Metabolism of glucose
The glucose molecules are subsequently digested by glycolysis to produce pyruvate in the microbial cell. Depending on microorganism kinds and environmental conditions, pyruvate is transformed into CO2 and water, ethanol, or organic acids.
Hydrolytic Mechanism of cellulose degradation
The hydrolytic mechanism of cellulose degradation involves the breaking of the glycosidic bonds between the glucose units that make up cellulose. This process is carried out by enzymes known as cellulases, which are produced by microorganisms such as bacteria and fungi.
Cellulases can be classified into three types based on their mode of action: endoglucanases, exoglucanases, and β-glucosidases. Endoglucanases randomly cleave the internal glycosidic bonds within the cellulose chain, creating shorter cellulose chains. Exoglucanases, on the other hand, act on the ends of the cellulose chains, cleaving off one glucose unit at a time. β-glucosidases then further break down the shorter cellulose chains into individual glucose molecules.
The hydrolysis reaction involves the addition of a water molecule to the glycosidic bond, breaking it and forming two new hydroxyl groups. This reaction requires the presence of a catalytic site on the enzyme, which brings the water molecule into contact with the glycosidic bond and orients it in the correct position for the reaction to occur.
The products of the hydrolysis reaction are shorter cellulose chains and glucose molecules, which can be further metabolized by the microorganisms to produce energy and other compounds. Overall, the hydrolytic mechanism of cellulose degradation is an essential process in the carbon cycle and plays a significant role in the recycling of organic materials in the environment.
The hydrolytic mechanism of cellulose degradation involves the following steps:
- Adsorption: The cellulose fibers are first adsorbed onto the surface of the microorganisms using their cell wall or specialized enzymes.
- Enzymatic hydrolysis: The microorganisms produce enzymes called cellulases that break down the cellulose fibers into smaller glucose molecules. The cellulases include three types of enzymes: endoglucanases, exoglucanases, and β-glucosidases.
- Action of endoglucanases: Endoglucanases randomly cleave the internal glycosidic bonds within the cellulose chain, creating shorter cellulose chains.
- Action of exoglucanases: Exoglucanases act on the ends of the shorter cellulose chains, cleaving off one glucose unit at a time.
- Action of β-glucosidases: β-glucosidases further break down the shorter cellulose chains into individual glucose molecules.
- Hydrolysis reaction: The hydrolysis reaction involves the addition of a water molecule to the glycosidic bond, breaking it and forming two new hydroxyl groups. This reaction requires the presence of a catalytic site on the enzyme, which brings the water molecule into contact with the glycosidic bond and orients it in the correct position for the reaction to occur.
- Product formation: The products of the hydrolysis reaction are shorter cellulose chains and glucose molecules, which can be further metabolized by the microorganisms to produce energy and other compounds.
