- The immune system is a finely-tuned system that operates around-the-clock throughout life to protect the body against pathogens and aberrant cells. Even though the immune system is constantly exposed to self-antigens, it does not mount a defence against them.
- Occasionally, these processes malfunction, resulting in damage to diverse tissues.
- Autoimmunity is the production of autoantibodies and immunologically competent T cells against the body’s own tissues.
- Autoimmune disorders are conditions in which the mechanisms of self-tolerance fail, and autoimmunity is the resulting condition.
- Autoimmunity literally translates as “protection against self,” yet in practise it results in “self-injury.”
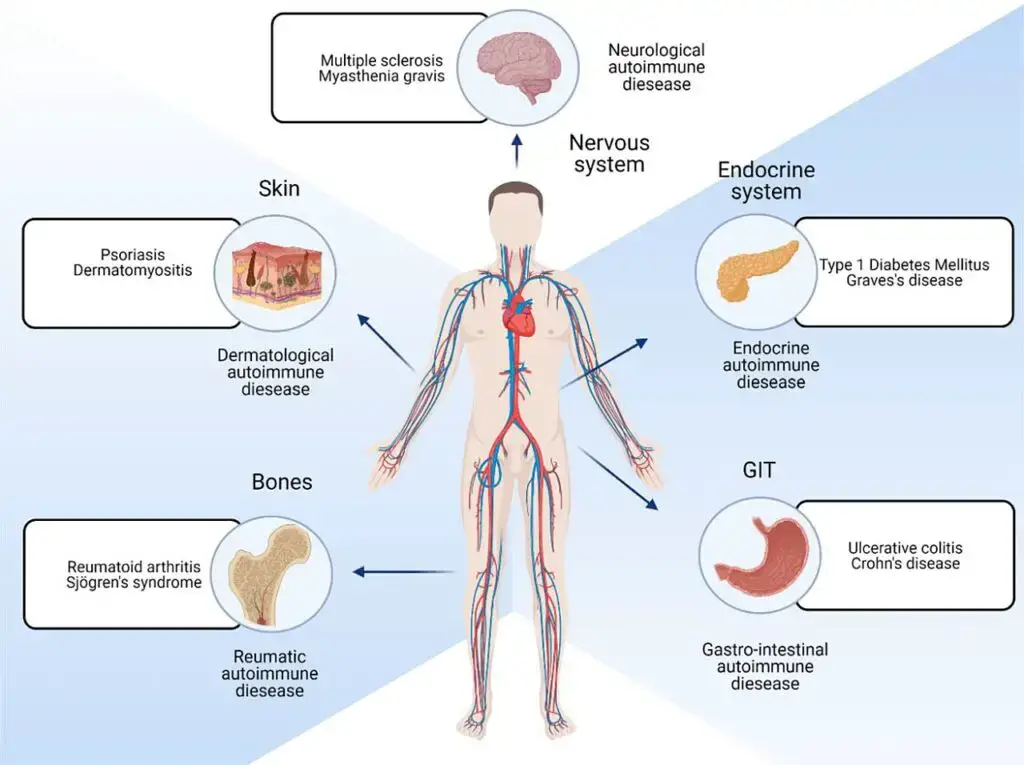
- At the clinical level, autoimmunity appears to play a role in a number of seemingly unrelated disorders, including systemic lupus erythematosus (SLE), insulin-dependent diabetic mellitus, myasthenia gravis, rheumatoid arthritis, multiple sclerosis, and hemolytic anemias.
- Ehrlich first hypothesised the possibility of tolerance to self-antigens in 1901, as well as the circumstances in which this mechanism would fail, resulting in “horror autotoxicus.”
- Understanding the many immunological systems and illnesses has led to the same conclusions in recent years.
What is Autoimmunity?
- Autoimmunity refers to the immune system’s response in which it mistakenly attacks the body’s own healthy cells, tissues, and other normal constituents. This immune response against self is known as an autoimmune response, and any disease that arises as a result is called an autoimmune disease. Examples of autoimmune diseases include celiac disease, post-infectious irritable bowel syndrome (IBS), type 1 diabetes mellitus, Henoch-Schönlein purpura (HSP), sarcoidosis, systemic lupus erythematosus (SLE), Sjögren syndrome, eosinophilic granulomatosis with polyangiitis, Hashimoto’s thyroiditis, Graves’ disease, idiopathic thrombocytopenic purpura, Addison’s disease, rheumatoid arthritis (RA), ankylosing spondylitis, polymyositis (PM), dermatomyositis (DM), and multiple sclerosis (MS). Steroids are often used in the treatment of autoimmune diseases.
- In a healthy state, individuals possess antibodies or T cells that react with self-proteins, which is a normal occurrence. However, if this self-reactivity leads to tissue damage, it can give rise to autoimmune diseases. Essentially, autoimmunity occurs when the immune system fails to distinguish between self and nonself, resulting in an attack on the body’s own tissues.
- The term “autoimmune disease” is used when there is evidence of specific immune components involved in the disease’s pathogenesis. This can include the presence of immunoglobulins (autoantibodies) or cytotoxic T-lymphocytes that display specificity towards self-antigens (autoantigens). These immune components contribute to the development and progression of the autoimmune disease.
- Overall, autoimmunity represents a breakdown in the immune system’s ability to differentiate between self and nonself, leading to the development of autoimmune diseases. Understanding the underlying mechanisms of autoimmunity is crucial for the diagnosis, treatment, and management of these diseases.
General Features Of Immunologic Tolerance
Autoimmunity, encompassing more than 80 different conditions, affects approximately 5% of the population. While each autoimmune disease may have unique characteristics, there are several common features shared among them:
- HLA genetic association: Many autoimmune diseases exhibit a genetic association with certain alleles of the human leukocyte antigen (HLA) gene family. These genetic variations can contribute to an individual’s susceptibility to developing autoimmune disorders.
- Higher incidence among females: Autoimmune diseases are more frequently observed in females compared to males, particularly in the reproductive age group. Hormonal factors, such as estrogen, may play a role in this sex disparity, although the precise mechanisms are not fully understood.
- Onset in young adulthood or middle age: Most autoimmune diseases manifest during young adulthood or middle age, although they can occur at any age. The exact age of onset varies depending on the specific autoimmune condition.
- Detectable autoantibody levels: Autoimmune diseases often involve the production of autoantibodies, which are antibodies that mistakenly target and attack the body’s own tissues or organs. These autoantibodies can be detected in blood tests and are specific to each autoimmune disease.
- Positive response to immunosuppressive treatments: Immunosuppressive therapies, such as corticosteroids and certain immunomodulatory drugs, are commonly used in the management of autoimmune diseases. The fact that these treatments often yield positive responses suggests an aberrant immune response as a contributing factor to autoimmunity.
- Fluctuations in symptom severity, with flare-ups and remissions: Autoimmune diseases are characterized by unpredictable fluctuations in symptom severity over time. Patients may experience periods of flare-ups, during which symptoms worsen, followed by periods of remission, during which symptoms improve or temporarily disappear.
Autoimmune diseases can be classified into two broad categories based on the nature of the targeted self-antigens: organ-specific and systemic.
In organ-specific autoimmune diseases, such as Hashimoto’s thyroiditis, the immune response is confined to specific organs or tissues. For example, in Hashimoto’s thyroiditis, the immune system specifically targets the thyroid gland, resulting in anti-thyroid peroxidase (TPO) antibodies.
In contrast, systemic autoimmune diseases like systemic lupus erythematosus (SLE) involve autoantibodies that recognize structures found in various cell types throughout the body. In SLE, autoantibodies can target double-stranded DNA and other cellular components. This widespread immune reactivity in SLE can lead to a diverse range of symptoms that may initially appear unrelated, making diagnosis challenging.
The features of autoimmunity outlined above provide a general framework for understanding the commonalities and variations observed across different autoimmune diseases. However, it is important to note that each autoimmune condition has its own unique characteristics and clinical manifestations, requiring individualized approaches to diagnosis and treatment.
Tolerance
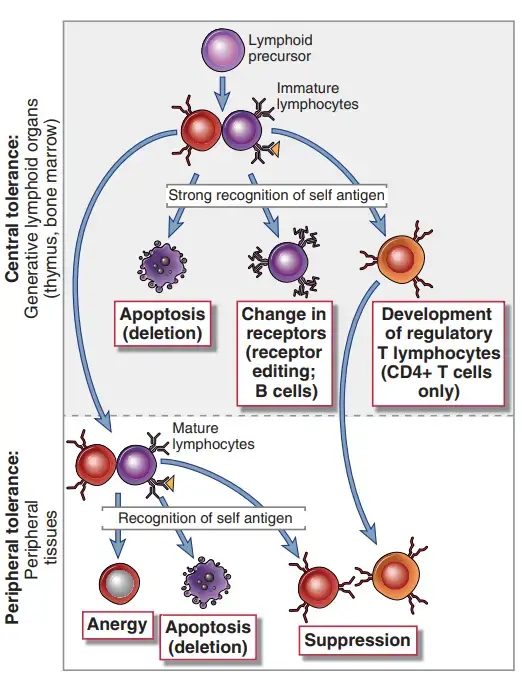
- Tolerance is a state of specific immunological insensitivity to a particular antigen or epitope despite otherwise normal immune function.
- Antigens present throughout embryonic development are typically regarded as self and do not provoke an immune response; hence, the host maintains tolerance to these antigens.
- Due to the elimination of self-reactive T-cell precursors in the thymus, the foetus does not mount an immunological response.
- On the other hand, antigens that are absent during the maturation process are deemed nonself and typically induce an immune reaction against them.
Mechanisms of Tolerance
T cells and B cells engage in tolerance, although T-cell tolerance plays the most significant function.
1. T-cell tolerance
The ideas of (a) clonal deletion, (b) clonal anergy, and (c) clonal ignorance explain T-cell tolerance.
a. Clonal deletion
- The earliest theory of tolerance was the theory of clonal deletion described by Burnet, Fenner, and Medawar based on their studies with mice.
