An Overview of Complement System
- The enormous complexity of the human immune system not only enables good defence against an astounding variety of pathogens, but also protects against an undesired response against self-components.
- The immune system is traditionally classified into two broad and interrelated sections, the innate and the adaptive. The innate immune system offers an instantaneous and non-specific initial line of defence via humoral, cellular, and mechanical processes, and plays a crucial role in pathogen challenge protection.
- The complement system is a crucial component of the innate immune system, having three roles that overlap: protection against infection, clearance of immune complexes and cell debris, and connection between innate and adaptive immunity.
- The complement system is comprised of over 35 plasma proteins, complement receptors on cell surfaces, and regulatory proteins. The vast majority of soluble proteins circulate as inactive proenzymes or zymogens.
- Inactive molecules become active upon proteolytic cleavage, resulting in a proteolytic cascade that induces several effector activities, such as phagocytosis, inflammation, cell lysis, and direction of the adaptive immune response.
- To protect host tissues from the potentially damaging effects of complement activation, many inhibitors closely regulate this system. Destroying invading pathogens and minimising damage to host cells and tissues are the two primary objectives of the complement system’s regulation and activation mechanisms.
- The disruption of this delicate equilibrium has detrimental repercussions on the host, with possibly dire consequences.
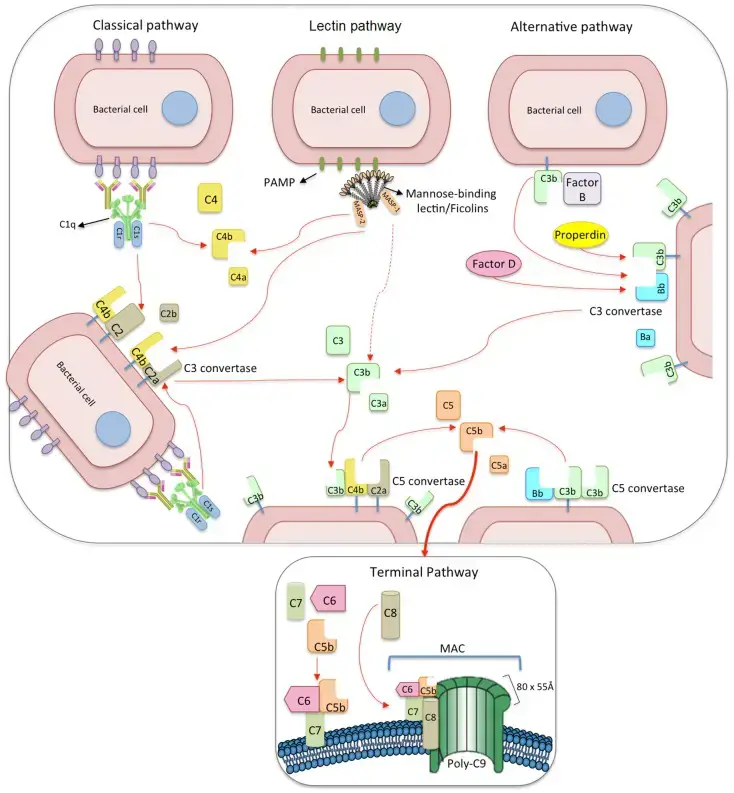
- On the surface of pathogens or damaged/infected cells, complement activation can occur via three separate but converging cascade pathways: classical, alternative, and lectin.
- Multiple inputs activate all three pathways independently, and the proteolytic cascades eventually converge on the activation of the main component C3, resulting in the construction of the membrane-attack complex (MAC).
- On immunological complexes, the activation of the classical pathway is begun by the binding of C1q to the Fc region of IgM or IgG.
- In contrast, the alternate route is activated by the spontaneous hydrolysis of C3 in plasma. Similarly to the alternate method, the lectin pathway can be activated without immune complexes.
- It is triggered by the binding of pattern-recognition plasma molecules such as mannose-binding lectin (MBL), collectin 11 (CL-K1), or ficolins to carbohydrates or acetylated residues present on microbes or to abnormal glycocalyx patterns on apoptotic, necrotic, or cancerous cells.
- Through the binding of MBL–MBL-associated serine proteases (MASPs) or ficolin–MASP complexes to fibrinogen or fibrin, the lectin pathway also plays a function in the coagulation system.
What is Lectin Pathway of Complement Activation?
- The lectin pathway, also known as the lectin complement pathway, is an essential component of the complement system. It operates similarly to the classical complement pathway, but with a distinct mechanism of activation. Unlike the classical pathway, the lectin pathway does not rely on antibody recognition of its target. Instead, it begins with the binding of mannose-binding lectin (MBL) or ficolin to specific sugars.
