The classical pathway is a key immune system mechanism that plays an important role in defending the body from foreign invaders. It consists of a series of sequential interactions among complement components that result in the killing of target cells via a process known as cell lysis.
The presence of an antibody attached to an antigen, generating an antigen-antibody complex, activates the classical route. It is critical to highlight that these unique complexes activate the classical route rather than native or free antibodies. Antigen-antibody complexes can be soluble or generated when an antibody attaches to antigenic determinants on the membranes of viruses, fungi, parasites, or bacteria, known as epitopes.
Immune complexes are widely used to describe soluble antibody-antigen complexes. It is worth noting, however, that only complexes generated by specific subclasses of IgG or IgM antibodies have the ability to activate the classical complement system. These subclasses have distinct structural properties that enable them to interact and activate the complement cascade.
When antigen-antibody complexes capable of activating the classical route are produced, a number of complement components are activated progressively. This chain of events is characterized by enzymatic processes in which each activated component acts as a catalyst for the activation of the next component in the pathway. The activated complement components function as enzymes or opsonins, which are chemicals that promote phagocytosis and label target cells for destruction.
Cell lysis is the end result of the classical route. A membrane attack complex (MAC) is created as the cascade advances. This MAC generates pores in the target cell’s membrane, causing the cell’s integrity to be disrupted and the cell to lyse or be destroyed. Cell lysis is an important immune system defensive mechanism used to remove germs and protect the body from illness.
In summary, the classical pathway is a set of complement events initiated by antigen-antibody complexes. It is activated by the presence of these complexes rather than by free antibodies. Only specific subclasses of IgG or IgM antibodies are capable of triggering the pathway, which culminates in the killing of target cells by cell lysis. The classical route is an important part of the immune system’s defense systems against infections.
Classical Pathway of Complement Activation
- The complement components react in a cascade in a specified order, culminating in cell lysis, and this process is known as the classical pathway.
- Antibody-antigen complexes, but not native or unbound antibodies, trigger this response.
- IgG and IgM antibodies are the primary initiators of the classical complement pathway.
- In order to cleave the C3 protein, a series of activation-induced protein recruitment events must occur, at which point C3 convertase (C4b2b, formerly known as C4b2a) is produced.
- C3 convertase (C4b2b) attaches to the C3b subunit of cleaved C3, generating C5 convertase (C4b2b3b), which cleaves the C5 protein.
- The cleavage products signal for the arrival of phagocytes and mark infected cells for phagocytic clearance.
- The membrane attack complex is assembled near the end of the complement system’s life cycle, which is triggered by the C5 convertase (MAC).
- By forming a breach in the membrane of the victim cell, the membrane assault complex triggers the cell’s internal organelles to lyse and kill it.
- In addition to apoptotic cells, necrotic cells, and acute phase proteins can activate the classical complement pathway.
Activators of the classical pathway
The classical pathway, a key component of the complement system, can be activated by various molecules and substances. These activators play a crucial role in initiating the cascade of complement reactions that lead to the destruction of target cells. Here are some important activators of the classical pathway:
- Immunoglobulin IgM and IgG: Both IgM and certain subclasses of IgG antibodies have the ability to activate the classical pathway. IgM antibodies, as the first antibody class produced during an immune response, are particularly effective activators of this pathway. Among the IgG subclasses, IgG3 is the most efficient in activating the complement system, followed by IgG1 and IgG2. However, IgG4 antibodies do not activate the classical pathway.
- Staphylococcal protein A: Staphylococcal protein A is a surface protein produced by Staphylococcus aureus bacteria. It has the ability to bind to the Fc region of IgG antibodies, leading to the activation of the classical pathway. This interaction between protein A and IgG antibodies triggers the complement cascade, enhancing the immune response against the bacteria.
- C-reactive protein: C-reactive protein (CRP) is an acute-phase protein produced by the liver in response to inflammation or infection. CRP can bind to certain microbial surfaces and damaged host cells. This interaction between CRP and these targets can activate the classical pathway, initiating complement-mediated immune responses against the pathogens or damaged cells.
