What is Enzyme?
- Enzymes are remarkable proteins that play a pivotal role in catalyzing non-spontaneous chemical reactions within biological systems. As vital components of an organism’s metabolic pathways, enzymes ensure the smooth execution of cellular tasks necessary for growth, repair, and energy acquisition.
- The metabolic network, a complex web of interconnected chemical reactions, adapts its activities in response to internal and external stimuli. For an organism to function and survive effectively, these metabolic responses must be specific in timing and circumstances. Regulatory enzymes are key players in this process, as they control the overall rate of metabolic pathways, determining the pace at which cellular tasks are accomplished.
- Enzymatic reactions are remarkably precise, occurring only in suitable cellular environments and proceeding at a rate proportional to the availability of required substrates or cofactors. The surrounding conditions heavily influence enzyme activity, with factors such as temperature, pH, substrate concentration, and the presence of inhibitors or activators affecting the rate of these chemical reactions.
- Biochemical reactions are the foundation of an organism’s growth, tissue repair, and energy generation. These essential reactions, collectively known as metabolism, continuously occur within all living organisms. If metabolism ceases to function, it inevitably leads to the organism’s demise.
- Metabolic reactions demand high activation energy to occur, which can be energetically costly for cells. To minimize energy consumption, nature has devised a solution – the enzyme. Enzymes serve as biological catalysts, consisting of large protein molecules that accelerate chemical reactions inside cells. Composed of chains of polypeptides made up of amino acids, enzymes remain unchanged during the reaction, enabling them to speed up reactions without being consumed.
- Enzymes exhibit a remarkable specificity compared to other catalysts, with each enzyme specialized for a particular substrate or a select few substrates, thereby ensuring the precise execution of specific reactions. By lowering the activation energy required to initiate a reaction, enzymes facilitate the rapid progress of essential cellular processes.
- Several factors influence the speed of an enzyme’s action. The concentration of the enzyme and substrate, as well as environmental factors like temperature and pH, play significant roles in determining the enzyme’s efficiency. Additionally, the presence of inhibitors can hinder the enzyme’s activity, while activators can enhance it.
- In summary, enzymes are essential and fascinating biological catalysts that orchestrate vital chemical reactions within living organisms. Through their specific and efficient actions, enzymes contribute to the overall functioning and survival of all life forms. Understanding the intricacies of enzymes not only advances our knowledge of biological systems but also opens the door to numerous applications in various fields, including medicine, biotechnology, and environmental science.
Factors That Affects Enzyme Activity
Enzyme activity, a crucial aspect of biological processes, is influenced by several key factors that dictate the rate and efficiency of enzymatic reactions. Understanding these factors is vital for comprehending the intricacies of enzyme functioning. Let us delve into the six main factors that affect enzyme activity:
- Concentration of Enzyme: The availability of enzymes significantly impacts the rate of enzymatic reactions. Higher enzyme concentrations generally lead to more rapid reactions, as there are more enzymes available to interact with substrates. Conversely, low enzyme concentrations can limit the reaction rate, even if an ample amount of substrate is present.
- Concentration of Substrate: The concentration of the substrate, the molecule upon which the enzyme acts, is another crucial determinant of enzyme activity. When substrate concentration increases, the probability of substrate-enzyme collisions rises, leading to more frequent enzyme-substrate interactions and, consequently, increased reaction rates. However, once the enzyme reaches its saturation point, further increases in substrate concentration will not enhance the reaction rate.
- Effect of Temperature: Temperature profoundly influences enzyme activity. As the temperature rises, so does the kinetic energy of molecules, including enzymes and substrates. This heightened energy results in more frequent collisions between the enzyme and substrate, thereby accelerating the reaction rate. However, excessively high temperatures can denature the enzyme, rendering it non-functional.
- Effect of pH: The pH level of the surrounding environment plays a critical role in enzyme activity. Enzymes exhibit optimal activity at specific pH values, which vary depending on the type of enzyme. Deviations from the optimal pH can denature the enzyme or alter its active site, leading to reduced catalytic efficiency.
- Effect of Product Concentration: The accumulation of reaction products can influence enzyme activity. In some cases, high product concentrations can inhibit enzyme activity through negative feedback mechanisms, ensuring that excessive product formation is controlled. This phenomenon helps maintain cellular homeostasis.
