Contents
What is Stem cell?
- Stem cells are undifferentiated cells that have the remarkable ability to self-renew and differentiate into specialized cell types. They serve as the building blocks of the human body, capable of generating all other cells with specialized functions. In the right experimental conditions, stem cells can divide and produce daughter cells that can either become new stem cells through self-renewal or mature into functional cells such as blood cells or brain cells.
- These cells hold immense potential in various fields of research, including developmental biology, disease modeling, and cell therapy. They offer a valuable tool for studying the development of organisms, understanding disease mechanisms, and exploring potential treatments. Unlike ordinary cell lines or immortalized cell lines, stem cells provide researchers with a means to investigate cellular processes while preserving the genetic integrity of the cells.
- Stem cells have the unique property of being able to differentiate into different cell types, making them highly versatile. This differentiation process is tightly regulated and controlled by various factors and signals within the cellular environment. By manipulating these cues, researchers can direct the fate of stem cells and guide them to develop into specific cell lineages.
- The study of stem cells has faced some challenges in its early stages, but significant progress has been made in understanding their properties and potential applications. To aid researchers in their investigations, stem cell culture guides and resources have been developed to provide the necessary techniques and tools for working with these cells.
- In summary, stem cells are a special type of undifferentiated cells that possess the ability to self-renew and differentiate into a wide range of specialized cell types. Their unique properties make them invaluable in scientific research, offering insights into development, diseases, and therapeutic approaches.
Classification of Stem Cells
Stem cells can be classified into different categories based on their origin, potency, and differentiation potential. Here are the main classifications of stem cells:
- Embryonic Stem Cells (ESCs): These stem cells are derived from the inner cell mass of blastocysts, which are 3 to 5-day-old embryos. Embryonic stem cells are considered pluripotent because they have the ability to differentiate into all cell types of the body. They are highly versatile and hold great potential for regenerative medicine and tissue engineering.
- Adult Stem Cells (ASCs) or Tissue-specific Stem Cells: These stem cells are found in specific adult tissues or organs throughout the body. They are responsible for maintaining and repairing the tissue in which they reside. Adult stem cells are generally multipotent or sometimes unipotent, meaning they can differentiate into a limited range of cell types related to their tissue of origin. Examples of adult stem cells include hematopoietic stem cells (found in bone marrow and responsible for blood cell production) and mesenchymal stem cells (found in various tissues like bone marrow, adipose tissue, and umbilical cord tissue).
- Induced Pluripotent Stem Cells (iPSCs): iPSCs are artificially generated from adult cells, typically by reprogramming the cells using genetic techniques. By introducing specific genes into the adult cells, they are transformed into a pluripotent state similar to embryonic stem cells. iPSCs exhibit the ability to differentiate into various cell types and hold promise for personalized medicine, disease modeling, and drug discovery.
- Fetal Stem Cells: These stem cells are derived from the tissues of developing fetuses, typically obtained during prenatal diagnosis or elective terminations. Fetal stem cells have intermediate properties between embryonic and adult stem cells, displaying greater differentiation potential than adult stem cells but less than embryonic stem cells.
- Umbilical Cord Stem Cells: Stem cells can be found in the umbilical cord blood and tissue. Umbilical cord blood contains hematopoietic stem cells, which are used in transplantations for treating certain blood disorders and cancers. Umbilical cord tissue also contains mesenchymal stem cells, which have potential applications in regenerative medicine.
- Multipotent Stem Cells: These stem cells have the ability to differentiate into a limited number of cell types within a particular lineage. Examples include neural stem cells, which can differentiate into various types of neural cells, and hematopoietic stem cells, which can give rise to different blood cell types.
It’s important to note that the classification of stem cells is not always rigid, as there can be overlap and ongoing research to explore the properties and capabilities of different stem cell types. Additionally, new discoveries and advancements in stem cell research may lead to further refinements in their classification.
