What is Immunoglobulin M (IgM Antibody)?
- Immunoglobulin M (IgM) is a crucial component of the immune response to exogenous pathogens. It is one of several isotypes of antibodies, also called immunoglobulins, produced by vertebrates. IgM is the first antibody produced during an immune response and functions as an antigen receptor on B cells.
- Mature B lymphocytes express IgM as a transmembrane antigen receptor at rest, constituting a portion of the B-cell receptor (BCR). B cell activation occurs when antigens bind to the BCR, leading to accelerated cell division and the expansion of activated B lymphocytes. These cells can differentiate into either antibody-secreting plasma cells or memory B lymphocytes, which are essential for the immune system’s capacity to mount a response to the same pathogen upon subsequent encounters.
- IgM is structurally comparable to transmembrane immunoglobulins but lacks a carboxy-terminal transmembrane segment. The predominant configuration of the secreted form of IgM is pentameric, and its molecular weight exceeds 900 kDa. IgM is predominantly found in the intravascular space, including the bloodstream and lymph fluid, due to its large size.
- IgM constitutes approximately 10% of the total immunoglobulin content in the bloodstream. This immunoglobulin is the third most prevalent in the human body. IgM is considered the most efficient complement-fixing immunoglobulin and plays a crucial role in primary immune responses to the majority of antigens. Complement fixation is the process by which the immune system activates a succession of pathogen-destroying proteins.
- Plasmablasts, a form of immune cell, are thought to reside in the spleen, which is the main site of specific IgM production. IgM is produced by these plasmablasts as part of the adaptive immune response. In 1937, IgM production was first observed in horses hyper-immunized with pneumococcus polysaccharide. Its original name, -macroglobulin, was derived from the fact that the newly discovered antibody was substantially larger than the typical rabbit -globulin. Due to its larger molecular weight of 990,000 daltons, it was renamed IgM, with the “M” standing for “macro.”
- Initially, the heterogeneity of the V domains in normal immunoglobulins presented obstacles to the detailed structure study of IgM. However, homogeneous IgM from multiple myeloma patients and engineered immunoglobulin genes expressed in tissue culture have provided sources of more homogeneous IgM, allowing for additional research and identification of specific molecular requirements.
- IgM is an essential antibody isotype that plays an important function in the immune response of the body. It is the initial antibody produced in response to an antigen and is primarily found in the circulation and lymph fluid. Its size and ability to repair complements make it an essential component of the immune system’s defense against pathogens.
Definition of Immunoglobulin M (IgM Antibody)
Immunoglobulin M (IgM) antibody is the first antibody produced by the immune system in response to an infection or foreign substance. It is a large pentameric antibody that plays a crucial role in the body’s initial immune response.
Fundamentals of IgM
IgM, or immunoglobulin M, is a fundamental component of the immune system and plays a crucial role in the recognition and response to antigens. Here are some key fundamentals of IgM:
- Structure: IgM is a heterotetramer composed of two heavy chains and two light chains that are linked together by covalent bonds. It has a Y-shaped structure, with two Fab regions that recognize antigens and an Fc tailpiece that determines the antibody’s biological activity.
- Genetic Origins: The genes encoding IgM are located on chromosome 14 for the heavy chain and on chromosomes 2 and 22 for the alternative light chain loci, kappa and lambda, respectively. The somatic DNA recombination process in B lymphocytes in the bone marrow generates a diverse range of immunoglobulins through the random recombination of different DNA segments.
- B Cell Receptor (BCR): Monomeric IgM, along with IgD, serves as the BCR on resting B lymphocytes before they encounter antigens. The BCR allows B cells to recognize specific antigens and initiate an immune response.
- Primary and Secondary Responses: Upon antigen recognition, B cells secrete pentameric IgM as the initial antibody response during a primary immune response. Although the affinity of primary IgM for foreign antigens is lower compared to secondary responses, the pentameric structure provides increased avidity with ten antigen-binding sites per IgM molecule. This avidity is particularly effective for binding pathogens with repetitive epitopes, such as those found on bacterial capsules.
