What is Lock and Key Model?
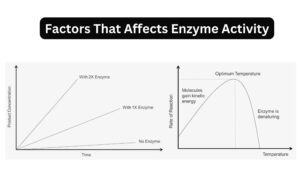
- The Lock and Key Model is a fundamental concept in the realm of biochemistry, elucidating the mechanism by which enzymes function. Enzymes, as we know, are biological catalysts predominantly made up of proteins. Their primary role is to accelerate chemical reactions by significantly reducing the activation energy required. This acceleration can be to the extent of making reactions occur millions of times faster than they would in the absence of enzymes.
- Therefore, for enzymes to carry out their functions, they must interact with specific molecules known as substrates. These substrates are the reactant molecules that enzymes act upon, transforming them into products. It’s essential to understand that while enzymes facilitate these reactions, they remain unchanged and reusable post-reaction.
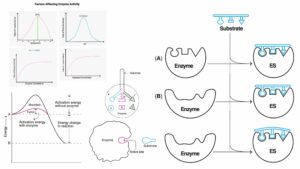
- The interaction between enzymes and substrates is highly specific. This specificity is explained by the Lock and Key Model. In this model, the enzyme is visualized as a lock, while the substrate is seen as a key. Just as a specific key fits into a particular lock, a substrate binds to its corresponding enzyme at a designated area called the active site. This binding ensures that chemical bond-breaking and bond-forming processes occur efficiently. Besides, it’s worth noting that while enzymes lower the energy of the transition state, they do not alter the overall energy change (∆G) of the reaction. This means that the energy released or absorbed during the reaction remains consistent, regardless of the enzyme’s presence.
- Then, as the substrate binds to the enzyme’s active site, it undergoes a transformation, resulting in the formation of products. This entire process emphasizes the enzyme’s role in ensuring substrate specificity and the smooth progression of biochemical reactions.
- In conclusion, the Lock and Key Model offers a clear and concise explanation of enzyme-substrate interactions. It underscores the importance of enzymes in regulating biochemical processes and highlights their specificity, ensuring that only the correct substrate binds to the appropriate active site. The model provides a descriptive detail of the enzyme’s function, emphasizing its role in facilitating and controlling biochemical reactions.
The Lock and Key Model Concept
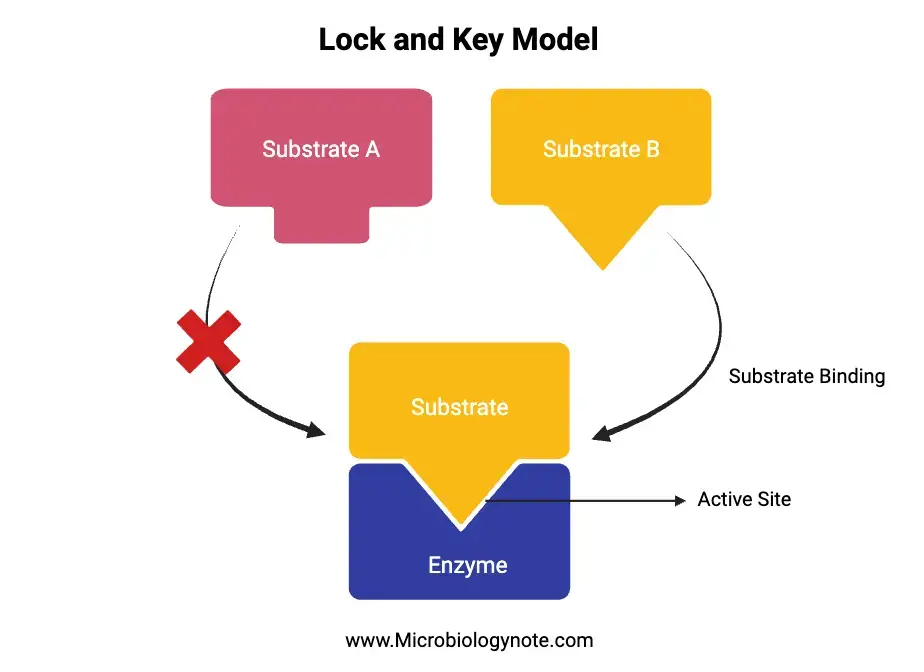
The “Lock and Key Model” is a fundamental concept in enzymology, introduced by Emil Fischer in 1899. This model provides an analogy to explain the specificity exhibited by enzymes during their interactions with substrates. In this analogy, the enzyme is likened to a lock, while the substrate is compared to a key. Just as a specific key is required to open a particular lock, a specific substrate binds to its corresponding enzyme.
The process of enzyme action, as described by the Lock and Key model, is sequential and detailed:
- The substrate approaches the enzyme and aligns itself with the enzyme’s active site, which is a specific region on the enzyme designed to bind substrates.