- DNA: While the primary role of DNA is to carry genetic information, it can also act as an activator of the classical pathway under certain conditions. DNA can stimulate the complement system by directly binding to and activating the complement component C1, which is the initial step in the classical pathway. This interaction triggers the subsequent cascade of complement reactions, contributing to the immune response.
These activators of the classical pathway play a crucial role in initiating the complement cascade and enhancing the immune response against pathogens and damaged cells. They interact with specific components of the complement system, such as antibodies, proteins, and DNA, triggering a sequence of reactions that ultimately leads to the destruction of target cells through cell lysis or other complement-mediated mechanisms. Understanding the role of these activators is important in comprehending the complex interplay between the immune system and its defense mechanisms.
Steps of activation of classical pathway
Typically, the conventional process of complement activation begins with the production of soluble antigen–antibody complexes (immune complexes) or with the binding of antibody to antigen on an appropriate target, such as a bacterial cell. The sequential steps in the activation of the classical route are as follows:

1. Activation of C1
- The activation of the classical pathway of the complement system is initiated by the activation of C1, a complex composed of three distinct molecules: C1q, C1r, and C1s. The activation process relies on the binding of C1q to the Fc domain of the attached antibody, which can be either IgM or IgG.
- The binding of C1q to the Fc region of the antibody is the initial step in the activation of C1. For C1 activation to occur, at least two contiguous Fc regions need to be bound by C1q. This requirement emphasizes the importance of having a high concentration of IgG antibodies and ensuring that the antigenic determinants recognized by these antibodies are in close proximity to each other.
- Pentameric IgM, which consists of five antibody subunits, assumes a stable conformation when bound to antigens on a target surface. In this conformation, at least three binding sites for C1q are accessible. As a result, only one IgM molecule is required to initiate the complement pathway.
- In contrast, due to the lower valency of IgG molecules (which typically have two antigen-binding sites), a significantly higher number of IgG molecules—roughly a thousand of them—are needed to achieve the same level of complement pathway initiation.
- The activation of C1 leads to the sequential activation of complement components C4, C2, and C3. This cascade of activations sets in motion the downstream complement pathways, resulting in various effector functions such as cell lysis, opsonization, and inflammation.
- In summary, the activation of the classical pathway begins with the activation of C1, a complex consisting of C1q, C1r, and C1s. C1q binds to the Fc domain of IgM or IgG antibodies, with at least two contiguous Fc regions needing to be bound for C1 activation. The activation of C1 triggers a series of complement reactions, leading to the initiation of downstream complement pathways and their associated immune functions.
2. Activation of C1r and C1s / C3 convertase formation
The activation of C1r and C1s, as well as the formation of C3 convertase, are important steps in the complement cascade following the binding of C1q. Here’s a breakdown of these processes:
- Binding of C1q and Activation of C1r and C1s: When C1q binds to the Fc region of the antibody-antigen complex in the presence of calcium ions, it triggers conformational changes in C1r and C1s, leading to their activation. These conformational changes result in the activation of the esterase activity of C1s.
- Cleavage of C4 by Activated C1s: The activated C1s enzyme cleaves the complement component C4 into two fragments: C4a and C4b. C4a is a small soluble fragment that acts as an anaphylatoxin, contributing to inflammation and immune cell recruitment. On the other hand, C4b, the larger fragment, attaches to the cell membrane alongside C1.
- Cleavage of C2 by C4b in the presence of Mg2+: The C4b molecule bound to the cell membrane serves as a platform for the cleavage of C2. In the presence of magnesium ions (Mg2+), C4b breaks down C2 into two fragments: C2a and C2b. C2b diffuses away from the membrane, while C2a remains bound to C4b.
- Formation of C3 Convertase (C4b2a): The complex formed by C4b and C2a is known as C4b2a, also referred to as the C3 convertase. The C4b2a complex possesses enzymatic activity, which allows it to cleave the complement component C3.