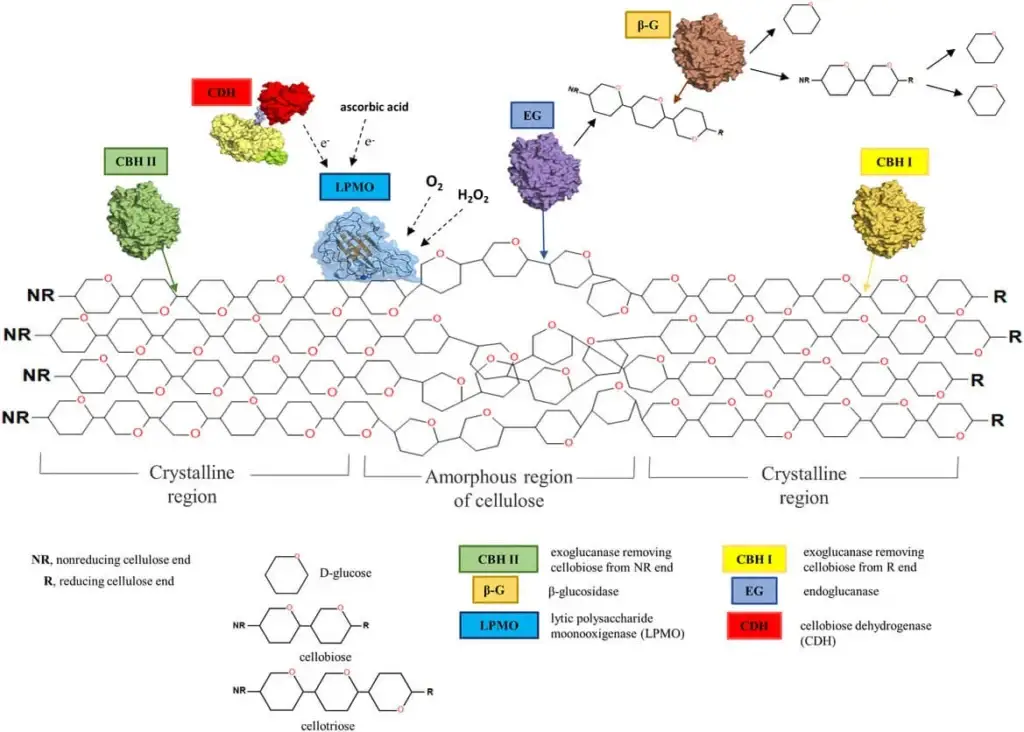
Example of Hydrolytic Mechanism
- One example of the hydrolytic mechanism of cellulose degradation can be seen in the action of the bacterium Cellulomonas fimi. This bacterium produces a variety of cellulases, including endoglucanases, exoglucanases, and β-glucosidases, which work together to break down cellulose into glucose molecules.
- The endoglucanases produced by C. fimi cleave the internal glycosidic bonds within the cellulose chain, creating shorter cellulose chains. These shorter chains are then acted upon by the exoglucanases, which cleave off one glucose unit at a time from the ends of the chains. Finally, the β-glucosidases further break down the shorter cellulose chains into individual glucose molecules.
- The hydrolysis reaction involves the addition of a water molecule to the glycosidic bond, breaking it and forming two new hydroxyl groups. This reaction requires the presence of a catalytic site on the enzyme, which brings the water molecule into contact with the glycosidic bond and orients it in the correct position for the reaction to occur.
- The glucose molecules produced by the hydrolysis reaction are then taken up by C. fimi and metabolized through the process of glycolysis, producing energy in the form of ATP for the bacterium to use. As a byproduct of respiration, carbon dioxide and water are produced and released into the environment.
- Overall, the hydrolytic mechanism of cellulose degradation plays an essential role in the recycling of organic materials in the environment and is a critical process for the growth and survival of microorganisms that are able to break down cellulose.
Oxidative Mechanism of cellulose degradation
The oxidative mechanism of cellulose degradation involves the use of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and hydroxyl radicals (•OH) to break down the cellulose fibers. This process is carried out by enzymes known as oxidases, which are produced by microorganisms such as bacteria and fungi.
The oxidative mechanism of cellulose degradation involves the following steps:
- Production of ROS: The microorganisms produce enzymes called oxidases, which generate ROS such as H2O2 and •OH.
- Activation of cellulose: The ROS react with the cellulose fibers, creating oxidized cellulose that is more susceptible to degradation.
- Action of lytic enzymes: The microorganisms produce lytic enzymes such as cellobiose dehydrogenase and lytic polysaccharide monooxygenase (LPMO) that bind to the oxidized cellulose and initiate the oxidative degradation process.
- Cleavage of glycosidic bonds: The LPMO enzymes break the glycosidic bonds between the glucose units in the cellulose fibers, creating shorter cellulose chains.
- Production of aldehydes: The cleavage of the glycosidic bonds by LPMO enzymes produces aldehydes, which are further oxidized by the oxidases to produce carboxylic acids and carbon dioxide.
- Production of H2O2: The reaction between the LPMO enzymes and the cellulose fibers also generates H2O2, which further oxidizes the cellulose fibers and promotes their degradation.