- In this pathway, MBL binds to sugars such as mannose, glucose, or other sugars that possess 3- and 4-OH groups arranged in the equatorial plane. These sugars are typically found in the carbohydrate or glycoprotein components of microorganisms, including bacteria like Salmonella, Listeria, and Neisseria strains. Additionally, MBL can also bind to fungal pathogens like Candida albicans and Cryptococcus neoformans, as well as certain viruses such as HIV-1 and Respiratory syncytial virus (RSV).
- Mannan-binding lectin, also referred to as mannose-binding protein, is a member of the collectin family and is produced by the liver. It plays a pivotal role in initiating the complement cascade by binding to the surfaces of pathogens. Once mannose-binding proteins bind to pathogen surfaces, the lectin pathway is activated, following a similar course as the classical pathway, resulting in the production of activated complement proteins further down the cascade. Apart from lectins, other pattern recognition molecules involved in the lectin pathway include collectins, ficolins H, L, and M.
- Within the lectin pathway, a series of enzymatic reactions occur in the innate immune system. Carbohydrate-binding proteins called lectins play a critical role by initiating the reactions upon binding to the surfaces of various pathogens. One key component of this pathway is the activation of mannose-binding lectin-associated serine proteases (MASPs), which are soluble serine proteases found in serum.
- In summary, the lectin pathway of complement activation operates through the binding of mannose-binding lectin or ficolin to specific sugars on the surface of pathogens. This triggers a cascade of enzymatic reactions, involving the activation of MASPs and the generation of activated complement proteins. The lectin pathway serves as an important defense mechanism within the innate immune system, providing a means to recognize and neutralize a wide range of microbial threats.
Components of Lectin Pathway
1. Mannose-binding lectin
- Mannose-binding lectin is a major recognition molecule in the lectin pathway, produced in liver cells and released as multimeric complexes with large molecular weight.
- It belongs to the collectin protein family, sharing collagen and carbohydrate-recognition domains (CRD).
- MBL is a C-type lectin due to its capacity to detect sugar moieties in a Ca2+-dependent manner; it is also referred to as “defensive collagen” due to its essential function in innate immunity and pathogen clearance.
- A trimer of identical polypeptide chains containing a cysteine-rich N-terminal domain, a collagen-like area, an alpha-helical coiled-coil neck domain, and a C-terminal CRD compose mannose-binding lectin.
- The disulfide bonds between the three chains constitute the structural unit of MBL, which polymerizes into higher-order MBL oligomers.
- Unlike dimers and higher-order oligomers, single MBL trimers are not fully functional, with tetramers predominating in circulation.
- The CRD domain is responsible for the recognition and binding of MBL to its ligands, and the oligomeric configuration confers multivalent and highly avid binding to targets.
- Mannose-binding lectin identifies repeating arrays of carbohydrate structures on pathogenic organisms such as viruses, bacteria, fungi, protozoa, multicellular parasites, and apoptotic/tumoral cells.
- MBL detects sugars with 3- and 4-OH groups positioned in the equatorial plane of the sugar ring, including glucose, L-fucose, N-acetylmannosamine (ManNAc), and N-acetylglucosamine (GlcNAc), but not galactose.
- MBL can also bind phospholipids, nucleic acids, and proteins that are not glycosylated.
- After binding to targets, MBL promotes multiple biological effects, including activation of the complement system via the lectin route, opsonophagocytosis, inflammatory regulation, and detection of changed self-structures.
- In addition, MBL may affect both mRNA and protein levels of cytokine production.
- By identifying damage-associated molecular patterns, mannose-binding lectin also contributes to the clearance of apoptotic cells (DAMPs).
- MBL enables the detection and phagocytosis of apoptotic cells by macrophages, resulting in their clearance, by binding to the terminal sugars of cytoskeletal proteins in apoptotic cells.
- The attachment of apoptotic cells to immature dendritic cells and macrophages is facilitated by both C1q and MBL.
- Defects in the clearance of apoptotic cells have been implicated in the aetiology of several autoimmune disorders, although the precise involvement of MBL in this process is uncertain.
- Experiments with MBL-deficient mice revealed an impairment in the elimination of apoptotic cells, but no association with autoimmune illness.