- Conversion of C3 into Active Form: The C3 convertase (C4b2a) catalyzes the cleavage of C3 into two fragments: C3a and C3b. C3a is a small soluble fragment that acts as an anaphylatoxin, contributing to inflammation. C3b, the larger fragment, plays a crucial role in opsonization, complement-mediated cell lysis, and immune complex clearance.
The formation of C3 convertase marks a critical point in the complement cascade, as it enables the amplification and continuation of complement activation. C3b generated by C3 convertase can bind to nearby surfaces, further propagating the cascade and leading to the formation of additional C3 convertases. This amplification loop ensures an efficient and robust complement response against pathogens.
In summary, the binding of C1q triggers the activation of C1r and C1s. Activated C1s cleaves C4 into C4a and C4b. C4b, in the presence of Mg2+, then cleaves C2 into C2a and C2b. The resulting C4b2a complex, known as C3 convertase, possesses enzymatic activity and cleaves C3 into C3a and C3b. The formation of C3 convertase initiates the amplification loop in the complement cascade, leading to further complement activation and effector functions.
3. Activation of C3 molecules
The activation of C3 molecules is a critical step in the complement cascade, initiated by the action of the C3 convertase. Here’s an overview of the activation of C3 and its biological significance:
- Activation and Cleavage of C3: The C3 convertase, formed by the interaction of C4b and C2a, activates and cleaves C3 into two fragments: C3a and C3b. The C3 convertase catalyzes the cleavage of hundreds of C3 molecules into C3a and C3b.
- Amplification of C3b: The generation of C3b through the cleavage of C3 is highly amplified. A single molecule of C3 convertase has the potential to produce over 200 molecules of C3b. This amplification results in a significant increase in the production of C3b molecules, contributing to the efficiency and potency of the complement system.
- Binding of C3b and C4b to C3b/C4b Receptors: Activated C3b and C4b have biological significance as they can bind to receptors called C3b/C4b receptors, which are now known as CR1 receptors. These receptors are present on various host cells, particularly phagocytes (cells specialized in engulfing and eliminating pathogens).
- Immune Adherence and Phagocytosis: The binding of C3b (or its degradation product iC3b) and C4b to CR1 receptors on phagocytic cells leads to a phenomenon known as immune adherence. This immune adherence increases the affinity of phagocytic cells for C3b/C4b-coated particles, such as pathogens or immune complexes.
The enhanced affinity resulting from immune adherence significantly promotes phagocytosis, which is one of the body’s primary defense mechanisms against pathogens. Phagocytes recognize and bind to the C3b/C4b-coated particles through the interaction with CR1 receptors, facilitating their engulfment and subsequent destruction.
Overall, the activation of C3 molecules by the C3 convertase and the production of C3b play a pivotal role in the complement system. The binding of C3b to CR1 receptors on phagocytic cells leads to immune adherence and an increased efficiency in phagocytosis. This process contributes to the body’s defense against pathogens and the removal of immune complexes, promoting immune clearance and maintaining immune homeostasis.
4. Formation of C5 convertase
The formation of the C5 convertase is an essential step in the complement cascade, leading to the breakdown of C5 and the subsequent initiation of the membrane attack complex (MAC). Here’s an explanation of the formation of C5 convertase and its downstream effects:
- Formation of C4b2a3b Complex (C5 Convertase): Some of the C3b molecules generated during complement activation bind to the pre-existing C4b2a complex (C3 convertase), resulting in the formation of the C4b2a3b complex, also known as C5 convertase. This complex consists of C4b, C2a, and additional C3b molecules.
- Cleavage of C5 by C5 Convertase: The C5 convertase enzymatically cleaves the complement component C5 into two fragments: C5a and C5b. C5a, similar to other anaphylatoxins, diffuses away and contributes to inflammatory responses. C5b, on the other hand, remains bound within the convertase complex.