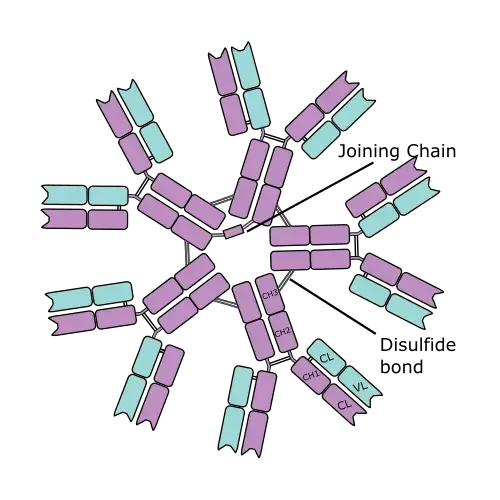
- Distribution: IgM is predominantly found in the blood but can also be present in the lymph and secreted across mucosal surfaces. Unlike IgG or IgE, multimeric IgM does not easily enter tissues from the bloodstream due to its larger size. The presence of the J chain, a small protein associated with secreted IgM and IgA, stabilizes pentameric IgM through covalent disulfide bonds and facilitates their transport across mucosal epithelia.
- Role in Mucosal Immunity: IgM, along with IgA, provides immune protection to mucosal surfaces. While IgA is the predominant antibody for mucosal defense, increased secretion of IgM can occur if IgA secretion is insufficient. The J chain protects secreted IgM and IgA from proteases and aids in their transport across mucosal epithelia.
Structure of IgM
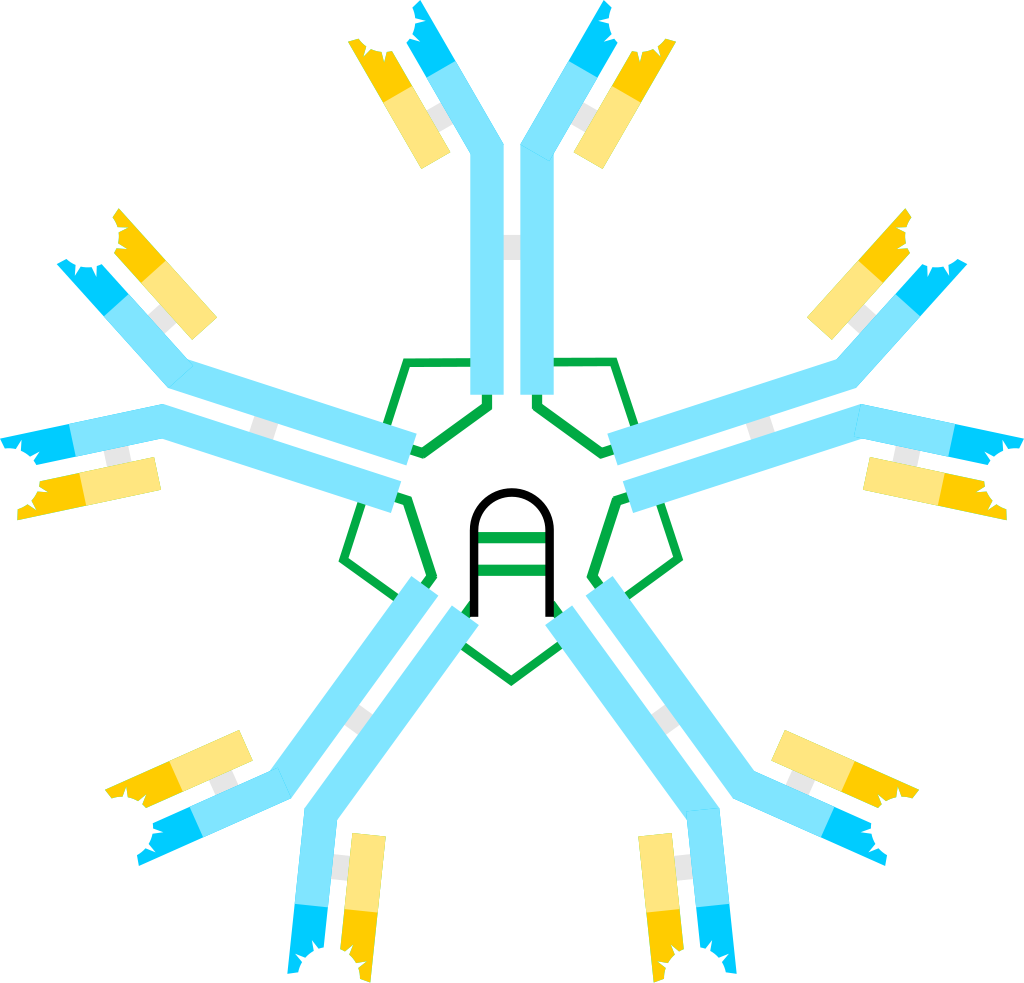
- The structure of Immunoglobulin M (IgM) exhibits distinct characteristics that contribute to its functionality as an essential component of the immune system. IgM exists in two forms: a pentamer and a monomer.