Structural subunits of mannan-binding lectin (MBL) and ficolins
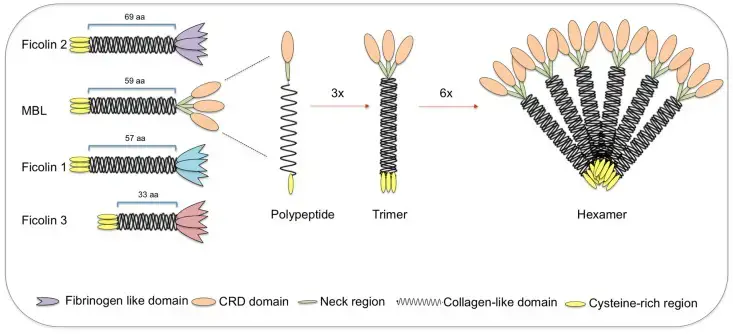
- MBL and ficolins both have a brief cysteine-rich region at the N-terminus, followed by a collagen-like sequence [length provided in number of amino acids (aa)].
- The C-terminal region consists of a carbohydrate-recognition binding domain for MBL (shown by ovals) and a fibrinogen-like domain for ficolins (shown as tulip forms).
- The polypeptides bind via their collagen-like region to produce triple helices (trimers), which then build higher oligomeric structures (tetramers to hexamers).
- Even though they have vastly different structures, ficolin polypeptides and trimers interact similarly to MBL, generating highly oligomeric forms (tetramers).
- MBL-associated serine proteases interact with collagen-like regions, triggering the complement lectin pathway.
2. MBL serum levels and MBL2 gene polymorphisms
- Mannose-binding lectin is encoded by the MBL2 gene, which is located on chromosome 10’s long arm (10q11.2–q21).
- It is considered an acute-phase reactant, and its levels can increase up to threefold during the acute phase of an immune response, primarily due to the upregulation of acute-phase mediators.
- Serum concentrations of MBL range from a few nanograms per millilitre to greater than 10 g/ml, with a typical value of approximately 0.8 g/ml.
- However, MBL levels are predominantly determined by MBL2 genetic polymorphisms, which account for up to 10-fold inter-individual differences in circulating MBL levels. In addition to genetic variance, MBL levels may also vary considerably over the course of a lifetime.
- Mannose-binding lectin insufficiency is quite prevalent, affecting 8–10% of the population and often characterised as 100 ng/ml in the circulation.
- MBL deficiency is more dangerous when other immunological abnormalities are present, while the majority of MBL-deficient individuals are otherwise healthy.
- MBL deficiency has been linked to upper respiratory tract infections in young infants and to an increased risk of serious infections in chemotherapy patients.
- Infections caused by intracellular pathogens, such as Mycobacterium spp. and Leishmania chagasi, which use C3 opsonization and C3 receptors to penetrate host cells, it may be advantageous.
- MBL2 is a highly polymorphic gene whose variants account for substantial differences in MBL levels and functional activity.
- These variants consist of at least one synonymous SNP (on codon 44 for asparagine) and eight non-synonymous variants situated in the first exon of the MBL2 gene (including B, C, and D, which are detailed in the next paragraph).
- MBL2*H and L alleles (due to a polymorphism at 550 bp), X and Y alleles (due to an SNP at 221 bp), and P and Q alleles (due to a non-coding SNP at +4 bp) also influence MBL levels.
- Sumiya et al. mapped the entire MBL2 gene in three British youngsters with recurrent bacterial infections and low MBL levels in 1991. Everyone carried the B allele (an exon 1 point mutation at codon 54, changing GGC to GAC and causing an amino acid change of glycine to aspartic acid – p.Gly54Asp).
- Others subsequently discovered two additional deficiency-causing frequent substitutions: allele D in codon 52 (CGT to TGT), which changes arginine to cysteine (p.Arg52Cys), and allele C in codon 57 (GGA to GAA), which replaces glycine with glutamic acid (p.Gly57Glu).
- In homozygous (e.g., B/B) or compound heterozygous (e.g., B/C) carriers, Exon 1 mutations drastically impair protein assembly and stability, increasing the proportion of weakly oligomerized MBL with diminished complement activation and ligand binding capacity.
- The wild allele at these locations is denoted by the letter A, while the collective designation for the D, B, and C alleles is 0.
- While 0/0 individuals have amounts of high-order MBL oligomers that are nearly undetectable, A/0 individuals may have lower plasma protein levels.
- In addition, a promoter mutation with X and Y alleles 221 kb before the transcription start site (g.602G > C) significantly reduces the amounts of otherwise completely functioning MBL proteins.
3. Ficolins
- Similarly to MBL, ficolins are pattern-recognition receptors that can bind to MASPs and activate the complement system via the lectin route; they play a crucial role in the immune response against clinically significant infections.
- In addition to activating complement, they limit infection by promoting macrophage secretion of interferon gamma (IFN-), interleukin-17 (IL-17), interleukin-6 (IL-6), tumour necrosis factor alpha (TNF-), and nitric oxide (NO).