- Initiation of the Membrane Attack Complex (MAC): The bound C5b in the C4b2a3b complex acts as a seed for the assembly of the membrane attack complex, also known as MAC. C5b recruits complement components C6 and C7 to form the C5b-6-7 complex. This complex serves as a docking site for subsequent complement components.
- Formation of Membrane Attack Complex (MAC) and Innocent-Bystander Lysis: The C5b-6-7 complex is followed by the recruitment and binding of C8. The binding of C8 stabilizes the complex’s adhesion to the target cell membrane. Finally, C9 is recruited and polymerizes to form a ring-like structure, resulting in the creation of the C5b-9 complex, also known as the membrane attack complex (MAC).
- Innocent-Bystander Lysis and Tissue Damage: The released C5b-9 complexes, capable of inserting into the membrane of surrounding cells, can trigger “innocent-bystander” lysis. In normal circumstances, regulator proteins in human sera prevent this process from causing harm to healthy cells and tissues. However, in certain disorders, the process of innocent-bystander lysis can lead to cell and tissue damage.
In summary, the formation of C5 convertase (C4b2a3b complex) allows for the cleavage of C5 into C5a and C5b. C5b initiates the assembly of the membrane attack complex (MAC) through the recruitment of C6, C7, C8, and subsequent polymerization of C9. The MAC can insert into cell membranes, leading to “innocent-bystander” lysis and potential tissue damage. Regulator proteins typically prevent harm to host cells, but dysregulation of this process can contribute to disorders and pathological conditions.

5. Formation of MAC
The formation of the membrane attack complex (MAC) involves the assembly and polymerization of complement components, leading to the creation of a transmembrane channel within the targeted cell. Here’s an explanation of the formation of MAC and its effects:
- C5b-8 Complex: The C5b component, which is generated through the cleavage of C5 by C5 convertase, binds to complement component C8. This results in the formation of the C5b-8 complex.
- Polymerization with C9: The C5b-8 complex undergoes polymerization upon the attachment of multiple C9 molecules. The binding and polymerization of C9 molecules cause the formation of the complete membrane attack complex, known as C5b-9 or MAC.
- Formation of Transmembrane Channel: The MAC forms a transmembrane channel within the targeted cell membrane. This channel spans across the lipid bilayer of the cell membrane, creating a pore or hole.
- Ion Exchange and Osmotic Pressure: The transmembrane channel formed by the MAC enables the exchange of ions between the intracellular and extracellular environments. Ions rapidly flow through the channel, leading to an increase in osmotic pressure inside the cell.
- Cell Swelling and Lysis: The rapid influx of ions and the subsequent increase in osmotic pressure inside the cell cause water to enter the cell, resulting in cell swelling. For certain cell types, the increased pressure can lead to the rupture and lysis of the cell membrane. This rupture and lysis can cause cell death and the release of cellular contents.
Overall, the formation of the membrane attack complex (MAC) involves the assembly of complement components and the polymerization of C9 molecules. The MAC creates a transmembrane channel within the targeted cell, allowing the exchange of ions. The increase in osmotic pressure inside the cell leads to cell swelling, and for certain cell types, the rupture and lysis of the cell membrane. The membrane attack complex is a crucial mechanism by which the complement system eliminates pathogens and damaged cells, contributing to immune defense.
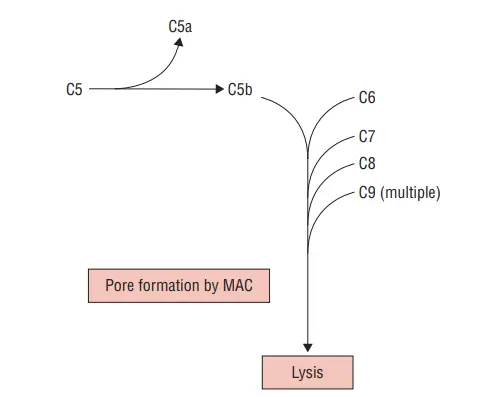
What is a Membrane attack complex?