Sources of Stem Cells
Stem cells can be obtained from various sources, each with its own advantages and limitations. Here are the main sources of stem cells:
- Embryonic Stem Cells (ESCs): Embryonic stem cells are derived from embryos at the blastocyst stage, typically around 3 to 5 days after fertilization. There are three primary sources of human ESCs:
- Existing embryonic stem cell lines: These are stem cell lines that have been established from previously created embryos for research purposes. These cell lines serve as a valuable resource for studying stem cell biology and potential therapeutic applications.
- Spare embryos from in vitro fertilization (IVF): In the process of IVF, multiple embryos are often created to increase the chances of successful implantation. However, not all embryos are used for implantation, and those remaining can be donated for research purposes with the informed consent of the donors.
- Embryos created by somatic cell nuclear transfer (SCNT): SCNT involves transferring the nucleus of an adult somatic cell into an emptied egg cell, resulting in the creation of an embryo with the genetic material from the somatic cell donor. These embryos can be used to generate embryonic stem cell lines for research purposes.
- Adult Stem Cells (ASCs): Adult stem cells, also known as tissue-specific stem cells or somatic stem cells, are found in various tissues and organs of the body. They can be obtained from different sources, including:
- Blood: Hematopoietic stem cells (HSCs) can be found in the bone marrow, peripheral blood, and umbilical cord blood. They are responsible for generating different blood cell types and are commonly used in hematopoietic stem cell transplantation.
- Bone marrow: Bone marrow contains a rich population of adult stem cells, including hematopoietic stem cells and mesenchymal stem cells (MSCs), which can differentiate into various cell types like bone, cartilage, and fat.
- Umbilical cord blood: The blood within the umbilical cord and placenta is a rich source of hematopoietic stem cells. It can be collected after childbirth and stored in cord blood banks for potential future use.
- Adipose tissue: Adipose tissue, commonly known as fat, contains a population of stem cells called adipose-derived stem cells (ADSCs). These cells can differentiate into several cell types and are relatively abundant in adipose tissue.
- Induced Pluripotent Stem Cells (iPSCs): iPSCs are generated by reprogramming adult cells, such as skin cells or blood cells, through genetic manipulation. By introducing specific genes, typically called pluripotency factors, into the adult cells, they can be transformed into a pluripotent state similar to embryonic stem cells.
Each source of stem cells has its own advantages and considerations, including availability, differentiation potential, ethical concerns, and potential for immune rejection. Scientists continue to explore and develop techniques to enhance the generation and utilization of different types of stem cells for various research and therapeutic applications.
Unique properties of all stem cells
Stem cells possess unique properties that distinguish them from other cells:
- Self-renewal: Stem cells have the remarkable ability to self-renew, meaning they can replicate and produce more stem cells. This characteristic allows stem cell populations to be maintained over time. When a stem cell divides, it can give rise to two identical daughter cells, both with the potential to remain as stem cells, or it can produce one stem cell and one more differentiated cell. The precise control mechanisms that regulate the balance between these types of divisions are still being studied. Understanding the process of self-renewal is crucial for comprehending how stem cells are regulated during development, aging, and disease. It may also enable scientists to improve the efficiency of stem cell production in the laboratory. Discovering the specific factors and conditions that maintain pluripotent stem cells in an undifferentiated state is an area of significant interest and ongoing research.
- Recreation of functional tissues: Stem cells possess the capacity to differentiate into specialized cells and recreate functional tissues. Pluripotent stem cells, such as embryonic stem cells, are undifferentiated and do not possess tissue-specific characteristics. Yet, they have the potential to give rise to all the different cell types in the body, including heart muscle cells, blood cells, and nerve cells. On the other hand, adult stem cells, also known as tissue-specific or somatic stem cells, reside in specific tissues or organs and have a more limited differentiation potential. They can differentiate into the specialized cell types of their respective tissue or organ. Adult stem cells play crucial roles in tissue maintenance, repair, and regeneration. The process of differentiation involves multiple stages where cells become progressively more specialized. Scientists are making progress in understanding the signals that trigger each step of this differentiation process. These signals can include factors secreted by neighboring cells, physical contact with other cells, and specific molecules in the microenvironment surrounding the stem cells.