- When present as a pentamer, all the heavy chains within IgM are identical, as are the light chains. This pentameric structure is found in the blood and is held together by disulfide bridges located at the carboxy-terminal end of the μ chains. Each pentamer consists of five four-chain units, wherein each unit comprises two light chains (either kappa or lambda) and two heavy chains.
- On the other hand, IgM can also exist as a monomer, which is expressed on the surface of B lymphocytes as B cell receptors. In this monomeric form, IgM is a crucial receptor for B cells, facilitating their interaction with antigens. The monomeric IgM on the B cell surface consists of four chain units: two μ H-chains and two L-chains. The H chains possess an additional hydrophobic domain, which aids in anchoring the molecule in the plasma membrane.
- The heavy chain in IgM monomers differs from that of IgG. It is composed of one variable region and four constant regions, with the additional constant domain replacing the hinge region found in IgG. This structural distinction highlights the unique properties and functionality of IgM.
- Furthermore, the J-chain plays an important role in IgM structure. It is associated with IgM in the bloodstream and initiates the polymerization of IgM subunits upon its secretion from plasma cells. This polymerization process allows the formation of IgM pentamers, enhancing their effectiveness in immune responses.
- In summary, the structure of IgM is characterized by its pentameric and monomeric forms. As a pentamer, IgM consists of five four-chain units held together by disulfide bridges. As a monomer, it functions as a B cell receptor and comprises four chain units with distinct heavy chain characteristics. The presence of the J-chain facilitates polymerization of IgM subunits, contributing to its overall function in the immune system.
Cellular Level Production of IgM
At the cellular level, IgM is produced by different subsets of B cells in various lymphoid organs.
- Bone Marrow-Derived B Cells:
- Developing B cells in the bone marrow produce a transmembrane IgM B cell receptor (BCR).
- Immature B cells expressing transmembrane IgM migrate to the spleen.
- In the spleen, immature B cells compete for access to follicles to receive survival signals.
- Only a portion of immature B cells survive to become mature, naive B cells known as B-2 cells.
- Mature B-2 cells have two fates: follicular B cells or marginal zone B cells.
- Follicular B cells express both IgM and IgD as transmembrane BCRs.
- Follicular B cells predominantly recognize peptide antigens and require help from T lymphocytes for activation.
- With T-cell assistance, follicular B cells undergo clonal expansion, producing progeny that differentiate into plasma cells or memory B lymphocytes.
- IgM is the primary antibody produced during the initial antigen challenge.
- Upon subsequent antigen exposure, follicular B cells undergo isotype switching to produce IgG, IgE, or IgA antibodies.
- Marginal Zone B Cells:
- Marginal zone B cells are also bone marrow-derived B-2 cells that complete maturation in the spleen.
- They localize in the marginal zone between the red and white pulp in the spleen.
- Marginal zone B cells provide the first line of defense against blood-borne pathogens.
- They primarily recognize non-peptide antigens like polysaccharides and lipids.
- Marginal zone B cells largely produce IgM and do not undergo isotype switching or affinity maturation.
- They contribute to the production of natural antibodies, which are predominantly IgM antibodies cross-reactive against common microbial antigens.
- B-1 Cells:
- B-1 cells are a subset of B cells derived from the fetal liver, not the bone marrow.
- They exhibit limited diversity in antibody production, mostly producing IgM.
- B-1 cells are self-renewing and mainly found in mucosal layers and the peritoneal cavity.
- Similar to marginal zone B cells, B-1 cells produce predominantly IgM antibodies against polysaccharides and lipids commonly found in microbes.
- They do not undergo isotype switching, affinity maturation, or memory lymphocyte formation upon subsequent antigen exposure.
Molecular Level
- At the molecular level, Immunoglobulin M (IgM) is composed of two heavy chains and two light chains, each containing variable and constant domains. The variable regions of the immunoglobulin determine the antigen-binding specificity, while the constant region of the heavy chain determines the cellular function.
- The heavy chain locus on chromosome 14 contains variable (V), diversity (D), and joining (J) regions. During B cell development, recombination between these segments occurs through the action of the RAG complex (RAG1 and RAG2 proteins). This process, known as somatic recombination, generates a diverse repertoire of immunoglobulins with different antigen-binding specificities.