The membrane attack complex (MAC) is a structure formed by the complement system, specifically the components C5b, C6, C7, C8, and C9. It represents the final stage where all three pathways of complement activation converge. Here’s an explanation of what the membrane attack complex is and how it is formed:
- Convergence of Complement Pathways: The membrane attack complex is the endpoint of all three pathways of complement activation: the classical pathway, lectin pathway, and alternative pathway. These pathways involve a cascade of reactions leading to the formation of the MAC.
- Contribution of Complement Components: The complement subcomponents C5b, C6, C7, C8, and C9 are involved in the formation of the MAC. These components come together to assemble and stabilize the complex.
- Formation on the Cell Membrane: In the soluble phase, C5b, C6, and C7 form a complex. This complex then binds to the cell membrane. C7, which is associated with the C5b-C6 complex, becomes exposed and interacts with the hydrophobic amino acid groups present on the cell membrane.
The subsequent steps in MAC formation involve the recruitment and polymerization of C8 and C9:
- Binding of C8: Once the C5b-C6-C7 complex is attached to the cell membrane, C8 is recruited to the complex. The binding of C8 further stabilizes the interaction between the MAC and the cell membrane.
- Polymerization of C9: Following the recruitment of C8, multiple molecules of C9 are added to the complex. C9 molecules polymerize, forming a ring-like structure.
- Formation of Transmembrane Channels: The polymerization of C9 molecules creates pores or channels that span the lipid bilayer of the cell membrane. These transmembrane channels allow for the exchange of ions and other molecules between the cell’s interior and the extracellular environment.
The membrane attack complex, once fully formed, can cause several effects, including disruption of the cell membrane, leading to cell lysis and destruction. The MAC’s transmembrane channels allow ions and other molecules to flow into the cell, leading to osmotic imbalances, cell swelling, and potentially cell death.
In summary, the membrane attack complex is the culmination of complement activation pathways, involving the coordinated assembly of C5b, C6, C7, C8, and C9. It forms on the cell membrane and creates transmembrane channels that can lead to cell lysis. The MAC represents an important defense mechanism of the immune system, targeting pathogens and damaged cells.

Deficiencies in the Classical Pathway: C1q, C1r, C1s, C4, C2, C1-Inh
Deficiencies in the classical pathway components, including C1q, C1r, C1s, C4, C2, and C1-Inh, can lead to various immune-related disorders and complications. Here’s an overview of the deficiencies and their associated conditions:
- C1q, C1r, C1s, and C4 Deficiencies:
- Deficiencies in these components can contribute to the development of systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA). In these conditions, the complement system fails to efficiently eliminate immune complexes and dying cells.
- When small complexes attach to complement receptors on macrophages in the spleen and liver, they are efficiently removed from circulation.
- In the absence of proper complement function, larger aggregates may form, triggering the alternative pathway and leading to the deposition of C3 in tissues. This can result in insoluble complexes that deposit in tissues and cause inflammation.
- C2 Deficiency:
- C2 deficiency is the most common complement deficiency, with prevalence estimates ranging from 1 in 10,000 to 1 in 20,000 for homozygous individuals.
- C2 deficiency is associated with an increased prevalence of SLE.
- In young children with primary immunodeficiency and recurrent infections, particularly upper respiratory infections with Streptococcus pneumoniae or related pathogens, C2 deficiency can be observed.
- C1-Inh Deficiency:
- Hereditary angioedema (HAE) is a condition caused by C1-Inh deficiency.
- Symptoms typically appear around puberty but can start earlier.
- Individuals with C1-Inh deficiency experience recurring swelling of the limbs, face, lips, throat, or gastrointestinal tract.
- Abdominal swelling can cause severe discomfort requiring exploratory surgery.
- The kinin-generating pathway, not the complement enzymes, is responsible for edema formation in HAE. Bradykinin synthesis via this pathway leads to changes in tissue permeability.
Management of these deficiencies and associated conditions may involve various treatments:
- Acute treatments for HAE include replacement therapy with C1 inhibitor, kallikrein inhibitor ecallantide, and bradykinin-2 receptor antagonist icatibant.