The unique properties of stem cells, including their self-renewal and differentiation abilities, hold tremendous potential for various fields of research and applications in regenerative medicine, disease modeling, and drug discovery. Understanding and harnessing these properties are essential for unlocking the full potential of stem cells in scientific and medical advancements.
Applications of Stem Cells
Stem cells have a wide range of applications in various fields of research and medicine. Here are some of the key areas where stem cells are utilized:
- Reparation and Reconstruction of Organs: Stem cells hold great promise for regenerative medicine. They can be used to repair and regenerate damaged or diseased tissues and organs. By directing the differentiation of stem cells into specific cell types, scientists aim to replace or restore the function of damaged organs, such as the heart, liver, kidneys, and spinal cord.
- Sources of Artificial Organs and Tissues: Stem cells can serve as a valuable source for creating artificial organs and tissues. Through tissue engineering techniques, stem cells can be guided to differentiate into specific cell types and assembled into functional organs or tissues in the laboratory. These engineered organs and tissues can potentially be used for transplantation, providing a solution to the shortage of donor organs.
- New Drug Development: Stem cells play a crucial role in drug discovery and development. They can be used to generate disease-specific cell models that closely mimic human tissues, allowing researchers to study the effects of drugs on specific cell types. This enables more accurate and efficient screening of potential therapeutic compounds and helps in the development of safer and more effective drugs.
- Gene Function Research: Stem cells provide a valuable tool for studying gene function and regulation. By manipulating the genetic material in stem cells, researchers can investigate how specific genes contribute to normal development, disease processes, and various cellular functions. This knowledge enhances our understanding of genetic disorders and can lead to the development of targeted therapies.
- Gene Therapy: Stem cells offer a promising avenue for gene therapy, a technique aimed at correcting genetic disorders. By introducing or modifying genes within stem cells, researchers can potentially replace defective genes or introduce therapeutic genes. These genetically modified stem cells can then be used for transplantation, providing a source of healthy cells that can restore normal function in the body.
- Toxicology and Pharmacology Research: Stem cells provide an alternative to animal testing in toxicology and pharmacology research. They can be used to model human tissues and organs, allowing for the assessment of drug toxicity and efficacy in a more relevant and ethical manner.
- Cancer Research: Stem cells play a critical role in cancer research, particularly in understanding the mechanisms of cancer development and progression. By studying cancer stem cells, which are a small population of cells within tumors with stem cell-like properties, researchers aim to unravel the processes involved in tumor growth, metastasis, and resistance to treatment. This knowledge contributes to the development of targeted cancer therapies.
- Technical Support: Stem cells provide technical support in various research areas. They serve as a valuable tool for testing new experimental techniques, optimizing protocols, and advancing scientific knowledge across different disciplines.
The applications of stem cells continue to expand as researchers make new discoveries and refine their understanding of stem cell biology. These versatile cells hold immense potential for revolutionizing medicine and advancing our ability to treat various diseases and injuries.
What is Stem cell culture?
Stem cell culture refers to the process of growing and maintaining stem cells in a laboratory setting. It involves providing the necessary conditions and nutrients to support the growth and proliferation of stem cells while preserving their unique properties and potential for differentiation.
Stem cell culture typically involves the following steps:
- Isolation: Stem cells can be obtained from various sources, such as embryos, adult tissues, or induced pluripotent stem cells (iPSCs). The first step is to isolate the stem cells from the source material using specific techniques and protocols.
- Expansion: Once isolated, stem cells are cultured in a suitable culture medium that provides the necessary nutrients, growth factors, and signaling molecules to support their survival and proliferation. The culture medium is carefully formulated to mimic the natural environment that stem cells require for growth.
- Feeding and Maintenance: Stem cell cultures need regular feeding and maintenance to ensure their viability and optimal growth. This involves regularly replacing the culture medium with fresh, nutrient-rich medium and removing waste products and dead cells.