- Recent studies reveal that early in life, T cells acquire the ability to differentiate between self and nonself by clonal deletion.
- This process involves the elimination of T lymphocytes (negative selection) that attack antigens, primarily self-MHC (major histocompatibility complex) molecules present in the foetus at the time.
- The self-reactive cells perish via apoptosis, a programmed cell death mechanism.
b. Clonal anergy
- Clonal anergy is the process that renders self-reactive T lymphocytes ineffective.
- The inability of these cells to develop an immune response due to insufficient costimulation is known as anergic.
c. Clonal ignorance
- This term refers to selfreactive T lymphocytes that disregard self-antigens.
- These self-reactive T cells disregard self-antigens due to their extremely low concentration.
- Also, these self-reactive cells are kept ignorant by physical separation from the target antigens, such as blood–brain barrier.
2. B-cell tolerance
B lymphocyte tolerance is required to preserve nonreactivity to thymus-independent self antigens, such as polysaccharides and lipids. Additionally, B cell tolerance prevents antibody responses to protein antigens. Multiple ways have been identified via which self antigens might inhibit the development and activation of B cells.
A. Central Tolerance in B Cells
Immature B cells that identify self antigens in the bone marrow with a high degree of affinity either alter their specificity or are eliminated. In experimental models, the mechanisms of central B cell tolerance have been best described.
a. Receptor editing
- If immature B cells recognise self antigens that are present in high concentration in the bone marrow, and especially if the antigen is displayed in multivalent form (e.g., on cell surfaces), a large number of antigen receptors on each B cell are cross-linked, thereby sending powerful signals to the cells.
- B cells reactivate their RAG1 and RAG2 genes and commence a new cycle of VJ recombination at the immunoglobulin (Ig) light chain gene locus in response to such signalling. A V segment upstream of the previously rearranged VJ unit is linked to a J segment downstream.
- As a result, the previously altered VκJκ exon in the self-reactive immature B cell is deleted and a new Ig light chain is produced, thus producing a B cell receptor with a new specificity.
- This process, known as receptor editing, is a crucial mechanism for removing self-reactivity from the mature B cell repertoire.
- If the edited light chain rearrangement is unsuccessful, the locus on the other chromosome may undergo rearrangement, and if that is unsuccessful, the light chain loci may undergo rearrangement.
- A B cell that expresses a light chain has commonly undergone receptor modification.
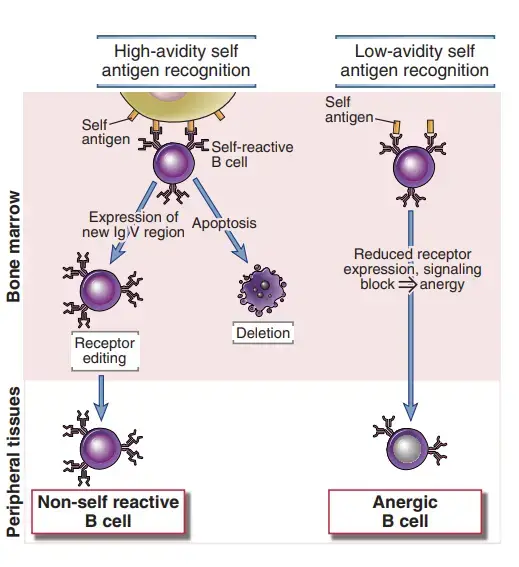
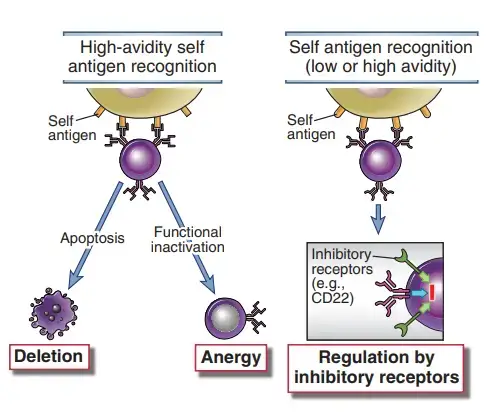
b. Deletion
- If editing fails, the immature B cells may be eliminated (i.e., they die by apoptosis) (i.e., they die by apoptosis). The mechanisms of deletion are not fully defined.
c. Anergy
- If developing B cells recognise self antigens poorly (for instance, if the antigen is soluble and does not cross-link many antigen receptors or if the B cell receptors recognise the antigen with low affinity), the cells become functionally unresponsive (anergic) and leave the bone marrow in this state.
- Anergy is caused by a decrease in antigen receptor expression and a block in antigen receptor signalling.
B. Peripheral B Cell Tolerance
Mature B lymphocytes that identify self antigens in peripheral tissues in the absence of appropriate T helper cells may become inactive or undergo apoptosis. If these T cells are destroyed or anergic, or if the self antigens are nonprotein antigens, helper T cell signals may be lacking. Since self antigens do not induce innate immune responses, B cells will not encounter any of the cytokines or other signals produced during such reactions. Thus, antigen identification without additional stimulation results in tolerance, just as it does in T cells. Peripheral tolerance mechanisms also remove autoreactive B cell clones that may be unintentionally created as a result of somatic mutation in germinal centres.
a. Anergy and deletion
- Some self-reactive B lymphocytes that have been repeatedly stimulated by self antigens lose their ability to respond to additional stimulation. These cells require high levels of the growth factor BAFF/BLys for survival and cannot effectively compete for survival in lymphoid follicles with typical naive B cells that are less dependent on BAFF.
- B cells that have encountered self antigens have a shorter lifespan and are destroyed more quickly than B cells that have not encountered self antigens.
- B cells that attach to self antigens in the peripheral with high avidity may also experience apoptosis via the mitochondrial route regardless of growth factor requirement.
- In germinal centres, the high rate of somatic mutation of Ig genes has the potential to generate self-reactive B cells.
- Through the interaction of FasL on helper T cells and Fas on activated B cells, these B cells may be actively destroyed.
- This interaction has previously been identified as the mechanism underlying the demise of self-reactive T cells.
- Failure of this peripheral B cell tolerance pathway may contribute to the autoimmunity caused by mutations in the Fas and FasL genes in mice, as well as in individuals with the previously mentioned autoimmune lymphoproliferative disease.
b. Signaling by inhibitory receptors
- Engaging multiple inhibitory receptors can decrease the response of B lymphocytes that identify self antigens with low affinity.
- The role of these inhibitory receptors is to establish a threshold for B cell activation, which allows responses to foreign antigens with the assistance of T cells or innate immunity but not to self antigens.
- Studies demonstrating that animals with abnormalities in the SHP-1 tyrosine phosphatase or the CD22 inhibitory receptor develop autoimmune uncovered this peripheral tolerance pathway.
- Lyn phosphorylates ITIM motifs in the cytoplasmic tail of CD22, and this inhibitory receptor then recruits SHP-1, so inhibiting B cell receptor activation.
- However, when inhibitory receptors like as CD22 are activated and what ligands they identify are unknown.
- Animal models, such as genetically modified mice, have contributed significantly to our understanding of the processes of tolerance in T and B cells.
- Active research focuses on applying this knowledge to comprehend the processes of tolerance to diverse self antigens in healthy persons and to determine why tolerance fails, giving rise to autoimmune disorders.
Loss of Immune Tolerance
Loss of immune tolerance refers to a breakdown in the body’s ability to distinguish between self and non-self antigens, leading to the development of autoimmune diseases. Despite the presence of various checkpoints in the immune system to prevent such occurrences, some individuals still experience an abnormal immune response that targets their own tissues and organs.
The underlying causes of loss of immune tolerance are believed to be a complex interplay between genetic and environmental factors. One significant risk factor is genetic predisposition, as autoimmune diseases often exhibit a familial pattern. The human leukocyte antigen (HLA) gene family has been identified as a major contributor to genetic predispositions, accounting for approximately half of the genetic component in autoimmune diseases.
Another noteworthy risk factor is the sex of the individual, with autoimmune diseases being more prevalent in females of childbearing age. This observation suggests a potential influence of hormones, particularly estrogen, on the immune response. The exact mechanisms by which estrogen affects immune function are not fully understood, but it is believed to play a role in modulating the immune system and contributing to the higher susceptibility of females to autoimmune diseases.
Impaired regulatory T cell (Treg) response is also implicated in the loss of immune tolerance. Tregs are a specialized subset of immune cells that play a crucial role in maintaining self-tolerance and preventing excessive immune reactions. In certain autoimmune diseases, the number or function of Tregs may be compromised, leading to an inadequate suppression of self-reactive immune cells and subsequent autoimmunity.
Infections have also been identified as potential triggers for autoimmune conditions. One intriguing mechanism by which infections may contribute to loss of immune tolerance is through molecular mimicry. In molecular mimicry, the immune system fails to distinguish between certain foreign antigens (typically derived from pathogens) and self-antigens that share structural similarities. Consequently, the immune response directed against the pathogen may also target and attack the self-antigens, leading to autoimmunity.
It is important to note that the development of autoimmune diseases is likely multifactorial, involving a combination of these and possibly other factors. The exact triggers and mechanisms may vary depending on the specific autoimmune condition. Ongoing research aims to unravel the complexities of immune tolerance loss and provide insights into potential therapeutic interventions for autoimmune diseases.
Tolerance Induced By Foreign Protein Antigens
- It is possible to give foreign antigens in a manner that induces tolerance rather than immune responses.
- The key to creating antigen-specific tolerance as a therapy option for immunologic illnesses is understanding how to induce tolerance through antigen delivery.
- Protein antigens delivered subcutaneously or intradermally with adjuvants tend to promote immunity, whereas antigens supplied systemically at large dosages without adjuvants tend to generate tolerance.
- In the absence of these second signals, T cells that detect the antigen may become anergic, die, or develop into regulatory cells.
- There are numerous antigen characteristics and administration methods that can affect the equilibrium between immunity and tolerance.
- When a protein antigen is administered orally, humoral and cell-mediated immune responses to immunisation with the same antigen are frequently suppressed. The term for this phenomena is oral tolerance.