- Similar to MBL, ficolins form oligomers of four structural subunits connected by disulfide bridges at the N-terminal regions, but higher or smaller oligomers appear to be less prevalent for ficolins.
- They should not be referred to as lectins, as they target acetylated molecules relatively independently of the structure of the acetylated molecule (carbohydrates being the preferred ligands for lectins). There are three human ficolins, each of which is encoded by its own gene.
Ficolin-1
- Ficolin-1 is linked to cell membranes or soluble in plasma at concentrations between 0.05 and 1.0 g/ml.
- It is also detected in monocyte secretory granules, neutrophil gelatinase granules, and type II alveolar epithelial cells.
- Ficolin-1 identifies common acetylated chemicals, such as GlcNAc and GalNAc, which bind to multiple Gram-positive (Staphylococcus aureus) and Gram-negative bacteria (Salmonella typhimurium LT2).
- It is the only human ficolin capable of binding to sialic acid, which is present on the surface of pathogens such as Streptococcus agalactiae and immune cells.
- Ficolin-1 is therefore believed to play a function in the regulation of immune cell interactions, blood coagulation, and/or fibrinolysis.
- Significantly, Ficolin-1 and pentraxin-3 heterocomplexes operate as non-inflammatory signals, boosting the clearance of changed self-cells and influencing the generation of IL-8.
- The FCN1 gene is on chromosome 9q34 and is composed of nine exons. At least eight SNPs described for the FCN1 gene are related with Ficolin-1 levels, four of which are located in the gene’s promoter and first exon.
- These polymorphisms contribute to the considerable variation (up to 15-fold) in Ficolin-1 plasma concentrations between individuals.
- FCN1 polymorphisms were related with an increased risk of death in patients with systemic inflammation and with rheumatoid arthritis susceptibility.
- Low levels of Ficolin-1 have been linked to a 12-fold greater risk of deadly necrotizing enterocolitis, the requirement for mechanical ventilation, and the development of serious infections in chemotherapy-treated cancer patients.
Ficolin-2
- Ficolin-2 is a plasma protein that is produced mostly in the liver, although low mRNA levels have also been detected in the bone marrow, gut, tonsils, and foetal lung.
- Ficolin-2 can bind N-acetylated molecules, such as Acetyl-d-glucosamine (GlcNAc), N-acetylgalactosamine, and N-acetyllactosamine, along with artificially acetylated substances.
- It also binds N-acetylneuraminic acid found on encapsulated opportunistic pathogens such as Group B streptococci (Streptococcus agalactiae), bacterial peptidoglycan (PGN), fungal 1,3-beta-D-glucan, and hepatitis C virus envelope glycoproteins.
- Ficolin-2 binds to Mycobacterium bovis and flagellated protozoa, such as Giardia intestinalis and Trypanosoma cruzi, and interacts with C-reactive protein, consolidating its binding to bacteria and activating complement.
- Three SNPs in the promoter region and one in exon 8 are associated with variance in Ficolin-2 plasma levels: 986G > A, 602G > A, and 4A > G and p.Ala258Ser. Two other SNPs, at locations 558 and 64, do not appear to alter gene expression.
- Ficolin-2 deficiency (1,200 ng/ml) has been associated with bronchiectasis and respiratory infection, particularly in the presence of atopy (94–96), but did not influence susceptibility to invasive pneumococcal illness.
- In neonates from Poland, low Ficolin-2 levels were also associated with prematurity, low birth weight, and perinatal infections, as well as susceptibility to chronic Chagas disease.
- And while not being connected with the development of malaria, children with severe malaria had higher levels of Ficolin-2 than those with moderate malaria.
- In contrast, FCN2 SNPs associated with normal Ficolin-2 levels provided protection against leprosy susceptibility.
Ficolin-3
- Ficolin-3 is the most abundant recognition molecule of the lectin-pathway, with a mean plasma concentration between 3 and 54 g/ml and a 10-fold variation across people.
- Ficolin-3 was shown to be significantly expressed in liver and lung tissues, demonstrating its importance in lectin pathway activation and pulmonary host defence.
- Ficolin-3 is therefore believed to play a significant role in both systemic and local innate immune responses.
- Numerous bacteria, including Salmonella typhimurium, Salmonella minnesota, Escherichia coli, and Aerococcus viridans, include acetyl groups. Ficolin-3 has been demonstrated to identify these acetyl groups.
- It was also discovered that it shares binding sites with Ficolin-2 and MBL on the Giardia intestinalis surface.
- In addition, Ficolin-3 may facilitate the elimination of late apoptotic cells and may have a protective effect against autoimmunity.