- Upon binding, an enzyme-substrate complex is formed. This complex is transient and facilitates the chemical transformation of the substrate.
- Depending on the nature of the reaction, the enzyme either aids in the synthesis of molecules by forming bonds or assists in digestion by breaking bonds, leading to the formation of new substances.
- Once the reaction is complete, the enzyme releases the product(s) and is free to participate in subsequent reactions, showcasing its reusable nature.
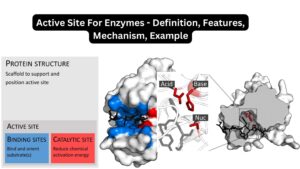
A critical aspect of the Lock and Key model is the emphasis on the specificity of the enzyme’s active site. This site is uniquely shaped to accommodate only a particular substrate, ensuring precise interactions and efficient catalysis.
However, it’s essential to note that while the Lock and Key model provides a foundational understanding of enzyme-substrate interactions, it has its limitations. Over time, it became evident that not all enzymes exhibit absolute specificity for a single substrate. Some enzymes can catalyze reactions with multiple substrates that have similar structures. For instance, digestive enzymes like pepsin and chymotrypsin in the stomach can break down various proteins. Additionally, certain synthetic molecules, if shaped similarly to the natural substrate, can also bind and react with enzymes.
Lock and Key Model Mechanism
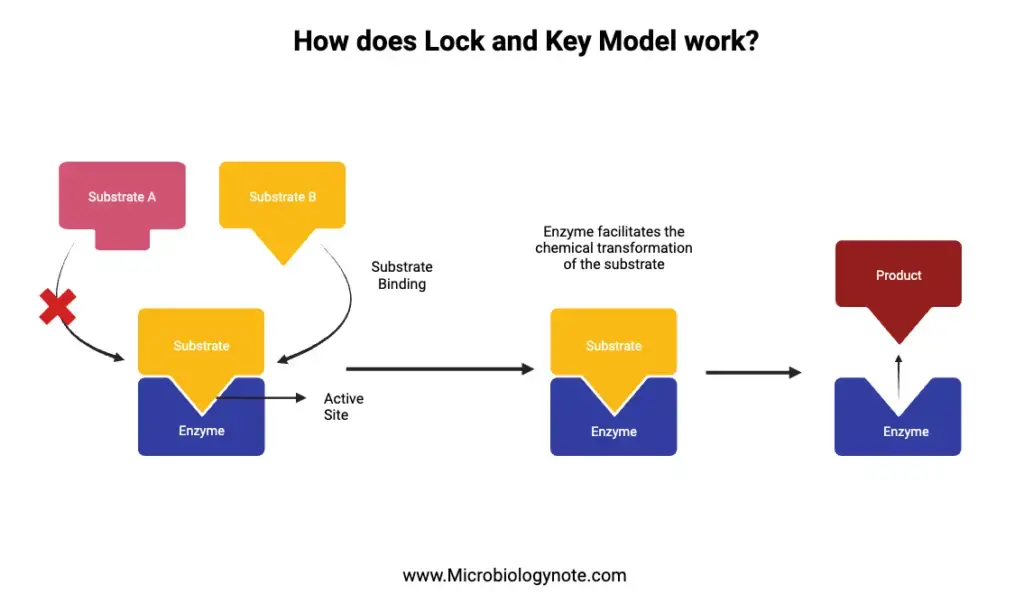
- Substrate Binding: Initially, the substrate approaches the enzyme, aligning itself with a specific region on the enzyme known as the active site. This region is uniquely structured, allowing only specific substrates to bind. Therefore, the enzyme’s active site and the substrate fit together precisely, much like a key fits into a lock. This precise interaction leads to the formation of an enzyme-substrate complex.
- Catalysis: Once the enzyme-substrate complex is formed, the enzyme facilitates the chemical transformation of the substrate. Depending on the nature of the enzyme and the reaction it catalyzes, two primary reactions can occur:
- Synthesis Reaction: In this type of reaction, the enzyme aids in the formation of bonds, leading to the synthesis of molecules.
- Decomposition Reaction: Contrarily, in decomposition reactions, the enzyme assists in breaking bonds, resulting in the formation of new, distinct substances.
- Product Release: Following the chemical transformation, the enzyme releases the product(s) of the reaction. This release signifies the completion of the enzyme’s role in that particular reaction. Besides, it’s noteworthy that enzymes are not consumed or altered during the reaction. Thus, they are free to participate in subsequent reactions, showcasing their reusable nature.
Limitations of Lock and Key Model
- Transition State Stabilization: One of the primary limitations of the Lock and Key Model is its inability to explain the stabilization of the enzyme-substrate complex during the transition state. The transition state is a critical phase in enzymatic reactions, and understanding its stabilization is essential for a comprehensive grasp of enzyme kinetics.