- Effect of Activators: Activators are molecules that enhance enzyme activity. These substances can bind to enzymes and induce conformational changes that increase the enzyme’s affinity for its substrate or stabilize the enzyme-substrate complex. As a result, the rate of the enzymatic reaction is boosted.
Factor 1: Enzyme Concentration
- Enzyme concentration is a critical factor that profoundly influences the rate and efficiency of enzymatic reactions. Enzymes play a pivotal role as catalysts, facilitating chemical reactions by forming transient bonds with their substrates, thus lowering the activation energy required for the reaction to occur. Moreover, enzymes stabilize the transition state, making the reaction more favorable.
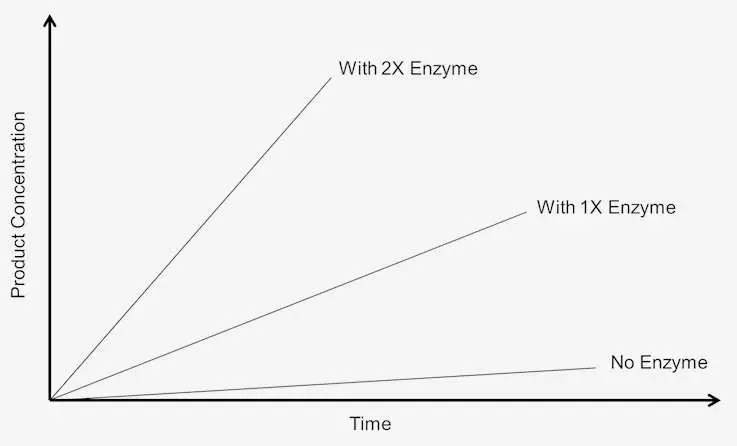
- When the enzyme concentration is high, more enzyme molecules are available to interact with the substrates. This abundance of enzyme-substrate complexes leads to a higher initial catalytic rate, giving the reaction a headstart in progressing towards reactant-product equilibrium. In contrast, reactions with the same enzyme but at lower concentrations may take longer to achieve equilibrium.
- Enzyme concentration becomes particularly significant when the substrate concentration is abundant, and the limiting factor becomes the availability of enzymes. In such cases, a higher enzyme concentration allows for more rapid formation of enzyme-substrate complexes, leading to a faster overall reaction rate.
- However, it is important to note that enzyme concentration has its limits. Beyond a certain point, increasing the enzyme concentration will not necessarily lead to a proportional increase in the reaction rate. This is because other factors, such as substrate concentration, reaction conditions, and the availability of cofactors, also play roles in governing the overall reaction rate.
- Enzyme concentration can be regulated by the cell in response to various physiological conditions. Cells may adjust the production of enzymes through gene regulation or control their activation and deactivation to maintain the appropriate balance between enzyme and substrate concentrations.
- In summary, enzyme concentration is a critical factor that directly impacts the rate of enzymatic reactions. A higher enzyme concentration results in a larger number of available enzymes to form enzyme-substrate complexes, leading to a more rapid initial catalytic rate. Understanding and controlling enzyme concentration are essential for regulating cellular processes and ensuring that biochemical reactions occur with precision and efficiency.
Factor 2: Substrate Concentration
- Substrate concentration is a crucial factor that significantly impacts the rate and efficiency of enzymatic reactions. For enzyme catalytic activity to occur, the substrate must be geometrically and electronically complementary to the enzyme’s catalytic or active site. When a substrate binds to the active site, the enzyme’s active residues transiently interact with the substrate, catalyzing its transformation into a product.