- The human FCN3 gene is located on 1p36.11 and is highly conserved. Five amino acid exchanges with allele frequencies of less than 5% were described: p.Leu12Val, p.Leu117fs, p.Thr125Ala, p.Glu166Asp, and p.Val287Ala.
- This high level of conservation suggests that Ficolin-3 may play a vital role in the immunological response. Indeed, Ficolin-3 deficiency is exceedingly uncommon and has been linked to necrotizing enterocolitis in premature infants.
4. MBL-associated serine proteases
- On binding of MBL, ficolins, and CL-K1 to carbohydrates or acetyl groups on the surface of pathogens or changed self-tissues, MBL-associated serine proteases serve as activators of the lectin pathway.
- Five proteins have been discovered thus far, including three MASP enzymes (MASP-1, MASP-2, and MASP-3) and two truncated proteins, MAp19 and MAp44, which lack the serine protease domain and, hence, functional activity.
- In the presence of Ca2+, all MASPs are capable of interacting with MBL, ficolins, and CL-K1 to create a proteolytic complex.
- Both MASP-1 and MASP-2 are essential for activating the lectin pathway. Recent research demonstrated that MASP-1 activation can trigger MASP-2 activation. MASP-2 is capable of autoactivation, although under physiological conditions, MASP-1 is the essential activator of MASP-2.
- MASP-2 is a protease that efficiently cleaves C4 and C2 to generate C3 convertase (113, 117). Due to competition for MASP binding sites on recognition molecules, MASP-3 appears to reduce the lectin-activity. pathway’s
- Additionally, MASP-3 is mainly complexed with Ficolin-3 and is believed to block complement activation mediated by Ficolin-3.
- MASP-3 also participate on developmental processes. MAp44 has been demonstrated to adversely control the lectin pathway by competing for the same binding sites as MASP-2 and MASP-1. The roles of MAp19 and MAp44 are currently poorly known, although MAp44 has been shown to negatively regulate the lectin pathway.
- MASP-1 was the first reported MASP. While MASP-1 and MASP-2 are primarily produced in the liver and are present in plasma at concentrations of 11 and 0.4 g/ml, respectively, MASP-3 is produced in multiple different tissues in addition to the liver.
- Both C1r and C1s share structural similarities with all three MASPs. MASP-1, MASP-3, and MAp44 are encoded by the MASP1 gene situated on chromosome 3q27–q28, whereas MASP-2 and MAp19 are encoded by the MASP2 gene on chromosome 1p36.23–31.
- Some MASP1 and MASP2 gene polymorphisms result in altered blood levels and functions of MASPs, consequently affecting complement activation via the lectin route.
- Ammitzbll et al. discovered 10 SNPs related with blood levels of MASP-1, MASP-3, and MAp44 in the MASP1 gene.
- In cystic fibrosis patients, MASP1 SNPs were related with 3MC syndrome (131–133) and Pseudomonas aeruginosa colonisation.
- In addition, MASP levels (MASP-1, MASP-2, and MASP-3) were found to be predictive of infection and prolonged intensive care reliance in critically unwell children.
- In contrast, MASP2 polymorphisms were related with leprosy, human T lymphotropic virus infection, malaria, Chagas disease, bacterial infections, and hepatitis C susceptibility.
- Several disorders, including schizophrenia, septic shock, acute lymphoblastic leukaemia, non-Hodgkin lymphoma, central nervous system malignancies, and colorectal cancer, have been linked to MASP-2 levels.
- Collectively, these investigations have provided evidence that MASPs have a growing and significant biological function in human illnesses.