- Some systemic infections (such as those caused by viruses) may generate an immune response, but the response is reduced before the virus is eliminated, resulting in a condition of persistent infection.
- In this case, there are virus-specific T cell clones, but they are unable to respond normally and remove the infection. This condition is referred to as clonal exhaustion, suggesting that antigen-specific lymphocyte clones provide an initial response before becoming anergic, or “exhausted.”
- On virus-specific CD8+ T lymphocytes, there is some evidence that clonal depletion is caused by the overexpression of inhibitory receptors such as PD-1.
- HIV-infected people and animal models of persistent viral infection have demonstrated this tendency. How certain bacteria upregulate T cell expression of inhibitory substances is unknown.
- Some pathogens exploit clonal fatigue as a means of evading the immune system, as it can facilitate viral persistence.
- Understanding this process could lead to the development of novel therapeutic approaches for some chronic viral illnesses, such as the use of PD-1– blocking antibodies.
Pathogenesis of Autoimmunity
The following pathogenic mechanisms have been hypothesised for autoimmunity:
- Release of sequestrated antigens
- Antigen alteration
- Epitope spreading
- Molecular mimicry
1. Release of sequestrated antigens
- Sperm, the central nervous system, and the lens and uveal system of the eye are examples of sequestered or concealed tissues.
- For different reasons, these locations are generally never exposed to the immune system. These locations are immunologically favoured.
- When these hidden or sequestered antigens are exposed as a result of an injury, the immune system of the host—both cellular and humoral—does not recognise them as self, but rather as foreign, and therefore assaults them.
- Lens protein, for instance, is contained within its capsule and has no interface with the circulatory system. Therefore, immune tolerance to lens protein does not develop during foetal development.
- When this antigen is released into the bloodstream after an injury or cataract surgery, it induces an immunological reaction that damages the lens of the other eye.
- Similarly, growing sperms reside within the lumen of the testicular tubules, which are walled off early in embryonic development, prior to the formation of the immune system.
- Because these developing sperms are encased in a sheath of densely connected Sertoli cells, immune cells are unable to access them.
- If these are exposed as a result of surgery, vasectomy, or injury, an immune response against the sperm results in aspermatogenesis, which can result in male sterility.
- Intracellular antigens that are generally sequestered from the immune system include DNA, histones, and mitochondrial enzymes.
- However, certain viral or bacterial infections, radiation exposure, and chemical exposure can destroy these cells and release sequestered intracellular antigens into the bloodstream. These antigens provoke a robust immunological response.
- Against these antigens, autoantibodies are generated, which interact with subsequently released sequestered antigens.
- This leads to the creation of immunological complexes, which are responsible for tissue damage. For instance, during a mumps infection, the virus damages the basement membrane of seminiferous tubules, provoking an immunological response and causing orchitis.
2. Antigen alteration
- Certain physical, chemical, or biological stimuli can change tissue antigens, leading to the production of neoantigens on the cell surface.
- These neoantigens are no longer identified as self; hence, they appear foreign to the immune system, provoking an immunological response.
- SLE triggered by procainamide is one example of an autoimmune illness resulting from this mechanism.
3. Epitope spreading
- Epitope spreading refers to the exposure of previously sequestered autoantigens as a result of viral infection-induced cell destruction.
- It is hypothesised that this process contributes to the aetiology of autoimmunity.
- These freshly exposed autoantigens or epitopes trigger autoreactive T cells, leading to the development of autoimmune disorders.
- In experimental animal infections, for instance, an encephalomyelitis virus causes a condition resembling multiple sclerosis.
- In this scenario, self-reactive T lymphocytes target cellular antigens, but not the virus responsible for the sclerosis-like sickness.
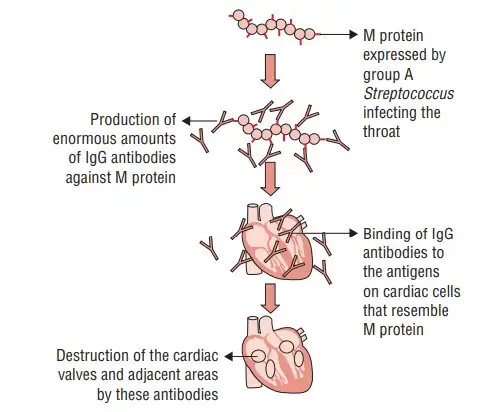
4. Molecular mimicry
- Molecular mimicry is the association between infection with a specific microbial pathogen and the development of specific autoimmune disorders.
Pathological Process of Autoimmune
The pathogenic process of autoimmunity may be begun and maintained by (a) autoantibodies, (b) immune complexes containing autoantigens, and (c) autoreactive T cells. Each of these immunological systems plays a significant role in a variety of diseases or may be synergistically related, especially in multiorgan, systemic autoimmune diseases.
1. Autoantibodies
- Diseases related with autoantibodies are defined by the presence of autoantibodies in the serum and the deposition of autoantibodies in tissues.
- In some diseases, autoantibodies may be actively engaged in the pathogenesis, but in others, they may simply serve as disease indicators with no recognised harmful effect.
- They may also have a role in initiating numerous pathogenic pathways that result in tissue damage and cell death.
- In the aetiology of (a) myasthenia gravis, (b) pemphigus vulgaris, and (c) different autoimmune cytopenias, autoantibodies play a crucial role.
2. Immune complexes containing autoantigens
- In autoimmune illnesses, the development of immunological complexes between self-antigens and autoantibodies, which results in organ destruction, is another pathogenic mechanism.
- Only sufficiently sized immune complexes are able to activate the complement system and participate in the pathogenesis of autoimmune disorders.
- Immune complexes play a significant part in the pathogenesis of systemic lupus erythematosus and polyarteritis nodosa, two exemplary autoimmune disorders.
3. Autoreactive T lymphocytes
- Self-tolerance is not induced by antigens that are sequestered from circulation and, as a result, are not recognised by growing T cells in the thymus.
- Later exposure of mature T cells to typically sequestered antigens could lead to their activation.
- Examples include the induction of autoantibodies to sperms following vasectomy, sympathetic ophthalmitis, and the development of antibodies to myocardial cells following myocardial infarction.
- In certain other circumstances, inappropriate expression of class II MHC molecules can also sensitise self-reactive T cells.
- This is corroborated by clinical observations indicating an increase in the incidence of autoimmune illnesses in families, as well as by higher rates of clinical concordance among monozygotic twins.
- Polyclonal B-cell activation can potentially initiate the onset of an autoimmune illness.
Types of Autoimmune Diseases
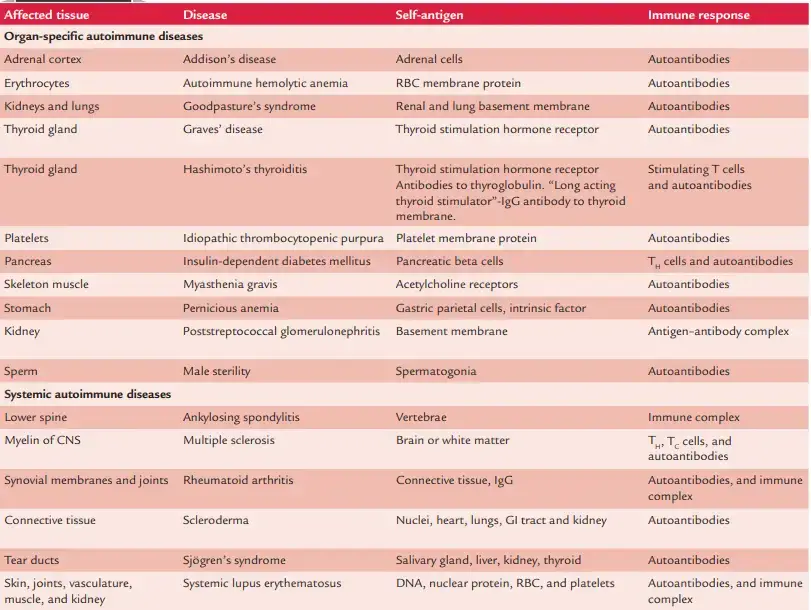
In autoimmune illnesses, different molecules, cells, and tissues are attacked. Table 20-1 = summarises damaged tissue, target antigens, and resulting autoimmune disorders. The autoimmune disorders can be classified generally as
- Organ-specific autoimmune disease.
- Systemic autoimmune diseases
1. Organ-Specific Autoimmune Diseases
These are disorders in which autoantibodies attack the tissue of a specific organ, hence harming only that organ. Addison’s disease, autoimmune hemolytic anaemia, Goodpasture’s syndrome, Graves’ disease, Hashimoto’s thyroiditis, idiopathic thrombocytopenic purpura, insulin-dependent diabetes mellitus, myasthenia gravis, pernicious anaemia, poststreptococcal glomerulonephritis, etc. are a few examples of such conditions. On the basis of tissue damage, these diseases can be further subcategorized as (a) diseases mediated by cell-mediated immunity and (b) autoantibody-mediated diseases.
A. Diseases mediated by the action of cell-mediated immunity
Some disorders where lymphocytes directly facilitate the primary mechanism of cell destruction are as follows:
a. Hashimoto’s thyroiditis
- Hashimoto’s thyroiditis is predominately a subclinical disease in which no thyroid dysfunction is apparent and no treatment is required until the latter stages of disease.
- It is suspected that a cell-mediated autoimmune response induced by unknown causes is responsible for the development of this disease.
- The condition is particularly prevalent in middle-aged women who produce autoantibodies and TH1 cells that are specific for thyroid antigens.
- It is the most prevalent kind of thyroiditis, and its progression is typically chronic. It happens most frequently between the third and fifth decades, with a 10:1 female to male ratio.
- Functionally, the condition is characterised by a sluggish development to hypothyroidism and a gradual onset of symptoms.
- The majority of hypothyroid individuals experience malaise, lethargy, cold intolerance, and constipation. Antithyroglobulin antibodies are typically utilised to confirm the diagnosis.
b. Addison’s disease (chronic primary hypoadrenalism)
- This condition may be caused by exogenous factors (e.g., infection of the adrenal glands by Mycobacterium tuberculosis) or be idiopathic.