- Rigidity of Enzymes: The model posits that enzymes are rigid structures, implying that their shape remains unchanged upon substrate binding. However, contemporary research challenges this notion. Studies have shown that the active site of an enzyme undergoes conformational changes upon substrate binding. Therefore, the idea of enzymes as static entities is not entirely accurate.
- Multiple Substrate Binding: The Lock and Key Model does not adequately address scenarios where multiple substrates bind to a single enzyme. This omission is significant, given that many enzymatic reactions involve more than one substrate.
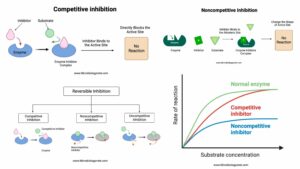
- Specificity Concerns: The model suggests that enzymes exhibit absolute specificity, binding exclusively to a single substrate. However, this is not universally true. While some enzymes do exhibit high specificity, others display broad specificity. For instance, enzymes like lipase can interact with various lipids, and proteases such as trypsin and chymotrypsin can degrade a range of proteins. This broad specificity contradicts the strict specificity proposed by the Lock and Key Model.
Advantages of Lock and Key Model
- Simplicity and Clarity: One of the primary advantages of the Lock and Key Model is its simplicity. It provides a clear and straightforward analogy that makes the concept of enzyme-substrate interaction easily understandable, even for those with limited knowledge of biochemistry.
- Visualization: The model offers a visual representation of how enzymes and substrates interact. By comparing enzymes to locks and substrates to keys, it provides a tangible analogy that aids in visualizing the specificity of these interactions.
- Foundation for Enzyme Specificity: The model emphasizes the specificity of enzymes, highlighting that each enzyme is tailored to bind with a particular substrate. This concept of specificity is fundamental in enzymology and has been confirmed by numerous experimental observations.
- Basis for Further Research: While the Lock and Key Model may not capture all the nuances of enzyme-substrate interactions, it laid the groundwork for further research in the field. The model prompted scientists to investigate the intricacies of these interactions, leading to the development of more refined models like the “Induced Fit Model.”
- Pedagogical Tool: The Lock and Key analogy has been a valuable educational tool. It is widely used in academic settings to introduce students to the concept of enzyme-substrate interactions. Its simplicity makes it an effective teaching aid.
- Highlighting Active Sites: The model underscores the importance of the active site in enzymes. It brings attention to the fact that only a specific part of the enzyme, the active site, is involved in binding with the substrate and facilitating the reaction.
- Explaining Enzyme-Substrate Complex Formation: The model provides a framework for understanding how enzymes and substrates come together to form a temporary complex, facilitating the chemical reaction.
Importance of Lock and Key Model
- Pioneering Concept: Introduced by Emil Fischer in 1899, the Lock and Key Model was one of the first attempts to explain the mechanism of enzyme-substrate interactions. It laid the foundation for our understanding of how enzymes recognize and bind to specific substrates.
- Promotion of Enzyme Specificity: The model emphasized the idea that enzymes are highly specific in their action. It proposed that each enzyme has a unique shape that matches a particular substrate, much like a key fits a specific lock. This concept of specificity is crucial in many biological processes and therapeutic applications.
- Educational Value: Due to its simplicity and clarity, the Lock and Key analogy has been an invaluable tool in education. It provides an easily understandable framework for students and those new to the field, helping them grasp the basic concept of enzyme-substrate interactions.
- Basis for Further Research: The Lock and Key Model sparked curiosity and led to more in-depth research into enzyme kinetics and mechanisms. While the model itself has been refined over time, it served as a starting point that encouraged further exploration and the development of more advanced models like the “Induced Fit Model.”
- Understanding Biological Processes: The model has been instrumental in explaining various biological processes at the molecular level. By understanding how enzymes interact with substrates, scientists have gained insights into metabolic pathways, drug interactions, and disease mechanisms.
- Therapeutic Implications: The concept of enzyme specificity, as highlighted by the Lock and Key Model, has implications in drug design and therapy. Drugs often target specific enzymes, and understanding the enzyme-substrate interaction can lead to the development of more effective and selective therapeutic agents.
- Biochemical Assays and Diagnostics: The principles derived from the Lock and Key Model have been applied in designing biochemical assays. These assays, based on specific enzyme-substrate interactions, are used in diagnostics, research, and various biotechnological applications.
- Catalysis and Industrial Applications: The model has also been influential in the field of biocatalysis. By understanding the specific interactions between enzymes and substrates, industries have been able to harness enzymes for various applications, from food processing to biofuel production.
References
- https://study.com/learn/lesson/lock-key-model-vs-induced-fit-model.html
- https://www.biologyonline.com/dictionary/lock-and-key-model
- https://www.learnatnoon.com/s/en-pk2/what-is-the-lock-and-key-model-of-enzymes/
- https://www.nagwa.com/en/videos/768109646750/
- https://www.ahmadcoaching.com/2020/12/lock-and-key-model-of-enzyme.html