Steps, Mechanism, Process of Lectin Pathway
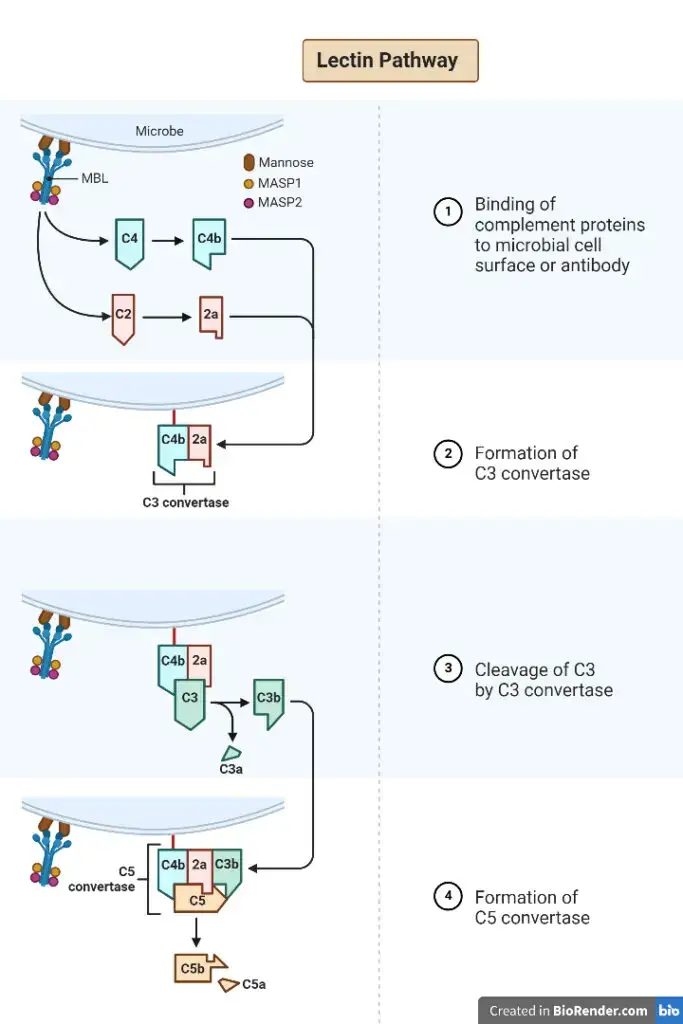
The lectin pathway, also known as the mannose binding lectin (MBL) pathway, consists of several steps that contribute to the immune response against pathogens. Here are the key steps involved in the lectin pathway:
- Recognition: The lectin pathway begins with the recognition of pathogens by pattern recognition receptors (PRRs) such as MBL or ficolins. These receptors are capable of binding to specific molecular patterns on the surface of pathogens, particularly those containing mannose sugars.
- Activation of MBL complex: When MBL binds to the pathogen’s surface, it forms a complex with two serine proteases called MASP-1 and MASP-2 (similar to C1r and C1s in the classical pathway). This MBL/MASP complex is crucial for the activation of the lectin pathway.
- Protease activation: Upon binding to the pathogen, MASP-1 and MASP-2 within the MBL complex are activated. MASP-1 is responsible for the cleavage and activation of MASP-2, and both proteases can cleave complement proteins.
- Cleavage of C4: The activated MASP-1 and MASP-2 proteases cleave the complement protein C4 into two fragments, C4a and C4b. C4b binds to the pathogen’s surface, facilitating further complement activation.
- Cleavage of C2: Following the binding of C4b, MASP-2 cleaves the complement protein C2 into C2a and C2b fragments. C2a then binds to C4b, forming a complex called C3 convertase.
- Cleavage of C3: The C3 convertase complex (C4b2a) generated in the previous step cleaves the complement protein C3 into C3a and C3b fragments. C3a is released into the surrounding fluid and participates in the inflammatory response.
- Amplification and cascade: The C3b fragment generated from the cleavage of C3 can bind to nearby surfaces, including the pathogen’s surface. This leads to the formation of additional C3 convertase complexes, amplifying the complement cascade and enhancing the immune response against the pathogen.
- Formation of membrane attack complex (MAC): The lectin pathway follows a similar pathway to the classical pathway, resulting in the formation of the membrane attack complex (MAC). The C5 convertase, formed by the sequential cleavage of C5 by C3 convertase, cleaves C5 into C5a and C5b. C5b recruits other complement proteins (C6, C7, C8, and C9) to assemble the MAC on the pathogen’s membrane.
- Cell lysis: The fully assembled MAC forms pores in the pathogen’s membrane, leading to cell lysis and destruction of the pathogen.
The lectin pathway plays a crucial role in the innate immune response, detecting and eliminating pathogens through the activation of the complement system. Understanding the steps involved in the lectin pathway provides insights into the mechanisms of immune defense and aids in the development of therapeutic interventions targeting complement activation.
Deficiencies of the Lectin Pathway Components
- Deficiencies in components of the lectin pathway, such as MBL (mannose-binding lectin), M-ficolin, L-ficolin, H-ficolin, CL-11, and MASPs, have been a subject of interest in the context of immunodeficiencies. However, it is important to note that MBL deficiency, in particular, does not constitute a primary immunodeficiency on its own.
- MBL is an integral part of the lectin pathway of the complement system, which is an essential component of the immune defense system. It is believed that the lectin pathway may be the first line of defense before the conventional immune response kicks in. It was previously assumed that increased susceptibility to bacterial infections might be linked to MBL deficiency.
- However, when a test was developed to measure MBL levels in the blood, it was discovered that low or absent MBL is actually quite common, affecting a significant portion of the population, ranging from 5 to 30%.
- Therefore, the absence of MBL alone cannot account for severe immunodeficiency. If that were the case, a large percentage of the global population would experience frequent and life-threatening infections. It is important to recognize that low or absent MBL levels do not necessarily indicate a primary immunodeficiency condition.