- It is hypothesised that the idiopathic type has an immunological basis, as 50% of patients develop autoantibodies to the microsomes of adrenal cells (compared to 5% of the normal population).
- It is hypothesised that autoantibodies directed against the adrenal glands perform the primary role in disease development.
- Addison’s disease is characterised by weakness, fatigue, anorexia, nausea, vomiting, weight loss, and diarrhoea.
- Increased skin pigmentation, vascular collapse, and hypotension are symptoms. The condition ultimately results in adrenal cortical atrophy and loss of function.
- By demonstrating antiadrenal antibodies with an indirect immunofluorescence test, the diagnosis is validated.
- Frequent associations exist between Addison’s disease and other autoimmune disorders, such as thyroiditis, pernicious anaemia, and diabetes mellitus.
c. Autoimmune Anemias
- Pernicious anaemia, autoimmune hemolytic anaemia, and drug-induced hemolytic anaemia are examples of autoimmune anemias.
- Autoantibodies to intrinsic factor, a membrane-bound intestinal protein on gastric parietal cells, cause pernicious anaemia. Intrinsic factor promotes vitamin B12 absorption from the small intestine.
- The binding of autoantibodies to intrinsic factor inhibits vitamin B12 absorption mediated by intrinsic factor. In the absence of adequate vitamin B12, which is required for healthy hematopoiesis, the number of mature, functional red blood cells declines below normal levels.
- Vitamin B12 injections are used to treat pernicious anaemia, thus avoiding the absorption deficiency.
- A person with autoimmune hemolytic anaemia produces autoantibodies to RBC antigens, resulting in complement-mediated lysis or antibody-mediated opsonization and phagocytosis of red blood cells.
- When certain medications, such as penicillin or the antihypertensive methyldopa, interact with red blood cells, the cells become antigenic.
- The immunodiagnostic test for autoimmune hemolytic anemias is often a Coombs test in which red blood cells are treated with anti–human IgG antiserum.
- If IgG autoantibodies are present on the red blood cells, the antiserum will agglutinate the cells.
d. Goodpasture’s Syndrome
- Autoantibodies specific for certain basement membrane antigens bind to the basement membranes of the kidney glomeruli and the alveoli in Goodpasture’s syndrome.
- Subsequent complement activation results in direct cellular injury and an inflammatory response mediated by the accumulation of complement split products.
- Progressive kidney failure and lung bleeding result from damage to the glomerular and alveolar basement membranes.
- Within a few months of the development of symptoms, death may occur. Along the basement membranes of Goodpasture’s syndrome patients’ biopsies stained with fluorescently labelled anti-IgG and antiC3b antibodies are linear deposits of IgG and C3b.
e. INSULIN-DEPENDENT DIABETES MELLITUS
- Insulin-dependent diabetic mellitus (IDDM), a disease affecting 0.2% of the population, is caused by an autoimmune attack on the pancreas.
- The attack targets specialised insulin-producing cells (beta cells) that are dispersed throughout the pancreas in spherical clusters known as the islets of Langerhans.
- The autoimmune onslaught kills beta cells, resulting in decreased insulin production and elevated blood glucose levels. Several causes contribute to the demise of beta cells.
- First, activated CTLs travel inside an islet and initiate an assault on insulin-producing cells. During this response, the local synthesis of cytokines includes IFN-, TNF-, and IL-1. The development of autoantibodies can also be a factor in IDDM.
- The initial CTL invasion and activation of macrophages, also known as insulitis, is followed by cytokine production and the presence of autoantibodies, which results in a cell-mediated DTH response.
- It is believed that cytokines generated during the DTH response and lytic enzymes secreted by activated macrophages are responsible for the eventual death of beta cells.
- Beta cell-specific autoantibodies may contribute to cell death by enhancing antibody-plus-complement lysis or antibody-dependent cell-mediated cytotoxicity (ADCC).
- The irregularities in glucose metabolism produced by the death of islet beta cells lead to severe metabolic issues, including ketoacidosis and an increase in urine output.
- The disease’s late stages are frequently characterised by atherosclerotic vascular lesions, which in turn induce gangrene of the extremities due to impaired vascular flow, renal failure, and blindness. Without treatment, death is possible.
- The most common treatment for diabetes is daily insulin delivery. This is very beneficial in managing the disease, but because random doses are not the same as metabolically regulated continuous and controlled release of the hormone, regularly injected doses of insulin do not completely eliminate the disease’s complications.
- A further complication of diabetes is that it can lie undiscovered for several years, allowing irreversible pancreatic tissue loss to develop prior to treatment.
B. Some Autoimmune Diseases Are Mediated by Stimulating or Blocking Auto-Antibodies
In certain autoimmune illnesses, antibodies function as agonists, attaching to hormone receptors in place of the normal ligand and promoting aberrant activity. This typically results in an increase in mediator production or cell proliferation. Autoantibodies may also behave as antagonists, binding hormone receptors but inhibiting receptor action. This typically results in decreased mediator secretion and progressive organ atrophy. Listed below are a few of this group’s most prominent representative disorders:
Myasthenia gravis
- Myasthenia gravis is the prototypical autoimmune disease caused by antibodies that inhibit the immune system. It is a neuromuscular transmission disorder.
- This condition is characterised by the production of autoantibodies that bind to acetylcholine receptors on the motor end-plates of muscles.
- These antibodies inhibit the normal binding of acetylcholine and cause complement-mediated cell death.
- Myasthenia gravis is typically characterised by an increase in fatigue and weakening in the muscles, which is exacerbated by physical activity.
- Typically, extraocular muscles are the first to show signs of weakness, resulting in diplopia or ptosis. Additionally, the cheeks, tongue, and upper extremities are usually affected. Typically, proximal skeletal muscle involvement is observed.
- Typically, the condition is characterised by spontaneous remission intervals. In myasthenia gravis, thymic anomalies are common. Approximately 10% of patients acquire malignant thymus tumours (thymomas).
- Antibodies against the antiacetylcholine receptor corroborate the diagnosis.
Graves’ disease
- Graves’ disease, also known as thyrotoxicosis, diffuse toxic goitre, and exophthalmic goitre, is caused by autoantibodies directed against the thyrotrophic hormone (thyroid-stimulating hormone [TSH]) receptor (TSH receptor antibodies).
- In Graves’ disease, the TSH receptor antibodies (also known as long-acting thyroid stimulator, thyroid-stimulating immunoglobulin, and thyroid-stimulating antibodies) boost thyroid gland activity.
- After attaching to the TSH receptor, these antibodies can increase the production of thyroid hormones by activating the adenylate cyclase system.
- Approximately 80–90% of patients with Graves’ illness exhibit these antibodies, which are often of the IgG isotype.
- Exophthalmos, often known as protruding eyeballs, is the typical manifestation of the illness.
- In addition to an accelerated metabolic rate and weight loss, other hyperthyroidism symptoms include nervousness, weakness, sweating, heat sensitivity, and loose stools.
- This condition is more prevalent in thirty-year-old women. Thyroid gland biopsies reveal widespread lymphoplasmacytic interstitial infiltration.
- There are elevated levels of thyroid hormones (triiodothyronine, or T3, and thyroxine, or T4), higher absorption of T3, and antithyroid receptor antibodies, as determined by laboratory tests.
2. Systemic Autoimmune Diseases
In systemic autoimmune disorders, the immune response is directed against a broad spectrum of target antigens and affects multiple organs and tissues. These disorders are the outcome of a systemic deficiency in immune control that causes T cells and B cells to be overactive. Widespread tissue damage results from both cell-mediated immune responses and direct cellular damage induced by autoantibodies or immune complex buildup.
Systemic Lupus Erythematosus Attacks Many Tissues
- The ratio of female to male patients is 10:1 for systemic lupus erythematosus (SLE), which is one of the greatest instances of a systemic autoimmune illness.
- Fever, weakness, arthritis, skin rashes, pleurisy, and renal dysfunction are characteristics of SLE.
- Lupus is more common among African-American and Hispanic women than in Caucasian women, but the reason for this is unknown.
- Affected individuals may manufacture autoantibodies against a wide range of tissue antigens, including DNA, histones, RBCs, platelets, leukocytes, and clotting factors; the interaction of these auto-antibodies with their specific antigens results in a variety of symptoms.
- For example, RBC- and platelet-specific autoantibodies can trigger complement-mediated lysis, resulting in hemolytic anaemia and thrombocytopenia, respectively.
- When immunological complexes of autoantibodies with diverse nuclear antigens are deposited along the walls of tiny blood vessels, a hypersensitive reaction of type III ensues.
- The complexes activate the complement system and form membrane-attack complexes and complement split products that cause vasculitis and glomerulonephritis by damaging the blood vessel wall.
- In individuals with severe SLE, excessive complement activation results in blood levels of the complement split products C3a and C5a that are three to four times greater than usual.
- C5a causes enhanced expression of complement receptor type 3 (CR3) on neutrophils, hence promoting neutrophil aggregation and adhesion to vascular endothelium.
- As neutrophils adhere to small blood vessels, the quantity of neutrophils in circulation decreases (neutropenia), and various occlusions of small blood vessels arise (vasculitis). These obstructions can cause extensive tissue harm.
- The typical antinuclear antibodies directed against double- or single-stranded DNA, nucleoprotein, histones, and nucleolar RNA are used to diagnose SLE in the laboratory. The indirect immunofluorescent labelling of nuclei with serum from SLE patients generates distinct nucleus staining patterns.
Multiple Sclerosis Attacks the Central Nervous System
- Multiple sclerosis (MS) is the most prevalent disease-related cause of neurologic impairment in Western nations.
- Mild symptoms include tingling in the limbs, whereas severe symptoms include paralysis or eyesight loss. The majority of MS diagnoses occur between the ages of 20 and 40.
- This condition is characterised by the production of autoreactive T lymphocytes that contribute to the development of inflammatory lesions along the myelin sheath of nerve fibres.