- Combinatorial diversity is achieved through random recombination events between V, D, and J coding segments. Additionally, junctional diversity occurs through the random incorporation of nucleotides at the junctions between these segments. Terminal deoxynucleotidyl transferase (TdT) enzyme adds random nucleotides (N nucleotides), increasing the diversity of the immunoglobulin repertoire.
- The successful production of a functional heavy chain is essential for B cell development. Developing B cells must pass the BCR receptor checkpoint by producing a heavy chain that can associate with a germline-encoded surrogate light chain, forming the pre-BCR receptor. This receptor transmits pro-survival and proliferative signals, promoting the development of B cells.
- After passing the checkpoint, recombination of the light chain loci (kappa and lambda) begins. The recombination of the kappa loci is attempted first, and if a non-self-reactive IgM molecule is produced, recombination of other light chain loci is inhibited through a process called allelic exclusion.
- Mature naive B cells express both IgM and IgD as transmembrane antigen receptors. Transmembrane immunoglobulins associate with Ig alpha and Ig beta proteins to form the B cell receptor (BCR). These proteins contain immunoreceptor tyrosine-based activation motifs (ITAMs), which are responsible for initiating B cell activation upon antigen recognition.
- Naive B cell activation in response to antigen recognition leads to the secretion of IgM antibodies. The primary RNA transcript undergoes alternative polyadenylation site selection, resulting in the secretion of IgM instead of a transmembrane form. During the secretory pathway, IgM can oligomerize to form pentameric IgM connected by a J chain or as a hexamer without the J chain.
- Structurally, IgM molecules consist of four constant heavy domains without a hinge region, making them less flexible compared to other antibody isotypes. The presence of cysteine residues in the carboxy terminus allows IgM to form pentamers or hexamers.
- In summary, at the molecular level, IgM is formed through recombination events between V, D, and J segments, resulting in a diverse repertoire of immunoglobulins. The successful production of a functional heavy chain is crucial for B cell development and passing the BCR receptor checkpoint. IgM is secreted as a pentamer or hexamer and plays an essential role in the primary immune response.
Clinical Significance
IgM (Immunoglobulin M) is an important antibody in the immune system’s response to foreign pathogens. It serves as the initial antibody produced during an immune response and is also a component of natural antibodies within the innate immune system. Several disorders are associated with IgM, each with its own clinical significance.
- X-linked Hyper-IgM Syndrome is a rare inherited disease characterized by elevated levels of IgM and deficient levels of other immunoglobulins, as well as defects in cellular immunity. The syndrome is caused by defects in genes involved in isotype switching, particularly the gene for CD40L on the X-chromosome. As a result, individuals with this syndrome have impaired production of antibodies other than IgM, leading to a compromised immune response against peptide antigens. They are susceptible to recurrent sinopulmonary and pyogenic infections, and their compromised cellular immunity increases the risk of opportunistic infections, such as Pneumocystis jirovecii pneumonia and infections by parasites like Cryptosporidium parvum. Additionally, they are at a higher risk of developing malignancies.
- Selective IgM Deficiency (SIGMD) is a rare disorder characterized by a specific deficiency of IgM while maintaining normal levels of other immunoglobulins. SIGMD can be asymptomatic or present with recurring infections, particularly those caused by encapsulated bacteria and viruses. It can also be associated with malignancy, autoimmunity, or allergy. Diagnosis of SIGMD involves excluding other diseases that result in low levels of multiple antibody isotypes. Treatment typically involves managing infections and addressing underlying conditions if present.
- Cold Agglutinin Disease (CAD) is an autoimmune hemolytic anemia mediated predominantly by IgM antibodies. IgM in CAD can agglutinate erythrocytes at colder temperatures, leading to the destruction of red blood cells. CAD is a rare disease associated with IgM autoantibodies targeting blood group antigens, primarily large I and small i. The binding of IgM to erythrocytes activates the complement pathway, resulting in erythrocyte destruction. CAD may occur as a result of underlying conditions or infections, such as Epstein-Barr virus or Mycoplasma pneumoniae. Management involves avoiding cold temperatures, treating severe anemia with transfusions, and plasmapheresis to remove IgM molecules.
- Monoclonal Gammopathies involve the proliferation of single clones of plasma cells and are characterized by the overproduction of IgM. Waldenström macroglobulinemia (WM) is a rare form of monoclonal gammopathy where elevated IgM levels are accompanied by the infiltration of the bone marrow by a lymphoplasmacytic lymphoma. Symptoms of WM include anemia, bleeding, organomegaly, and systemic complaints. Treatment options include rituximab, plasmapheresis for hyperviscosity symptoms, and chemotherapy. Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant clonal plasma cell disorder characterized by low levels of monoclonal immunological protein (M protein). MGUS requires monitoring as it can progress to multiple myeloma or Waldenström macroglobulinemia.