- Product formation: The products of the oxidative mechanism of cellulose degradation are shorter cellulose chains, carboxylic acids, and carbon dioxide. These products can be further metabolized by the microorganisms to produce energy and other compounds.
Overall, the oxidative mechanism of cellulose degradation is a complex process that involves the production of ROS by oxidases and the cleavage of glycosidic bonds by LPMO enzymes. This mechanism plays an important role in the recycling of organic materials in the environment and is a critical process for the growth and survival of microorganisms that are able to break down cellulose.
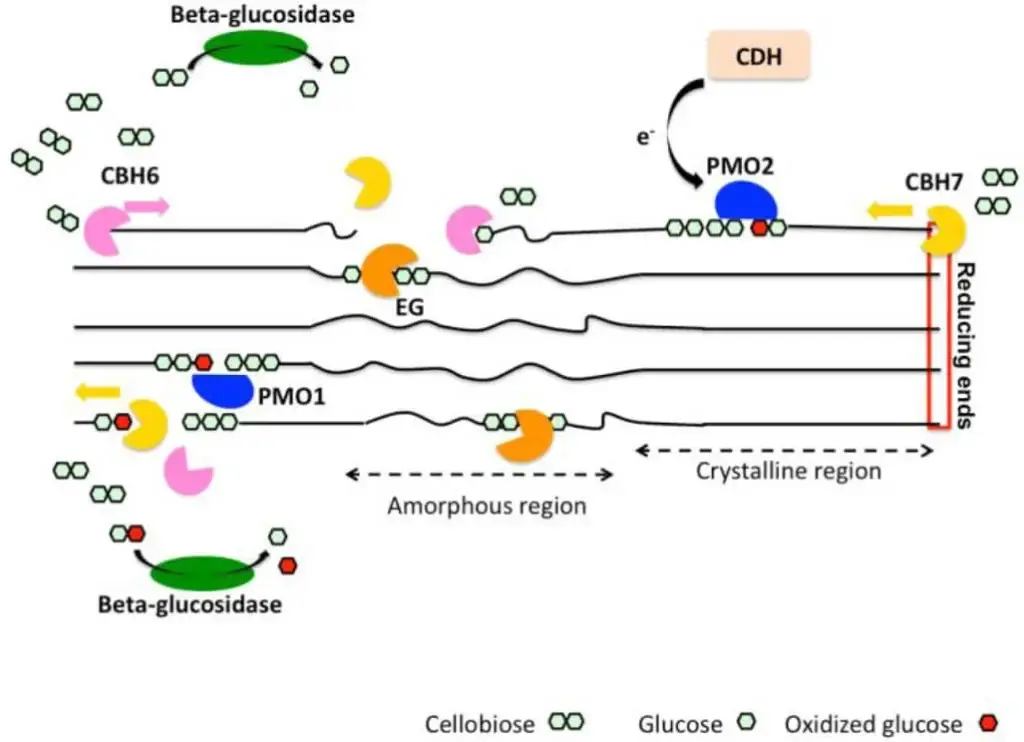
Example of Oxidative Mechanism
An example of the oxidative mechanism of cellulose degradation can be seen in the action of the fungus Trichoderma reesei. This fungus produces a variety of enzymes, including cellobiose dehydrogenase (CDH) and lytic polysaccharide monooxygenase (LPMO), which work together to break down cellulose into smaller units.
The oxidative mechanism of cellulose degradation by T. reesei involves the following steps:
- Production of ROS: The fungus produces CDH, which generates H2O2 as a byproduct of the reaction with cellobiose. H2O2 is a reactive oxygen species that can break down the cellulose fibers.
- Activation of cellulose: The H2O2 reacts with the cellulose fibers, creating oxidized cellulose that is more susceptible to degradation.
- Action of LPMO: The fungus produces LPMO, which binds to the oxidized cellulose and initiates the oxidative degradation process.
- Cleavage of glycosidic bonds: The LPMO enzymes break the glycosidic bonds between the glucose units in the cellulose fibers, creating shorter cellulose chains.