- Patients with active MS have activated T cells in their cerebrospinal fluid, which infiltrate brain tissue and generate distinctive inflammatory lesions that degrade myelin.
- Myelin insulates the nerve fibres; hence, a breakdown in the myelin sheath results in a variety of neurologic dysfunctions. Epidemiological research reveal that MS is more prevalent in the Northern hemisphere and, surprisingly, the United States.
- Those living north of the 37th parallel have a prevalence of 110–140 cases per 100,000 people, whereas those living south of the 37th parallel have a prevalence of 57–78 cases per 100,000 people.
- And individuals from south of the 37th parallel who migrate north before the age of 15 take a new risk.
- These startling statistics show that the risk of developing MS is influenced by environmental factors.
- However, this is not the whole picture, as genetic impacts are equally significant. While the average person in the United States has a 1 in 1000 probability of acquiring MS, close relatives, such as children and siblings, have a 1 in 50 to 100 chance of developing the disease.
- One in three identical twins of a person with MS will develop the condition. These findings are highly suggestive of the hereditary component of the disease. In addition, as mentioned in this chapter’s Clinical Focus, MS affects women two to three times more commonly than males.
- As with most autoimmune disorders, the cause of MS is poorly understood. Nevertheless, there is some evidence that infection with some viruses may predispose a person to MS.
- Certainly some viruses can cause demyelinating disorders, and it is tempting to hypothesise that virus infection plays a substantial role in MS, however there is currently no conclusive evidence implicating a specific virus.
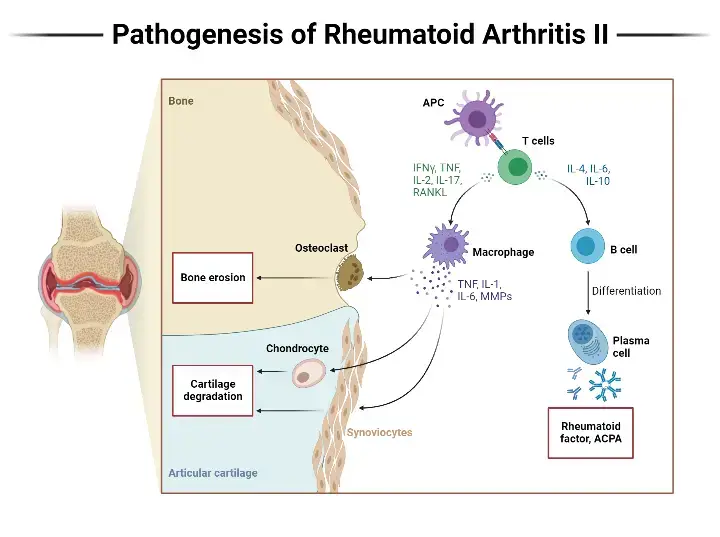
Rheumatoid Arthritis Attacks Joints
- Rheumatoid arthritis is a prevalent autoimmune illness that affects women between the ages of 40 and 60.
- However, the hematologic, cardiovascular, and pulmonary systems are usually affected as well.
- Numerous patients with rheumatoid arthritis develop rheumatoid factors, a collection of autoantibodies reacting with determinants in the Fc region of IgG.
- This specific IgM antibody is the conventional rheumatoid factor. These autoantibodies combine with normal circulating IgG to generate IgM-IgG complexes that are deposited in the joints.
- These immune complexes can activate the complement cascade, resulting in a type III hypersensitivity reaction that causes chronic joint inflammation.
Treatment of Autoimmune Diseases
- Ideally, treatment for autoimmune illnesses should target only the autoimmune response while leaving the remainder of the immune system intact. This aim has not yet been achieved.
- Current treatments for autoimmune disorders are not curative, but rather palliative, with the goal of lowering symptoms to offer an acceptable quality of life for the patient.
- In general, these treatments inhibit the immune system in a non-specific manner and, as a result, cannot discriminate between a pathological autoimmune response and a protective immunological response.
- Immunosuppressive medicines (e.g., corticosteroids, azathioprine, and cyclophosphamide) are frequently administered to inhibit lymphocyte growth.
- Such medications can lower the severity of autoimmune symptoms by suppressing the immune response in general.
- The general decrease in immunological reactivity, however, increases the patient’s susceptibility to infection and malignancy. Using cyclosporin A or FK506 to treat autoimmunity is a considerably more targeted method.
- These drugs inhibit only antigen-activated T cells while sparing nonactivated T cells by blocking signal transduction mediated by the T-cell receptor.
- In some cases of myasthenia gravis, the excision of the thymus has proven to be an effective therapeutic method.
- Because patients with this condition frequently have thymic abnormalities (such as thymic hyperplasia or thymomas), adult thymectomy frequently increases the probability of symptom remission.
- Plasmapheresis may provide patients with Graves’ illness, myasthenia gravis, rheumatoid arthritis, or systemic lupus erythematosus a short-term benefit. Using continuous-flow centrifugation, plasma is separated from a patient’s blood in this procedure.
- The red blood cells are subsequently reconstituted in an appropriate medium and returned to the patient. Plasmapheresis has been advantageous for patients with autoimmune illnesses involving antigen-antibody complexes, which are eliminated with the plasma.
- Even though removal of the complexes is transitory, it can result in a transient reduction in symptoms.
- Positively, animal models of experimental autoimmunity have demonstrated that it is possible to establish specific immunity against the development of autoimmunity.
T-Cell Vaccination Is a Possible Therapy
- Experiments with the EAE animal model provided the foundation for T-cell vaccination as a therapy for several autoimmune disorders.
- EAE symptoms were not observed in rats treated with low concentrations (10–4) of cloned T cells specific for MBP. In contrast, when they were later confronted with a lethal dose of activated MBP-specific T cells or MBP in adjuvant, they became resistant to the development of EAE.
- By crosslinking the cell-membrane components with formaldehyde or glutaraldehyde, it was discovered that the efficiency of these autoimmune T-cell clones as a vaccine may be improved.
- When mice with active EAE were injected with crosslinked T cells, lasting remission of symptoms was found.
- Apparently, the crosslinked T cells induce regulatory T cells specific for TCR variable-region determinants of autoimmune clones.
- Presumably, these regulatory T cells suppress the autoantibody-producing T cells that cause EAE.
Peptide Blockade of MHC Molecules Can Modulate Autoimmune Responses
- The identification and sequencing of numerous autoantigens has led to the development of novel techniques for modulating the activity of autoimmune T-cells.
- For instance, in EAE, the encephalitogenic peptides of MBP have been thoroughly described.
- It has been demonstrated that synthetic peptides varying from their MBP equivalent by a single amino acid can attach to the corresponding MHC molecule.
- In addition, the clinical development of EAE was prevented when significant doses of this peptide were delivered with the comparable encephalitogenic MBP peptide.
- Presumably, the synthetic peptide acts as a competitor by occupying the antigen-binding cleft on MHC molecules, so preventing the MBP peptide from binding.
- In other research, inhibiting peptides complexed to soluble class II MHC molecules prevented the clinical development of EAE in mice by generating clonal anergy in the autoimmune T cells.
Monoclonal Antibodies May Be Used to Treat Autoimmunity
- Several animal models have been successfully treated with monoclonal antibodies for autoimmunity. High percentages of (NZB NZW) F1 mice who received weekly injections of high doses of monoclonal antibody specific for the CD4 membrane molecule were able to recover from their lupus-like autoimmune symptoms.
- Anti-CD4 monoclonal antibody treatment resulted in the elimination of lymphocytic infiltration and diabetes symptoms in NOD mice.
- Due to the fact that anti-CD4 monoclonal antibodies inhibit or deplete all TH cells, independent of their specificity, they pose a hazard to the recipient’s overall immunological response.
- Attempting to block only antigen-activated TH cells, as these cells are engaged in the autoimmune state, is one solution for this drawback.
- To do this, researchers employed a monoclonal antibody directed against the component of the highaffinity IL-2 receptor, which is only produced by antigen-activated TH cells.
- Due to the increased expression of the IL-2R subunit on autoimmune T cells, monoclonal antibody against the subunit (anti-TAC) may preferentially inhibit autoreactive T cells.
- This strategy was evaluated on adult rats injected with activated MBP-specific T lymphocytes with or without anti-TAC. All of the control rats died from EAE, but six of the nine rats treated with anti-TAC exhibited no symptoms and the remaining three exhibited minor symptoms.
- Several animal models demonstrating a link between autoimmune illness and restricted TCR expression have encouraged researchers to investigate whether blocking the favoured receptors with monoclonal antibody could be therapeutic.
- By injecting PL/J mice with a monoclonal antibody specific for the V 8.2 T-cell receptor, MBP-induced EAE was avoided.
- Even more encouraging was the discovery that the V 8.2 monoclonal antibody could also correct the symptoms of autoimmunity in mice with induced EAE, and that these mice displayed long-lasting remission.
- Clearly, the use of monoclonal antibodies as a therapy for autoimmune disorders in humans presents promising prospects. Similarly, the relationship between certain MHC alleles and autoimmunity, as well as the evidence for excessive or improper MHC expression in some autoimmune diseases, suggests that monoclonal antibodies against appropriate MHC molecules may inhibit the development of autoimmunity.
- Furthermore, since antigen-presenting cells express a variety of class II MHC molecules, it should be able to selectively inhibit an MHC molecule linked with autoimmunity while sparing the others.
- In one study, the development of EAE was prevented by injecting mice with monoclonal antibodies to class II MHC molecules before administering MBP.
- If the antibody was administered after MBP injection, EAE development was delayed but not halted. Monoclonal antibodies against HLA-DR and HLA-DQ have been found to reverse EAE in nonhuman monkeys.
Oral Antigens Can Induce Tolerance
- When antigens are delivered orally, they tend to elicit tolerance, a condition of immunologic indifference. As indicated earlier in this chapter, mice fed MBP do not develop EAE after receiving a subsequent MBP injection.
- This finding led to a double-blind pilot study in which 30 patients with multiple sclerosis were given a placebo or 300 mg of bovine myelin daily for a year.