Functions of Immunoglobulin M (IgM Antibody)
IgM antibody serves several important functions and holds significant significance in the immune system:
- Primary Immune Response: IgM is the first antibody to be produced during an immune response. It plays a crucial role as the prime mediator of the primary immune response, initiating the defense against pathogens.
- Antigen Binding and Neutralization: IgM efficiently binds with antigens, facilitating agglutination, neutralizing reactions, and cytolytic reactions. It can directly target and neutralize pathogens, preventing their harmful effects.
- Complement Activation: IgM is essential for complement activation, a cascade of proteins that leads to the destruction of pathogens. It interacts with complement component C1 and triggers the classical pathway, enhancing the immune response against infections.
- Mucosal Defense: IgM interacts with the polyimmunoglobulin receptor (pIgR), enabling its transport to mucosal surfaces such as the gut lumen and breast milk. This involvement in mucosal defense helps protect these areas from pathogenic invasion.
- Neonatal Immunity: IgM is the first immunoglobulin class synthesized by newborns. It plays a crucial role in providing initial protection against pathogens, contributing to the neonate’s immune defense.
- Autoimmune Diseases: IgM antibodies are implicated in the pathogenesis of some autoimmune diseases, where the immune system mistakenly targets self-components. Understanding the role of IgM in these diseases can help in their diagnosis and management.
- High Avidity and Efficiency: Although IgM antibodies usually have low-affinity binding sites for antigens, their ten combining sites per molecule allow for synergistic interactions. This high avidity enables IgM to efficiently deal with pathogens, particularly during the early stages of the immune response.
- Diagnostic Marker: Elevated levels of IgM in the bloodstream can indicate recent infection or exposure to antigens. Monitoring IgM levels can help identify and diagnose certain infectious diseases.
FAQ
What is Immunoglobulin M (IgM) antibody?
Immunoglobulin M (IgM) is an antibody produced by the adaptive immune system in response to an infection or foreign pathogen. It is the first antibody produced during an initial immune response.
What is the clinical significance of IgM?
IgM plays a crucial role in the immune system’s defense against pathogens. Elevated levels of IgM can indicate an ongoing infection or an immune disorder.
What disorders are associated with IgM?
Some disorders associated with IgM include X-linked Hyper-IgM Syndrome, Selective IgM Deficiency, Cold Agglutinin Disease, and Monoclonal Gammopathies.
What is X-linked Hyper-IgM Syndrome?
X-linked Hyper-IgM Syndrome is a rare inherited disorder characterized by elevated levels of IgM, deficient levels of other immunoglobulins, and defects in cellular immunity. It results in increased susceptibility to infections and compromised immune responses.
What is Selective IgM Deficiency?
Selective IgM Deficiency is a rare disorder where individuals have a deficiency in IgM while maintaining normal levels of other immunoglobulins. It can lead to recurring infections, autoimmunity, and an increased risk of malignancy.
What is Cold Agglutinin Disease (CAD)?
Cold Agglutinin Disease is a form of autoimmune hemolytic anemia primarily mediated by IgM antibodies. These antibodies can cause red blood cell destruction, especially at lower temperatures. CAD can be associated with infections, drug-induced reactions, or underlying conditions.
What are Monoclonal Gammopathies?
Monoclonal Gammopathies are characterized by the overproduction of a single clone of plasma cells. Some forms of monoclonal gammopathies, such as Waldenström macroglobulinemia and Monoclonal Gammopathy of Undetermined Significance (MGUS), can result in elevated levels of IgM.
How is X-linked Hyper-IgM Syndrome diagnosed?
The diagnosis of X-linked Hyper-IgM Syndrome is primarily based on clinical symptoms, such as recurrent infections and elevated IgM levels, along with deficient IgG and IgA levels. Genetic testing can help identify specific mutations responsible for the condition.
How is Selective IgM Deficiency diagnosed?
Selective IgM Deficiency is diagnosed by demonstrating isolated low levels of IgM alongside normal levels of other immunoglobulins. Other conditions with reduced levels of multiple isotypes must be excluded through testing.
How are disorders associated with IgM treated?
The treatment for disorders associated with IgM varies depending on the specific condition. Treatment approaches may include intravenous gammaglobulin (IVIG) therapy, prophylactic measures against infections, targeted therapies, plasmapheresis, and close monitoring for disease progression or malignancies.