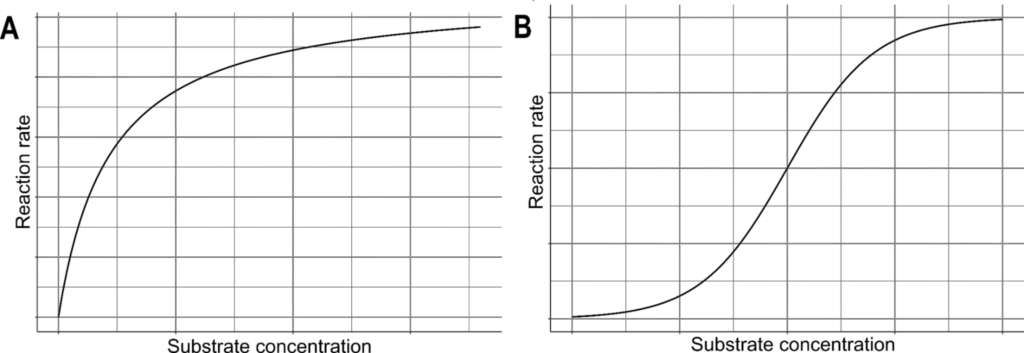
- The relationship between enzyme activity and substrate concentration can be described by different kinetic models, depending on the type of enzyme. Most enzymes follow the Michaelis-Menten kinetics, which consists of two stages. Initially, the relationship between enzyme activity and substrate concentration shows a linear association, where the reaction rate increases with increasing substrate concentration. However, this trend eventually plateaus as the number of unbound active sites decreases, reaching a maximum rate known as the maximum velocity (Vmax).
- On the other hand, allosteric enzymes exhibit a sigmoidal kinetic pattern. In the initial stages of the reaction, the relationship between the rate of an allosteric enzyme-catalyzed reaction and substrate concentration is exponential. As catalysis progresses and substrate binding saturates the enzyme’s active sites, the rate becomes linear, eventually reaching a plateau.
- In the presence of a fixed amount of enzyme, the rate of an enzymatic reaction initially increases with increasing substrate concentration until it reaches a limiting rate. At this point, the enzyme’s active sites become fully occupied by substrate molecules, and the enzyme is said to be saturated with substrate. Any further increase in substrate concentration does not significantly affect the reaction rate because the excess substrate molecules cannot react until the substrate already bound to the enzymes has undergone the reaction and been released.
- This phenomenon highlights the importance of proper substrate regulation in enzymatic reactions. Cells carefully control substrate concentrations to maintain the optimal efficiency of enzymatic processes. Substrate concentration is influenced by factors such as substrate availability, cellular demand, and the presence of regulatory molecules.
- In conclusion, substrate concentration plays a fundamental role in enzymatic reactions. The availability of substrate molecules dictates the rate at which enzyme-substrate complexes form and, subsequently, the overall reaction rate. Proper understanding and regulation of substrate concentration are essential for cells to maintain precise control over biochemical processes and ensure efficient utilization of resources.
Factor 3: Effect of Temperature
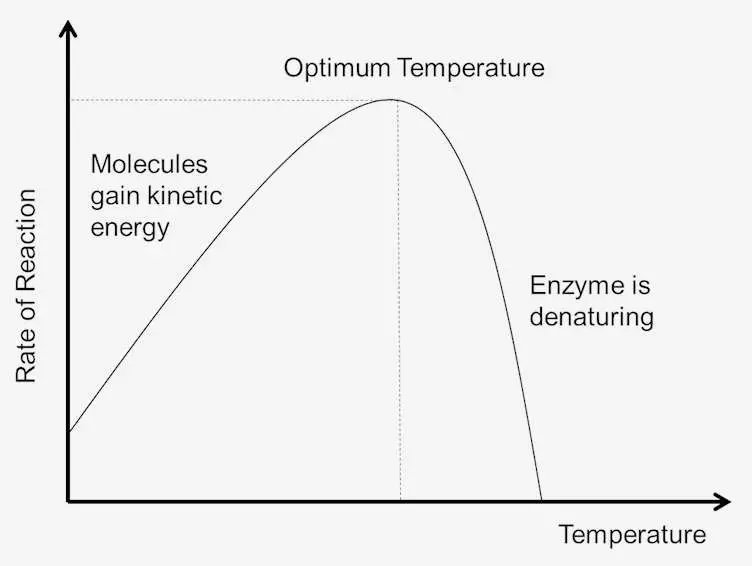
- The effect of temperature on enzyme activity is a critical factor that significantly influences the efficiency of enzymatic reactions. Like pH, temperature plays a crucial role in the stability of an enzyme’s intramolecular bonds, which in turn affects its overall activity. Generally, enzymes exhibit optimal activity at their specific optimal temperature.