- Production of aldehydes: The cleavage of the glycosidic bonds by LPMO enzymes produces aldehydes, which are further oxidized by CDH to produce carboxylic acids and carbon dioxide.
- Production of H2O2: The reaction between CDH and cellobiose also generates H2O2, which further oxidizes the cellulose fibers and promotes their degradation.
- Product formation: The products of the oxidative mechanism of cellulose degradation by T. reesei are shorter cellulose chains, carboxylic acids, and carbon dioxide. These products can be further metabolized by the fungus to produce energy and other compounds.
FAQ
What is microbial degradation of cellulose?
Microbial degradation of cellulose is the process by which microorganisms break down cellulose fibers into simpler compounds, such as glucose and other sugars, through the action of enzymes.
Which microorganisms are involved in the degradation of cellulose?
A wide range of microorganisms, including bacteria, fungi, and protozoa, are capable of degrading cellulose.
What are the enzymes involved in the degradation of cellulose?
The primary enzymes involved in the degradation of cellulose are endoglucanases, exoglucanases, and cellobiohydrolases, which break down cellulose into smaller oligosaccharides, and β-glucosidases, which hydrolyze these oligosaccharides into glucose.
How does microbial degradation of cellulose affect the environment?
Microbial degradation of cellulose is an important process in the carbon cycle, as it helps to release carbon trapped in cellulose fibers back into the ecosystem.
What are the applications of microbial degradation of cellulose?
Microbial degradation of cellulose has a wide range of applications, including in the production of biofuels, biodegradation of agricultural and industrial waste, and the production of paper and textiles.
How is microbial degradation of cellulose measured?
Cellulose degradation can be measured using a variety of methods, including gravimetric analysis, spectrophotometric analysis, gas chromatography, and nucleic acid sequencing.
What factors affect the rate of microbial degradation of cellulose?
Factors that affect the rate of microbial degradation of cellulose include temperature, pH, oxygen concentration, and the presence of inhibitors or other substances that may interfere with the degradation process.
What is the role of microbial consortia in cellulose degradation?
Microbial consortia, or communities of microorganisms working together, can increase the rate of cellulose degradation by providing a range of enzymes and metabolic pathways.
What are some challenges in the microbial degradation of cellulose?
Some challenges in the microbial degradation of cellulose include slow rates of degradation, susceptibility to inhibition by toxic substances, and the limited range of microorganisms capable of degrading cellulose.
How can the efficiency of microbial cellulose degradation be improved?
Efforts to improve the efficiency of microbial cellulose degradation include the identification and selection of cellulose-degrading microorganisms, optimization of environmental conditions, and genetic engineering of microorganisms to enhance their cellulose-degrading capabilities.
References
- Lakhundi S, Siddiqui R, Khan NA. Cellulose degradation: a therapeutic strategy in the improved treatment of Acanthamoeba infections. Parasit Vectors. 2015 Jan 14;8:23. doi: 10.1186/s13071-015-0642-7. PMID: 25586209; PMCID: PMC4300153.
- de Souza, W. R. (2013). Microbial Degradation of Lignocellulosic Biomass. InTech. doi: 10.5772/54325
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/cellulose-decomposition
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/cellulose-decomposition
- https://bioenergycenter.org/besc/publications/wilson_microbial.pdf
- https://pubs.acs.org/doi/10.1021/acs.estlett.1c00843
- https://www.jstor.org/stable/4353503
- https://www.frontiersin.org/articles/10.3389/fmicb.2019.00204/full
- https://www.onlinebiologynotes.com/cellulose-decomposition-microbial-decomposition-of-cellulose-in-soil/
Related Posts
- Agrobacterium-Mediated Gene Transfer – Mechanism, Applications, Advantages
- Organic Waste Recycling – Definition, Characteristics, Methods, Steps, Significance
- Microbial Degradation of Lignin – Microorganisms, Enzymes, Steps, Mechanisms, Challenges
- Phyllosphere Microorganisms – Examples, Factors, Effects
- Soil Formation (Pedogenesis)- Definition, Factors, Process, Steps, Examples