- Unfortunately, the MBL test is not commonly ordered during immunodeficiency evaluations, and a low or absent MBL level is sometimes mistakenly considered evidence of a primary immunodeficiency disorder.
- Expert immunologists who specialize in caring for patients with primary immunodeficiencies have a different perspective. They believe that low or absent components of the lectin pathway, including MBL, do not cause immunodeficiency on their own.
- Currently, there is no recommended treatment for low or absent MBL, and immunoglobulin replacement therapy is not appropriate for this condition. It is crucial to emphasize that the detection of low or missing MBL does not provide a definitive explanation for an individual’s infections. The diagnostic process should continue until an accurate diagnosis is established.
- In such cases, the Immune Deficiency Foundation (IDF) recommends consulting with an experienced immunologist to assist with the diagnostic evaluation. These specialists can provide valuable insights and guidance to ensure a thorough and accurate diagnosis is reached.
Inhibitors of Lectin Pathway
While the Lectin Pathway plays a critical role in the immune response, uncontrolled activation of this pathway can lead to detrimental consequences. To prevent excessive and potentially harmful activation, various inhibitors are employed to regulate the lectin pathway.
One of the key inhibitors is C1-inhibitor, which acts as a control mechanism for several complement pathways, including the lectin pathway. C1-inhibitor effectively blocks the activity of C1, a critical component in the initiation of the classical and lectin pathways. By inhibiting C1, the excessive activation of the lectin pathway can be controlled, preventing the potential damage caused by uncontrolled complement activation.
Antithrombin (AT), another inhibitor, also plays a role in regulating the lectin pathway. AT is a naturally occurring molecule that regulates the coagulation cascade. It has been found to inhibit the serine proteases MASP-1 and MASP-2, which are essential components of the lectin pathway. By inhibiting these proteases, AT effectively modulates the lectin pathway’s activity, preventing excessive complement activation and maintaining the delicate balance of the immune response.
Furthermore, α(2)-macroglobulin acts as an inhibitor of MASP-1 and MASP-2. This molecule binds to and inhibits the activity of these proteases, preventing their involvement in the activation of the lectin pathway. By inhibiting MASP-1 and MASP-2, α(2)-macroglobulin helps regulate the lectin pathway and prevent uncontrolled complement activation.
An additional crucial inhibitor of the lectin pathway is Map44 (Mannose-binding lectin-associated protein of 44 Kilodalton). This protein acts as a competitive inhibitor by displacing MASP-2 from Mannose-binding lectin (MBL), a key recognition molecule in the lectin pathway. By doing so, Map44 prevents the cleavage of complement components C4 and C2, which are crucial steps in the activation of the lectin pathway. Through its competitive inhibition, Map44 effectively regulates the lectin pathway, ensuring controlled complement activation.
These inhibitors of the lectin pathway play vital roles in maintaining the appropriate balance of complement activation and preventing uncontrolled inflammation and tissue damage. By targeting specific components of the lectin pathway, they serve as important therapeutic targets for various complement-mediated diseases and conditions.
In summary, the lectin pathway, like other complement activation pathways, requires tight regulation to avoid excessive activation. Inhibitors such as C1-inhibitor, antithrombin, α(2)-macroglobulin, and Map44 are crucial in modulating the activity of the lectin pathway. Their roles in preventing uncontrolled complement activation highlight their significance in maintaining immune homeostasis and protecting against complement-mediated damage.
Applications and Significance of Lectin Pathway
The lectin pathway is a part of the innate immune system that helps in the recognition and elimination of pathogens. It is one of the three complement activation pathways, along with the classical pathway and the alternative pathway. The lectin pathway is primarily activated by the binding of specific pattern recognition molecules called lectins to certain carbohydrate structures present on the surface of pathogens. Here are some applications and significance of the lectin pathway:
- Pathogen recognition: The lectin pathway plays a crucial role in recognizing and initiating an immune response against pathogens such as bacteria, viruses, and fungi. Lectins, such as mannose-binding lectin (MBL), bind to specific carbohydrate patterns on the pathogen’s surface, marking it for destruction by the complement system.
- Opsonization and phagocytosis: Activation of the lectin pathway leads to the deposition of complement components on the surface of pathogens. This process, known as opsonization, enhances the recognition and uptake of pathogens by phagocytic cells, such as macrophages and neutrophils, which engulf and destroy the opsonized pathogens.
- Inflammatory response: Activation of the lectin pathway results in the generation of bioactive molecules, such as anaphylatoxins (C3a and C5a), which induce local inflammation. This inflammation helps in recruiting immune cells to the site of infection and promotes a more efficient immune response.