- A slight increase in temperature can accelerate the reaction rate as the reactants gain more kinetic energy, leading to more frequent collisions between enzymes and substrates. This results in an increase in the rate of enzyme-substrate interactions and, consequently, a faster reaction rate. However, significant deviations from the optimal temperature can have adverse effects on enzyme activity.
- At extremely high temperatures, the intramolecular bonds and enzyme conformation can be disrupted, leading to permanent denaturation and rendering the enzyme non-functional. This phenomenon is commonly observed in extreme thermophiles, such as Thermococcus hydrothermalis and Sulfolobus solfataricus, which thrive in high-temperature environments and possess enzymes adapted to such conditions.
- Conversely, at low temperatures, the kinetic energy of the system decreases, leading to reduced reaction rates. Enzyme activity declines as the temperature falls below the optimal range. Unlike high temperatures, low temperatures do not necessarily cause permanent enzyme denaturation, and enzyme activity may be restored once the temperature returns to the optimal range.
- However, excessively low temperatures can lead to decreased solubility of enzymes in aqueous solutions, causing them to unfold and become inactive. Furthermore, freezing and thawing processes can damage enzymes irreversibly, particularly when ice crystals form and disrupt the enzyme’s protein structure. To minimize freeze-thaw damage, it is essential to minimize freeze-thaw cycles, control freezing or thawing durations, and use additives like sucrose or glycerol to protect the enzyme during freezing.
- Enzymes’ protein nature makes them extremely sensitive to thermal changes, and their activity is restricted to a narrow range of temperatures compared to ordinary chemical reactions. Each enzyme has an optimal temperature, typically ranging from 37 to 40°C. Beyond this range, enzyme activity gradually decreases until it reaches a temperature at which the enzyme becomes completely inactive due to alterations in its natural composition.
- In summary, temperature is a significant factor affecting enzyme activity. Enzymes exhibit optimal activity at specific temperatures, and deviations from this range can lead to reduced enzymatic efficiency or permanent denaturation. Proper temperature regulation is crucial for maintaining optimal enzyme activity and ensuring the precise and efficient execution of biochemical reactions in living organisms.
| Name | Description/habitat | Optimal pH | Optimal temperature |
|---|---|---|---|
| 1. Thermococcus hydrothermalis | Prokaryotic archaea found in the East Pacific hydrothermal vent | 5.5 | 85°C |
| 2. Sulfolobus solfataricus | Prokaryotic archaea found in sulfur-rich volcanic fields | 3.0 | 80°C |
| 3. Halomonas meridiana | Gram-negative bacteria found in Antarctica salt lake | 7.0 | 37°C |
| 4. Pseudoalteromonas haloplanktis | Fast-growing bacteria found in Antarctic seawater | 7.6 | 4°C |
Factor 4: Effect of pH
- The effect of pH on enzyme activity is a critical factor that significantly influences the efficiency of enzymatic reactions. Enzymes, being protein molecules, consist of a chain of amino acids with electrical charges derived from the sequence of their residues. The specific arrangement of these amino acids determines the enzyme’s three-dimensional structure and its functional residues, including those found at the active site.
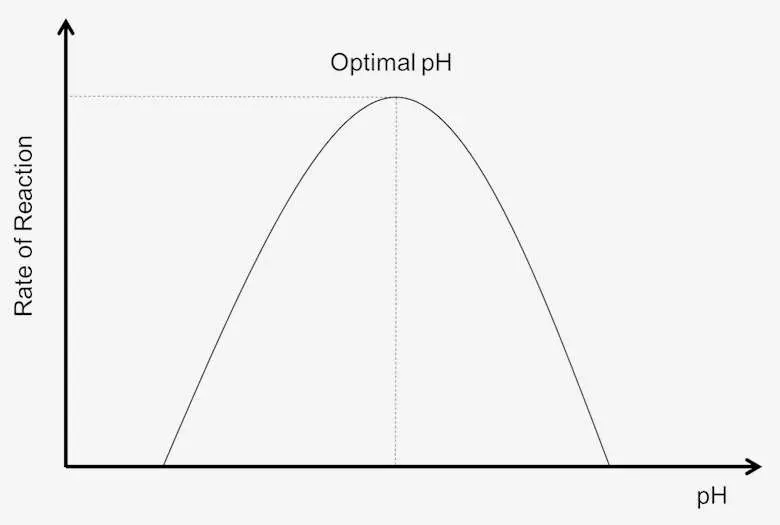
- Enzymes exhibit a remarkable degree of substrate specificity, allowing them to function optimally within a narrow range of pH. Most enzymes perform at their best in slightly acidic or basic pH conditions. However, some enzymes are naturally adapted to extreme acidic or basic environments, and they exhibit maximum activity within these specific pH ranges.