- The results of this trial indicated that T lymphocytes specific for MBP were reduced in the myelin-fed group; there was also some evidence that MS symptoms were reduced in male receivers (albeit the reduction was not statistically significant) but not in female recipients.
- While the results of oral tolerance induction in mice were promising, it does not appear that the same is true for humans.
- However, human clinical trials are still in their infancy, and it is possible that the peptides utilised thus far were not the most effective, or that the dosages were incorrect.
- In the coming years, it is probable that further clinical trials will be done due to the promise demonstrated by animal research.
Examples of autoimmune disorders
1. Multiple Sclerosis
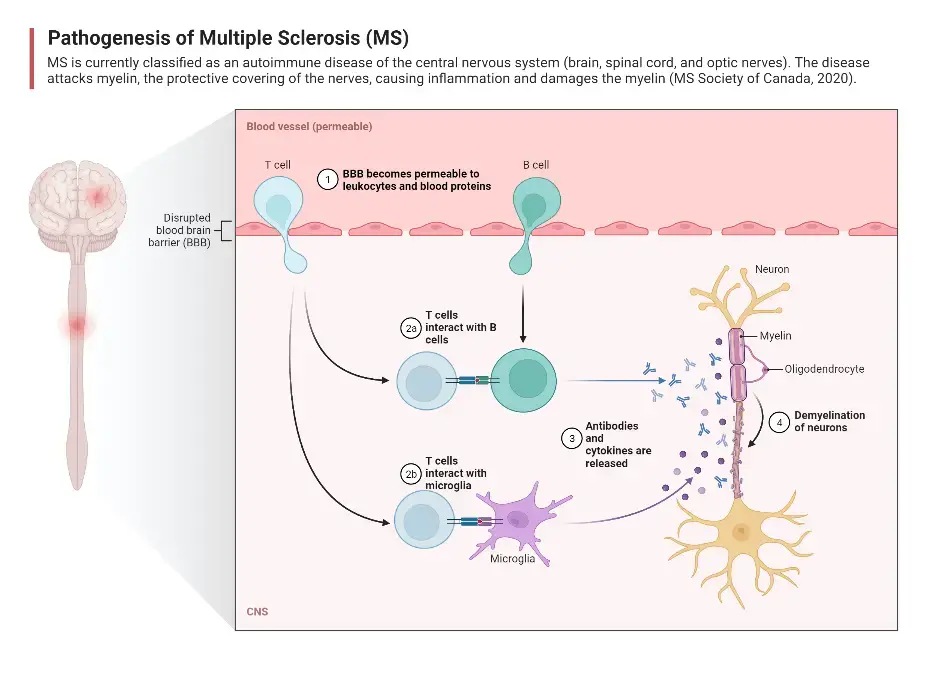
- Multiple Sclerosis (MS) is a chronic inflammatory demyelinating disease that primarily affects the central nervous system (CNS). In MS, the immune system mistakenly targets and damages the myelin sheath, a protective layering that surrounds nerve fibers in the CNS. This immune attack leads to inflammation and disrupts the normal transmission of signals between the brain and the rest of the body.
- The exact cause of MS is still unknown, but it is believed to involve a combination of genetic and environmental factors. It is thought that activated T cells and macrophages, immune cells normally responsible for defending against infections, mistakenly enter the CNS by crossing the blood-brain barrier. Once inside, they target and attack the myelin sheath, leading to inflammation and damage to nerve fibers.
- The symptoms of MS can vary greatly from person to person, depending on the location and extent of the damage within the CNS. Common symptoms include numbness or weakness in one side of the body, including the hands and legs. Some individuals may experience electric shock-like sensations while bending the neck. Other symptoms can include a lack of coordination, difficulty walking properly, blurry vision, and fatigue.
- It is important to note that while MS is a chronic condition with no known permanent cure, there are treatments available to manage and alleviate symptoms. These treatments aim to reduce inflammation, modify the immune response, and provide symptomatic relief. Medications such as corticosteroids, immunomodulators, and disease-modifying therapies may be prescribed based on the individual’s specific needs and disease progression.
- In addition to medical treatment, individuals with MS may benefit from various supportive measures. Physical therapy, occupational therapy, and exercise can help improve mobility, strength, and overall well-being. Additionally, lifestyle modifications, such as stress management techniques and a healthy diet, may contribute to symptom management and overall quality of life.
2. Rheumatoid Arthritis
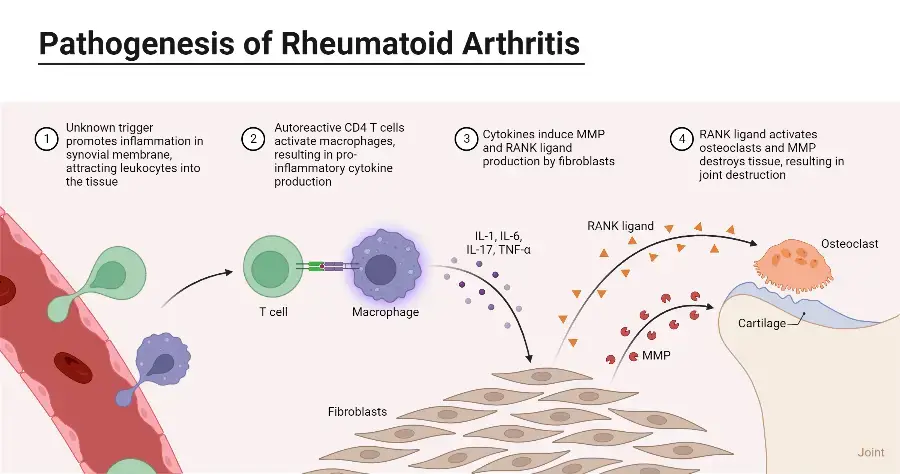
- Rheumatoid Arthritis (RA) is a chronic systemic autoimmune disorder that primarily affects the synovial joints’ lining. It is characterized by inflammation in the joints, which can lead to progressive disability, premature death, and significant socioeconomic burdens.
- RA is more commonly observed in females than males, and it is more prevalent in elderly individuals. The exact cause of RA is still unknown, but it is believed to involve a combination of genetic and environmental factors. The immune system mistakenly attacks the body’s own tissues, particularly the synovial membrane lining the joints, leading to chronic inflammation and damage.
- Symptoms of rheumatoid arthritis can vary in severity and may include arthralgia (joint pain), swelling and redness in the joints, limited joint motion, weight loss, fatigue, and fever. The symptoms can fluctuate, with periods of increased inflammation known as disease flares, and periods of remission or decreased symptoms.
- While there is currently no permanent cure for rheumatoid arthritis, various treatments are available to manage the symptoms and slow down disease progression. The primary goal of treatment is to reduce inflammation, relieve pain, preserve joint function, and improve the individual’s quality of life. Medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), and biologic therapies may be prescribed based on the severity of the disease and the individual’s specific needs.
- In addition to medical treatment, lifestyle modifications can play a significant role in managing RA symptoms. Regular exercise, physical therapy, and occupational therapy can help improve joint flexibility, strength, and overall function. Assistive devices and adaptive strategies may be recommended to support daily activities and reduce joint stress. Additionally, managing stress, maintaining a balanced diet, and getting adequate rest and sleep can contribute to overall well-being.
- Interestingly, breastfeeding has been associated with a decreased rate of occurrence of rheumatoid arthritis in females. It is believed that hormonal changes during pregnancy and breastfeeding may have a protective effect against the development of RA.
3. Type 1 Diabetes
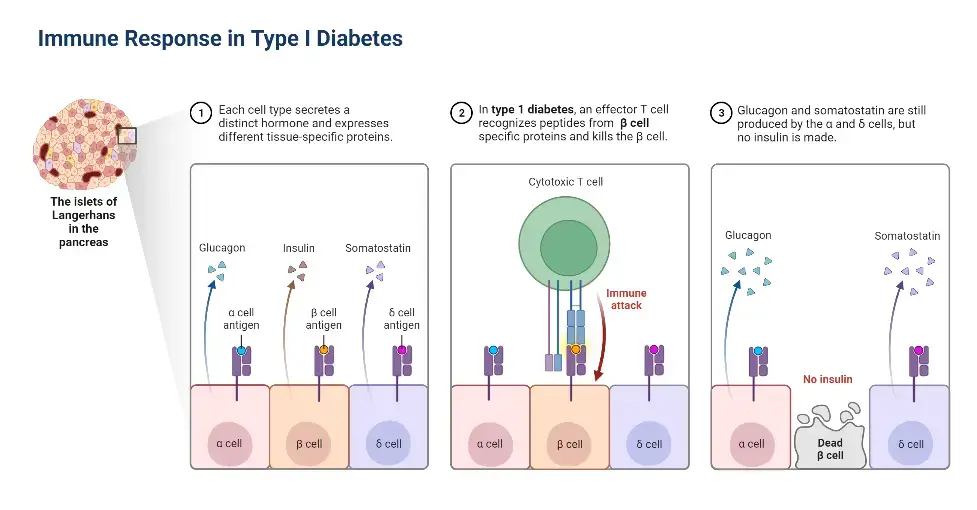
- Type 1 Diabetes (T1D) is a T-cell-mediated autoimmune disease characterized by the destruction of pancreatic β-cells, which results in insulin deficiency. Insulin is crucial for regulating blood sugar levels, so the lack of insulin leads to hyperglycemia and a tendency towards ketoacidosis.
- T1D commonly presents in childhood or adolescence, but it can develop at any age. It represents about 5-10% of all diabetes cases, with the majority of individuals having type 2 diabetes or other forms of diabetes.
- In T1D, autoimmune cells target and destroy the beta cells in the pancreas, which are responsible for producing insulin. Without insulin, glucose cannot enter the cells properly, resulting in elevated blood sugar levels.
- There are specific antibody markers of autoimmunity associated with T1D. These include islet-cell autoantibodies, which are antibodies targeting various components of the pancreatic islets, such as insulin, glutamic acid decarboxylase (GAD), and tyrosine phosphatases IA-2 and IA-2β. Another antibody marker is ZnT8 (Zinc Transporter 8).