- Clearance of apoptotic cells: The lectin pathway also contributes to the clearance of apoptotic cells, which are dying or dead cells that need to be efficiently removed to prevent the release of harmful cellular contents. MBL and other lectins can recognize specific carbohydrate patterns exposed on apoptotic cells and trigger their clearance by phagocytes.
- Clinical significance: The lectin pathway has clinical significance in various ways. Deficiencies or dysfunction of lectins, such as MBL, have been associated with increased susceptibility to infections. Additionally, excessive activation of the lectin pathway can contribute to inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus.
- Diagnostic and therapeutic potential: The lectin pathway and its components, such as MBL, can be used as diagnostic markers for certain diseases. Measuring MBL levels in blood can help identify individuals with MBL deficiency and assess their susceptibility to infections. Moreover, targeting the lectin pathway components can have therapeutic potential in modulating immune responses in various diseases.
References
- Beltrame MH, Catarino SJ, Goeldner I, Boldt AB, de Messias-Reason IJ. The lectin pathway of complement and rheumatic heart disease. Front Pediatr. 2015 Jan 21;2:148. doi: 10.3389/fped.2014.00148. PMID: 25654073; PMCID: PMC4300866.
- Larsen F, Madsen HO, Sim RB, Koch C, Garred P. Disease-associated mutations in human mannose-binding lectin compromise oligomerization and activity of the final protein. J Biol Chem (2004) 279:21302–11. 10.1074/jbc.M400520200
- Ambrosio AR, De Messias-Reason IJ. Leishmania (Viannia) braziliensis: interaction of mannose-binding lectin with surface glycoconjugates and complement activation. An antibody-independent defence mechanism. Parasite Immunol (2005) 27:333–40. 10.1111/j.1365-3024.2005.00782.x
- Jack D, Turner M. Anti-microbial activities of mannose-binding lectin. Biochem Soc Trans (2003) 31:753–7. 10.1042/BST0310753
- Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, et al. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol (2004) 173:3044–50. 10.4049/jimmunol.173.5.3044
- Estabrook MM, Jack DL, Klein NJ, Jarvis GA. Mannose-binding lectin binds to two major outer membrane proteins, opacity protein and porin, of Neisseria meningitidis. J Immunol (2004) 172:3784–92. 10.4049/jimmunol.172.6.3784
- Kang HJ, Lee S-M, Lee H-H, Kim JY, Lee B-C, Yum J-S, et al. Mannose-binding lectin without the aid of its associated serine proteases alters lipopolysaccharide-mediated cytokine/chemokine secretion from human endothelial cells. Immunology (2007) 122:335–42. 10.1111/j.1365-2567.2007.02644.x
- Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol (2005) 174:3220–6. 10.4049/jimmunol.174.6.3220
- Dahl M, Tybjaerg-Hansen A, Schnohr P, Nordestgaard BG. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J Exp Med (2004) 199:1391–9. 10.1084/jem.20040111
- Søborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis (2003) 188:777–82 10.1086/377183
- Boldt AB, Messias-Reason IJ, Meyer D, Schrago CG, Lang F, Lell B, et al. Phylogenetic nomenclature and evolution of mannose-binding lectin (MBL2) haplotypes. BMC Genet (2010) 11:38. 10.1186/1471-2156-11-38
- Boldt AB, Culpi L, Tsuneto LT, de Souza IR, Kun JF, Petzl-Erler ML. Diversity of the MBL2 gene in various Brazilian populations and the case of selection at the mannose-binding lectin locus. Hum Immunol (2006) 67:722–34. 10.1016/j.humimm.2006.05.009
- Boldt AB, Luty A, Grobusch MP, Dietz K, Dzeing A, Kombila M, et al. Association of a new mannose-binding lectin variant with severe malaria in Gabonese children. Genes Immun (2006) 7:393–400. 10.1038/sj.gene.6364312
- Jensenius JC. The manna-binding lectin (MBL) pathway of complement activation: biochemistry, biology and clinical implications. Adv Exp Med Biol (2005) 564:21–2 10.1007/0-387-25515-X_6
- Jack D, Turner M. Anti-microbial activities of mannose-binding lectin. Biochem Soc Trans (2003) 31:753–7. 10.1042/BST0310753
- https://www.sinobiological.com/research/complement-system/complement-activation-lectin-pathway
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/lectin-pathway
- https://www.svarlifescience.com/knowledge/focus-areas/complement-system-overview/lectin-pathway
- https://en.wikipedia.org/wiki/Lectin_pathway
- https://www.creative-biolabs.com/complement-therapeutics/lectin-pathway.htm