- A change in the pH value, whether it becomes more acidic or basic, affects the ionization of amino acid residues within the enzyme. This, in turn, leads to alterations in the three-dimensional structure of the enzyme, which directly impacts its interaction with substrates and, consequently, reduces its overall activity.
- Another significant effect of pH changes is on the enzyme’s catalytic capability. Acid-base and covalent catalysis mechanisms can be hindered or suppressed by shifts in pH, thereby influencing the enzyme’s ability to catalyze reactions effectively. In extreme cases, substantial pH changes can lead to the denaturation of the enzyme, disrupting its three-dimensional structure and rendering it permanently non-functional.
- The pH scale is used to measure the concentration of hydrogen ions (H+) in a solution, determining whether a liquid is acidic, basic, or neutral. A pH below 7 is considered acidic, while a pH above 7 is alkaline or basic. A pH of 7 represents neutrality, similar to the acidity of pure water at 25°C. Enzymes are particularly affected by changes in pH due to the presence of acidic carboxylic groups (COOH-) and basic amino groups (NH2) in their structure.
- Each enzyme has an optimal pH value at which it operates with maximum efficiency. Deviations from this optimal pH result in a decrease in enzyme activity until it eventually stops working. For example, pepsin, a digestive enzyme, operates optimally at low pH levels (high acidity), while trypsin, another digestive enzyme, functions best at high pH levels (basic conditions). Many enzymes work optimally at a neutral pH of 7.4, reflecting the physiological pH of the human body.
- In conclusion, pH is a critical factor influencing enzyme activity due to its impact on the enzyme’s three-dimensional structure, substrate specificity, and catalytic capability. Proper regulation of pH is essential for maintaining the optimal activity of enzymes in different cellular environments, ensuring the precise and efficient execution of biochemical reactions necessary for the functioning and survival of living organisms.
| Enzyme | Function | pH range | Optimal pH |
|---|---|---|---|
| 1. ɑ-Amylase | In saliva, amylase breaks down most polysaccharides in human diets. | 6.4 – 7.0 | 6.6 |
| 2. Pepsin | Pepsin is one of the many proteases found in the stomach’s gastric juice. It hydrolyzes peptide bonds in the protein’s amino acid chains. | 1.5 – 4.5 | 2 |
| 3. Trypsin | Found in the small intestine, trypsin is another protease that digests proteins. | 7.5 – 8.5 | 7.8 |
| 4. Alkaline Phosphatase (ALP) | ALP catalyzes the removal of phosphate groups from its substrate. It is found in all human tissue and is most abundant in the intestine and placenta. | 8 – 10 | 10 |
Factor 5: Effect of Effector or Inhibitor
- The effect of effectors or inhibitors is a critical factor that regulates the catalytic function of many enzymes. Enzymes often require the presence of non-substrate and non-enzyme molecules to initiate or modulate their activity. For instance, certain enzymes rely on metal ions or cofactors to establish their catalytic activity, while others depend on effectors to regulate their catalytic function, and this is especially evident in allosteric enzymes.
- Effectors play a vital role in activating or inhibiting enzyme activity, promoting or preventing their binding to substrates. Allosteric enzymes are a prime example, where effectors can bind to regulatory sites away from the active site, inducing conformational changes that either enhance or hinder the enzyme’s catalytic activity. This allows cells to fine-tune enzyme function in response to changing metabolic demands or external signals.
- On the other hand, inhibitors can bind to enzymes or their substrates, interfering with the ongoing enzymatic activity and preventing further catalytic events. The impact of inhibitors on enzyme activity can be either reversible or irreversible. Reversible inhibitors only render the enzyme inactive when bound to it and can be displaced by other molecules. In contrast, irreversible inhibitors form strong bonds with the enzyme’s functional groups, leading to permanent inactivation of the enzyme.