- Studies have shown a genetic predisposition to T1D, with certain human leukocyte antigen (HLA) genes, particularly DQA and DQB, being linked to susceptibility to the disease. However, genetics alone cannot explain the development of T1D, and environmental triggers likely play a role.
- The symptoms of T1D include weight loss, frequent urination, impaired vision, and hunger attacks. These symptoms arise due to the inability of the body’s cells to effectively utilize glucose as an energy source and the resultant high levels of sugar in the bloodstream.
- Currently, there is no cure for T1D, and treatment involves lifelong insulin therapy to replace the deficient insulin. This can be administered through injections or insulin pumps. Additionally, blood sugar monitoring, a balanced diet, regular exercise, and proper diabetes management are essential for maintaining stable blood sugar levels and preventing complications.
- Managing T1D requires a multidisciplinary approach involving healthcare professionals, such as endocrinologists, diabetes educators, and dietitians. Ongoing research aims to better understand the underlying mechanisms of T1D and develop more advanced treatment options, including potential immunotherapies and technologies to improve glucose control and reduce the burden of the disease on individuals living with T1D.
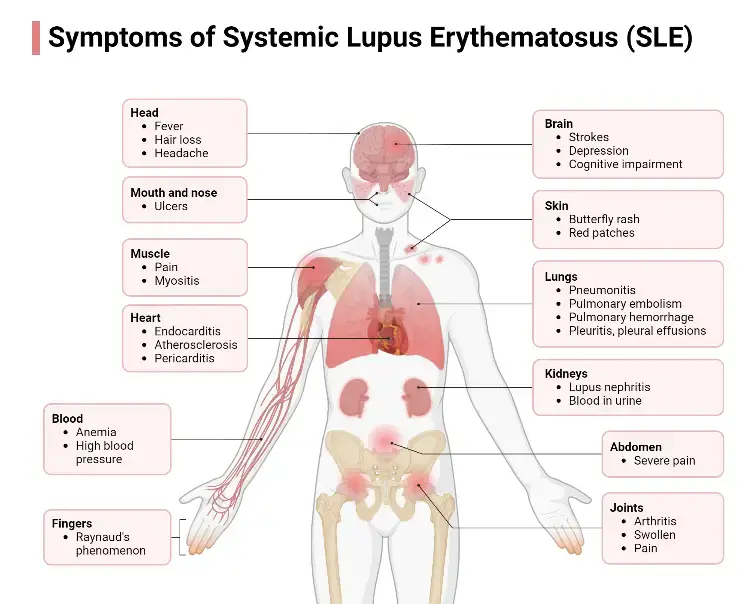
4. Systemic Lupus Erythematosus
- Systemic Lupus Erythematosus (SLE) is a systemic autoimmune disease characterized by inflammation that can affect multiple organs throughout the body. The immune system produces autoantibodies that target nucleic acids and their binding proteins, leading to a loss of self-tolerance.
- In SLE, the innate immune system plays a significant role in initiating the inflammatory response. It produces inflammatory cytokines that contribute to tissue injury and activates autoreactive B and T cells, which further contribute to organ damage.
- The symptoms of SLE can vary widely among individuals. Common symptoms include fever, skin rashes (such as the characteristic butterfly rash across the face), pain or swelling in the joints, sensitivity to sunlight, and oral ulcers. SLE can also affect various organs, including the heart, kidneys, and lungs, leading to complications in these systems. In some cases, individuals with SLE may experience neurological symptoms, such as psychosis.
- The diagnosis of SLE is based on a combination of clinical symptoms, laboratory tests, and the presence of specific autoantibodies. These autoantibodies, such as antinuclear antibodies (ANA) and anti-double-stranded DNA antibodies (anti-dsDNA), are commonly found in individuals with SLE and aid in confirming the diagnosis.
- The management of SLE involves a combination of medications and lifestyle modifications. Medications may include nonsteroidal anti-inflammatory drugs (NSAIDs) for pain and inflammation, corticosteroids to suppress the immune response during flares, and immunosuppressants to reduce the activity of the immune system. Additionally, individuals with SLE are advised to protect themselves from sun exposure, as ultraviolet (UV) light can trigger or worsen symptoms.
- A multidisciplinary approach involving rheumatologists, dermatologists, nephrologists, and other specialists is often required to manage SLE effectively. Regular monitoring of organ function and disease activity is important to adjust treatment as needed and minimize potential complications.
- Ongoing research aims to improve our understanding of the underlying mechanisms of SLE and develop more targeted therapies. While there is currently no cure for SLE, advancements in treatment options and strategies continue to provide hope for better management and improved quality of life for individuals living with this complex autoimmune disease.
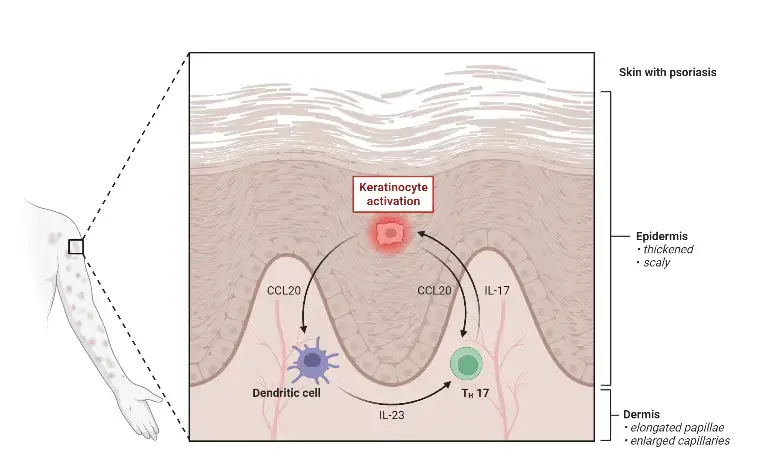
5. Psoriasis
- Psoriasis is a chronic inflammatory skin disease characterized by the development of scaly red patches on the skin. It is considered an autoimmune disease and is often associated with comorbidities, such as psoriatic arthritis, which affects the joints.
- The pathogenesis of psoriasis involves the dysregulation of the immune system, particularly the interleukin-23 (IL-23) and interleukin-17 (IL-17) axis. This inflammatory pathway plays a crucial role in the development and progression of psoriasis. Figure: The IL-23/IL-17 Axis in Psoriasis.
- The symptoms of psoriasis can vary in severity and presentation. The most common symptom is the presence of scaly red patches on the skin, often with defined edges and silvery-white scales. These patches can appear on any part of the body but are commonly found on the elbows, knees, scalp, and lower back. Psoriasis can also affect the nails, causing them to become thick, pitted, or ridged.
- In addition to skin manifestations, individuals with psoriasis may experience symptoms related to psoriatic arthritis, such as stiff and swollen joints. Psoriasis can also lead to extreme dryness of the skin, which may result in bleeding or cracking. Itching, burning, and pain can accompany the lesions, further impacting the quality of life for affected individuals.
- While there is no permanent cure for psoriasis, several treatment options are available to manage symptoms and improve the condition. These treatments aim to reduce inflammation, slow down skin cell turnover, and alleviate discomfort. Topical medications, such as corticosteroids and retinoids, can be used to target localized areas. Phototherapy, which involves exposing the skin to ultraviolet light, is another treatment option. In more severe cases, systemic medications like methotrexate (an immunosuppressant) or biologic agents may be prescribed to target the underlying immune dysfunction.
- It is important to note that psoriasis is a chronic condition that can show symptoms irregularly and may even remain dormant for extended periods. Treatment plans may need to be adjusted over time to address changing symptoms and disease activity.
- Living with psoriasis involves self-care measures to manage symptoms and minimize triggers. These include keeping the skin moisturized, avoiding irritants, managing stress levels, and maintaining a healthy lifestyle.
- Regular follow-up with a dermatologist or healthcare provider who specializes in psoriasis is essential to monitor the condition, adjust treatment plans as needed, and address any concerns or complications that may arise.
- While there is currently no permanent cure for psoriasis, ongoing research continues to enhance our understanding of the disease and improve treatment options, offering hope for better management and improved quality of life for individuals living with psoriasis.
6. Dermatomytosis
- Dermatomyositis is an autoimmune disease characterized by the body’s immune system mistakenly targeting its own cells as foreign. It can result from various factors, including genetic predisposition, environmental triggers, infections, medications, or even cancer, particularly in older individuals.
- The condition leads to inflammation in the muscles and skin. The exact cause of dermatomyositis is not fully understood, but it is believed to involve an abnormal immune response.
- The symptoms of dermatomyositis can vary from person to person. Common symptoms include painful rashes resembling a sunburn, swelling of the upper eyelids, the presence of red and purple patches on the knees, elbows, knuckles, and toes, stiffness and pain in the joints, hair thinning accompanied by dry skin, red fingernails, weakness in the muscles around the hips, back, shoulders, and neck, weight loss, and fatigue.
- Diagnosing dermatomyositis typically involves a combination of medical history evaluation, physical examination, blood tests to check for specific markers of inflammation and autoimmunity, electromyogram (EMG) to assess muscle function, magnetic resonance imaging (MRI) to visualize muscle and tissue abnormalities, and in some cases, a biopsy of the skin or muscle to confirm the diagnosis.
- The treatment of dermatomyositis aims to alleviate symptoms and suppress the autoimmune response. Immunosuppressive drugs are commonly prescribed, such as azathioprine, methotrexate, or tacrolimus, to reduce the activity of the immune system. Corticosteroids may also be used to control inflammation.
- In addition to medication, physical therapy and exercise programs can help improve muscle strength and mobility. Sun protection measures, including wearing sunscreen and protective clothing, are crucial due to increased photosensitivity in individuals with dermatomyositis.
- Regular follow-up with healthcare professionals, including dermatologists and rheumatologists, is important to monitor disease activity, adjust treatment plans as needed, and address any complications or concerns that may arise.
- Living with dermatomyositis requires a multidisciplinary approach and self-care measures. It is important to maintain overall health, manage stress, and communicate openly with healthcare providers to optimize management of the condition and improve quality of life.