- Preventative therapy may include attenuated androgens and C1 inhibitor.
In summary, deficiencies in components of the classical pathway, such as C1q, C1r, C1s, C4, C2, and C1-Inh, can lead to immune disorders like SLE, RA, and HAE. These deficiencies can impair the complement system’s ability to eliminate immune complexes and dying cells, resulting in inflammation, recurrent infections, and other associated symptoms. Proper management and treatment are essential to mitigate the complications of these deficiencies.
FAQ
What is the classical pathway of complement activation?
The classical pathway is one of the three pathways (classical, lectin, and alternative) by which the complement system, a part of the immune system, can be activated. It is initiated by the binding of antibodies (IgM or IgG) to antigens, leading to a cascade of complement component reactions.
How is the classical pathway activated?
The classical pathway is activated when antibodies bind to antigens, forming antigen-antibody complexes. This binding triggers conformational changes in the Fc region of the antibody, allowing the complement component C1 to bind.
What is the role of C1 in the classical pathway?
C1 is a complex composed of three distinct molecules: C1q, C1r, and C1s. C1q binds to the Fc region of the antibody-antigen complex, leading to the activation of C1r and C1s.
What happens after C1 is activated?
Activation of C1 results in the sequential activation of other complement components, including C4, C2, and C3. These components undergo proteolytic cleavage and generate active fragments that participate in immune responses and inflammation.
What is the role of C3 in the classical pathway?
C3 is a crucial complement component. When activated, it can initiate the formation of C3 convertase, which further amplifies the complement cascade and leads to the opsonization of pathogens, recruitment of immune cells, and formation of the membrane attack complex (MAC).
What is the membrane attack complex (MAC)?
The MAC is a structure formed by complement components, including C5b, C6, C7, C8, and multiple C9 molecules. It forms pores in the cell membranes of target cells, leading to cell lysis and destruction.
Can deficiencies in the classical pathway occur?
Yes, deficiencies in various components of the classical pathway, such as C1q, C1r, C1s, C4, C2, and C1-inhibitor, can occur. These deficiencies can contribute to autoimmune disorders and increase the risk of infections.
How are deficiencies in the classical pathway diagnosed?
Deficiencies in the classical pathway components can be diagnosed through laboratory tests that measure the levels and activity of these complement proteins in the blood.
What are the consequences of deficiencies in the classical pathway?
Deficiencies in the classical pathway can impair the immune system’s ability to clear immune complexes and dying cells, leading to increased susceptibility to infections and autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
Can deficiencies in the classical pathway be treated?
Treatment options for deficiencies in the classical pathway depend on the specific component affected. In some cases, replacement therapy with deficient complement proteins or inhibitors may be used to manage symptoms and prevent complications. However, treatment approaches can vary, and it’s important to consult with a healthcare professional for personalized guidance.
References
- Textbook of Microbiology & Immunology by Subhash Chandra Parija
- Holsbach Beltrame, Marcia & Catarino, Sandra & Goeldner, Isabela & Boldt, Angelica & Reason, Iara. (2014). The Lectin Pathway of Complement and Rheumatic Heart Disease. Frontiers in pediatrics. 2. 148. 10.3389/fped.2014.00148.
- Brown, J. S., Hussell, T., Gilliland, S. M., Holden, D. W., Paton, J. C., Ehrenstein, M. R., … Botto, M. (2002). The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proceedings of the National Academy of Sciences, 99(26), 16969–16974. doi:10.1073/pnas.012669199
- https://www.creative-diagnostics.com/complement-system.htm
- https://www.svarlifescience.com/knowledge/focus-areas/complement-system-overview/classical-pathway
- https://www.creative-diagnostics.com/complement-system.htm
- https://www.creative-biolabs.com/complement-therapeutics/classical-pathway.htm
- https://aklectures.com/lecture/immunology/classical-pathway-of-complement-system
- https://en.wikipedia.org/wiki/Classical_complement_pathway
- https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/complement-deficiencies