- Competitive inhibitors are a type of reversible inhibitor that competes with substrates for binding to the enzyme’s catalytic sites. By occupying the active site, they prevent the substrate from accessing it, reducing the enzyme’s catalytic activity. Non-competitive inhibitors, another type of reversible inhibitor, do not bind to the active site but instead bind to an allosteric site, leading to conformational changes that decrease the enzyme’s activity.
- Uncompetitive inhibitors are another form of reversible inhibition that binds only to the enzyme-substrate complex. By doing so, they prevent the release of the product, leading to a reduction in the overall reaction rate.
- Understanding the role of effectors and inhibitors is essential for regulating enzyme activity and maintaining the delicate balance of biochemical processes within cells. The presence or absence of specific effectors or inhibitors can modulate enzyme activity and influence cellular functions in various physiological and pathological conditions.
- Moreover, enzymes often rely on certain inorganic metallic cations, such as Mg2+, Mn2+, Zn2+, Ca2+, Co2+, Cu2+, Na+, and K+, for their optimal activity. In some cases, rare anions, like chloride ions (CI-), are also needed for specific enzyme activities, such as amylase.
- In summary, the effect of effectors or inhibitors is a critical regulatory factor that governs enzyme activity. These non-substrate and non-enzyme molecules can either activate or inhibit enzyme function, ensuring precise control over biochemical reactions in response to cellular demands and external stimuli. Understanding the interplay between effectors, inhibitors, and cofactors is fundamental to advancing our knowledge of enzyme regulation and developing potential therapeutic interventions for various medical conditions.
Factor 6: Effect of Product Concentration
- The effect of product concentration is a critical factor that can influence the velocity and efficiency of enzymatic reactions. As enzymes catalyze reactions, they convert substrates into products. The accumulation of reaction products can have a significant impact on enzyme activity.
- In some cases, the reaction products may bind to the active site of the enzyme, forming a loose complex. This product-enzyme complex can inhibit the enzyme’s activity, reducing its catalytic efficiency. This type of inhibition is known as product inhibition.
- Product inhibition is an important regulatory mechanism in living systems. In order to prevent the negative effects of product inhibition, living organisms have evolved mechanisms to rapidly remove or utilize the products formed during enzymatic reactions. This quick removal of products prevents the accumulation of inhibitory molecules at the enzyme’s active site, allowing the enzyme to continue functioning effectively.
- There are various ways in which living systems prevent or mitigate the effects of product inhibition. One common strategy is the presence of specific enzymes dedicated to the degradation or utilization of the reaction products. These secondary enzymes act on the products, converting them into other substances that can be further utilized or excreted from the cell.
- Additionally, regulatory mechanisms, such as feedback inhibition, play a role in preventing excessive product accumulation. Feedback inhibition occurs when the final product of a metabolic pathway acts as an inhibitor of one of the earlier enzymes in the pathway. This creates a negative feedback loop that regulates the rate of the entire pathway, preventing the overproduction of the final product.
- Overall, the effect of product concentration on enzyme activity is a crucial consideration in cellular processes. Product inhibition can slow down enzymatic reactions and potentially disrupt metabolic pathways. However, living systems have evolved sophisticated mechanisms to mitigate the effects of product inhibition, ensuring that enzymatic reactions proceed with precision and efficiency, and maintaining the delicate balance necessary for proper cellular functioning.
Examples
- Factor 1: Enzyme Concentration Example: Imagine a scenario where you have an enzyme that breaks down a complex sugar molecule into simpler sugars. If you increase the concentration of this enzyme in a test tube containing the substrate (complex sugar), the reaction rate will increase as more enzyme molecules are available to interact with the substrate. Conversely, if you decrease the enzyme concentration, the reaction rate will slow down due to a lower number of available enzymes to catalyze the reaction.
- Factor 2: Substrate Concentration Example: Let’s consider an enzyme that converts hydrogen peroxide (H2O2) into water (H2O) and oxygen gas (O2). If you increase the concentration of hydrogen peroxide in the reaction mixture, the enzyme’s activity will increase, as there will be more substrate molecules available for the enzyme to act upon. However, once all the enzyme’s active sites are fully occupied with substrate molecules, further increases in hydrogen peroxide concentration will not affect the reaction rate.