- While dermatomyositis is a chronic condition, proper treatment and ongoing care can help individuals manage symptoms, reduce inflammation, and minimize the impact of the disease on daily life activities.
7. Celiac Disease
- Celiac disease, also known as celiac sprue, nontropical sprue, or gluten-sensitive enteropathy, is a chronic autoimmune disorder that primarily affects the small intestine. It is triggered by the consumption of gluten, a protein found in wheat, barley, rye, and their derivatives.
- Celiac disease is estimated to affect approximately 1% of the world’s population. When individuals with celiac disease consume gluten, their immune system responds by attacking the lining of the small intestine, leading to inflammation and damage to the villi, which are small finger-like projections responsible for nutrient absorption.
- The symptoms of celiac disease can vary widely among individuals. Common symptoms include bloating, chronic diarrhea, constipation, gas, lactose intolerance, nausea and vomiting, abdominal pain, and fatigue. Some individuals may also experience weight loss, anemia, bone or joint pain, skin rashes, or even neurological symptoms.
- Diagnosing celiac disease typically involves a combination of genetic testing, blood tests to detect specific antibodies associated with the condition, and biopsies of the small intestine. Genetic testing can help identify certain genetic markers associated with celiac disease, while blood tests can detect antibodies that are typically elevated in individuals with the condition. Confirmation of the diagnosis is often done through an endoscopic biopsy, where small tissue samples are taken from the small intestine for microscopic examination.
- The only treatment for celiac disease is strict adherence to a lifelong gluten-free diet. This involves completely avoiding foods and products that contain gluten, including wheat, barley, rye, and their derivatives. With a gluten-free diet, the inflammation in the small intestine subsides, and the intestinal lining can heal, leading to a reduction or resolution of symptoms. It is important to work with a registered dietitian or healthcare professional with expertise in celiac disease to ensure a well-balanced and nutritious gluten-free diet.
- While there is no permanent cure for celiac disease, following a strict gluten-free diet can effectively manage the condition and prevent long-term complications. It is essential for individuals with celiac disease to educate themselves about hidden sources of gluten and carefully read food labels. Regular follow-up with healthcare providers is crucial to monitor the response to the gluten-free diet, assess nutritional status, and address any concerns or complications.
- Living with celiac disease may require adjustments in lifestyle, dining habits, and social interactions to maintain a gluten-free diet. With proper management and adherence to a gluten-free lifestyle, individuals with celiac disease can lead healthy and fulfilling lives.
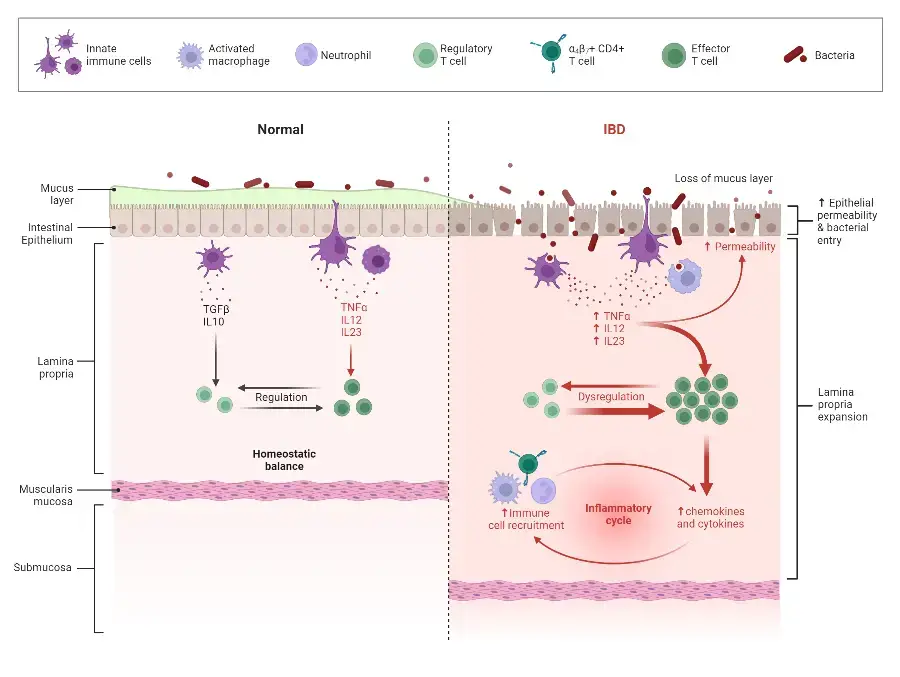
8. Inflammatory Bowel Disease: Ulcerative Colitis and Crohn’s Disease
- Inflammatory Bowel Disease (IBD) is a term used to describe two chronic inflammatory conditions of the gastrointestinal (GI) tract: Crohn’s disease and ulcerative colitis.
- Crohn’s disease can affect any part of the GI tract, although it most commonly affects the small intestine and the area of damage appears in patches. The inflammation associated with Crohn’s disease can involve all layers of the GI tract, leading to a variety of symptoms and potential complications.
- Ulcerative colitis, on the other hand, specifically affects the large intestine (colon) and rectum. Unlike Crohn’s disease, the inflammation in ulcerative colitis is continuous and does not appear in patches. It primarily affects the innermost layer of the large intestine, leading to characteristic symptoms and complications.
- The symptoms of both Crohn’s disease and ulcerative colitis can vary from person to person, but common symptoms include persistent diarrhea, abdominal pain, rectal bleeding or bloody stools, weight loss, and fatigue. Other symptoms may include fever, loss of appetite, and anemia.
- The diagnosis of IBD is typically made through a combination of medical history evaluation, physical examination, and diagnostic procedures such as endoscopy and colonoscopy. These procedures allow the healthcare provider to directly visualize the GI tract and obtain tissue samples for further analysis.
- Treatment for IBD aims to reduce inflammation, control symptoms, and promote long-term remission. Medications such as anti-inflammatory drugs, immune system suppressors, and biologic therapies may be prescribed, depending on the severity and specific characteristics of the disease. In some cases, surgery may be necessary to remove damaged portions of the GI tract or address complications.
- Managing IBD also involves lifestyle modifications and dietary changes. Stress management techniques, regular exercise, and a well-balanced diet can help improve overall well-being and minimize symptom flare-ups. Some individuals may benefit from working with a registered dietitian to identify trigger foods and optimize their nutritional intake.
- Living with IBD requires ongoing medical care and regular follow-up with healthcare professionals specializing in gastroenterology. These specialists can monitor disease activity, adjust treatment plans as needed, and provide guidance on managing symptoms and preventing complications.
- While there is currently no known cure for Crohn’s disease or ulcerative colitis, with appropriate treatment and self-care measures, many individuals with IBD are able to achieve and maintain remission, leading productive and fulfilling lives. It is important for individuals with IBD to stay informed, seek support from patient advocacy groups, and actively participate in their own care to effectively manage the condition.
FAQ
What is autoimmunity?
Autoimmunity is a condition in which the immune system mistakenly attacks and damages the body’s own healthy cells, tissues, and organs.
What are some common autoimmune diseases?
Common autoimmune diseases include rheumatoid arthritis, lupus, multiple sclerosis, type 1 diabetes, celiac disease, psoriasis, and Hashimoto’s thyroiditis, among others.
How does autoimmunity affect the body?
Autoimmunity can cause inflammation, tissue damage, and dysfunction in the affected organs or tissues. The specific symptoms depend on the autoimmune disease and the organs or tissues involved.
Is autoimmunity hereditary?
There is a genetic component to autoimmunity, meaning that certain genes can increase the risk of developing autoimmune diseases. However, not everyone with these genes will develop an autoimmune condition, indicating that other factors are involved.
Can autoimmunity be cured?
Currently, there is no cure for most autoimmune diseases. Treatment focuses on managing symptoms, reducing inflammation, and suppressing the immune system to minimize damage to the body.
How is autoimmunity diagnosed?
Diagnosing autoimmunity often involves a combination of medical history assessment, physical examination, blood tests to detect specific autoantibodies, and sometimes imaging or tissue biopsy.
What causes autoimmunity?
The exact cause of autoimmunity is not fully understood, but it is believed to involve a combination of genetic predisposition and environmental triggers, such as infections, toxins, or certain medications.
Are there any risk factors for developing autoimmunity?
Besides genetic predisposition, factors such as gender (many autoimmune diseases are more common in women), certain infections, hormonal changes, and exposure to certain environmental triggers may increase the risk of developing autoimmunity.
Can lifestyle choices influence autoimmunity?
While lifestyle choices alone may not cause autoimmunity, they can play a role in managing symptoms and overall well-being. Adopting a healthy lifestyle, including regular exercise, a balanced diet, stress management, and avoiding smoking, may help support the immune system and improve quality of life for individuals with autoimmune diseases.
Are there any experimental treatments for autoimmunity?
Researchers are continuously exploring new treatment options, including targeted immunotherapies and biologic medications, to modulate the immune response and improve outcomes for individuals with autoimmune diseases. However, these treatments are still being studied and may not be widely available or approved for all conditions.
References
- Nagy, Z. A. (2014). Autoimmunity. A History of Modern Immunology, 281–325. doi:10.1016/b978-0-12-416974-6.00010-7
- Kono, D. H., & Theofilopoulos, A. N. (2017). Autoimmunity. Kelley and Firestein’s Textbook of Rheumatology, 301–317.e5. doi:10.1016/b978-0-323-31696-5.00019-x
- Delves, P. J. (1998). Autoimmunity. Encyclopedia of Immunology, 292–296. doi:10.1006/rwei.1999.0075
- Actor, J. K. (2014). Autoimmunity. Introductory Immunology, 86–96. doi:10.1016/b978-0-12-420030-2.00007-x
- Fujinami, R. S. (1999). Autoimmunity. Encyclopedia of Virology, 108–112. doi:10.1006/rwvi.1999.0018
- Silverstein, A. M. (2014). Autoimmunity. The Autoimmune Diseases, 11–17. doi:10.1016/b978-0-12-384929-8.00002-2