- Factor 3: Effect of Temperature Example: Take the example of an enzyme that functions optimally at body temperature (around 37°C). At this temperature, the enzyme’s activity is at its highest because the kinetic energy of molecules is sufficient for efficient enzyme-substrate collisions. However, if you increase the temperature beyond a certain point, such as 50°C, the enzyme’s three-dimensional structure may start to denature, leading to a significant reduction in enzyme activity.
- Factor 4: Effect of pH Example: Consider an enzyme responsible for breaking down proteins in the stomach. This enzyme, known as pepsin, works optimally at a low pH (acidic environment) of around 2.0. If you change the pH to a more basic environment (higher pH), pepsin’s activity will decrease significantly because the enzyme’s active site is adapted to function best in an acidic environment.
- Factor 5: Effect of Activators Example: An example of an enzyme that requires an activator is the enzyme hexokinase, which catalyzes the first step in glucose metabolism. Hexokinase requires the presence of magnesium ions (Mg2+) as a cofactor to function properly. The magnesium ions serve as activators, enhancing the enzyme’s catalytic activity and facilitating the conversion of glucose to glucose-6-phosphate.
- Factor 6: Effect of Product Concentration Example: Imagine an enzyme that converts a precursor molecule (A) into a product (B). As the enzyme catalyzes the reaction, the concentration of product B starts to increase. If the product concentration becomes too high, B molecules may start to bind to the enzyme’s active site, leading to product inhibition. This inhibitory effect slows down the enzyme’s activity, preventing the excessive accumulation of product B. The cell may have additional enzymes or regulatory mechanisms to utilize or remove product B from the reaction to maintain enzyme activity.
FAQ
What factors affect enzyme activity?
Enzyme activity can be influenced by several factors, including temperature, pH, enzyme concentration, substrate concentration, presence of activators, and the concentration of reaction products.
How does temperature affect enzyme activity?
Temperature can significantly impact enzyme activity. Enzymes generally have an optimal temperature at which they work most efficiently. Deviations from this optimal temperature can either increase or decrease enzyme activity, with extreme temperatures leading to denaturation and loss of function.
How does pH affect enzyme activity?
The pH level of the environment in which enzymes operate can affect their activity. Each enzyme has an optimal pH at which it functions best. Deviations from this pH can alter the enzyme’s conformation and reduce its catalytic efficiency.
What is the relationship between enzyme concentration and activity?
Higher enzyme concentrations usually result in faster enzymatic reactions, as more enzyme molecules are available to interact with substrates. However, beyond a certain point, increasing enzyme concentration may not lead to a proportional increase in reaction rate.
How does substrate concentration influence enzyme activity?
Increasing substrate concentration generally increases enzyme activity until a saturation point is reached. At this point, all enzyme active sites are occupied by substrates, and further increases in substrate concentration do not significantly affect the reaction rate.
What are enzyme activators?
Enzyme activators are molecules that enhance the catalytic activity of enzymes. They may bind to the enzyme and induce conformational changes that increase the enzyme’s affinity for its substrate, leading to more efficient reactions.
How do inhibitors affect enzyme activity?
Inhibitors are molecules that reduce or completely halt enzyme activity. They can either bind to the active site of the enzyme (competitive inhibition) or to an allosteric site, leading to conformational changes that hinder catalytic activity (non-competitive inhibition).
What is product inhibition?
Product inhibition occurs when the accumulation of reaction products inhibits the enzyme’s activity. The products can bind to the enzyme’s active site or allosteric sites, reducing its ability to catalyze further reactions.
How do living systems prevent product inhibition?
Living systems have evolved mechanisms to prevent or mitigate product inhibition. These mechanisms include rapid removal of products, utilization of secondary enzymes to degrade or utilize products, and feedback inhibition, where the final product of a metabolic pathway regulates an earlier enzyme in the pathway.
Why is enzyme activity regulation important?
Regulation of enzyme activity is crucial for maintaining proper metabolic balance and cellular function. Precise control of enzymatic reactions ensures that biochemical processes occur at the right time, in the right place, and with the appropriate rate to meet the demands of the organism.