- Contamination Control: Maintaining a sterile and contamination-free environment is crucial in stem cell culture. Strict aseptic techniques and appropriate culture conditions, such as using a laminar flow hood and sterile equipment, are employed to prevent the introduction of contaminants that could affect the growth and integrity of the stem cells.
- Differentiation and Manipulation: Depending on the research or application goals, stem cells may be induced to differentiate into specific cell types by altering the culture conditions or adding specific differentiation-inducing factors. This allows researchers to generate specialized cell types for various purposes, such as tissue engineering or disease modeling.
Stem cell culture requires specialized expertise and adherence to strict protocols to ensure the success of the culture process and maintain the characteristics of the stem cells. It is a critical component of stem cell research, as it enables the expansion, manipulation, and study of stem cells in controlled laboratory conditions, opening up numerous possibilities for scientific investigation and therapeutic applications.
Types of Stem cell culture
There are two main types of stem cell culture systems: feeder-dependent and feeder-free.
1. Feeder-dependent Stem Cell Culture
Feeder-dependent stem cell culture involves co-culturing stem cells with “feeder cells,” such as fibroblasts, to support their pluripotency and proliferation. In this system, plates are prepared in advance with fibroblast-seeded layers. The feeder cells condition the culture medium through metabolic leakage and provide necessary proteins, including growth factors and extracellular matrix proteins, which support cell attachment and proliferation.
Mouse embryonic fibroblasts (MEFs) or human foreskin fibroblasts (HFFs) treated with mitomycin C or irradiated are commonly used as feeder cells. The inactivation of feeder cells prevents their overgrowth and competition with the slower-growing pluripotent stem cells. The plates for feeder cells are typically coated with a 0.1% gelatin solution.
2. Feeder-free Stem Cell Culture
Feeder-free stem cell culture systems utilize extracellular matrices instead of feeder cells to culture pluripotent stem cells. This approach has been made possible through advancements in stem cell culture technology. The feeder-free system achieves a balance between promoting pluripotent cell growth and inhibiting spontaneous cellular differentiation by precisely formulating the culture medium with essential amino acids, salts, nutritional elements, and growth factors.
Feeder-free culture conditions offer several advantages. They are easier to use, more reproducible, and amenable to larger scales. Additionally, concerns related to mixed cultures of stem cells and feeder cells are eliminated.
Feeder-dependent culture can be more labor-intensive and may carry a risk of transmitting animal pathogens if using MEFs. Feeder-free culture systems provide a more controlled environment for maintaining undifferentiated stem cells and offer greater flexibility in large-scale applications.
Both feeder-dependent and feeder-free stem cell culture systems are employed based on the specific research needs and preferences of researchers and the nature of the stem cells being cultured.
How do you culture stem cells in the laboratory?
Culturing stem cells in the laboratory involves specific techniques and conditions to support their growth and maintenance. Here’s an overview of how stem cells are cultured in the lab:
- Cell Culture: Stem cells are grown using a method called cell culture. The process begins by placing the stem cells in a culture dish that contains a nutrient-rich liquid called culture medium. The culture medium is carefully formulated to provide the necessary nutrients and growth factors required for the specific type of stem cells being cultured.
- Attachment and Proliferation: Most stem cells require a surface for attachment and growth. In the culture dish, the stem cells attach to the surface and start dividing and spreading. As they multiply, the culture dish can become overcrowded, requiring the cells to be transferred to new dishes in a process called subculturing. This process, also known as passaging, allows the stem cells to continue their growth and prevents overcrowding.
- Reprogramming to Induced Pluripotent Stem Cells (iPSCs): Regular cells, such as skin cells, can be reprogrammed to a pluripotent state to generate induced pluripotent stem cells (iPSCs). This reprogramming is achieved by introducing specific genes known as master regulators of pluripotency into the cells. Over time, these genes remodel the expression of multiple genes, transforming the differentiated cells into a pluripotent state.
- Differentiation: To generate specific types of differentiated cells from pluripotent stem cells, scientists manipulate the culture conditions. They may modify the composition of the culture medium, alter the surface of the culture dish, or induce gene expression changes. These modifications can direct the stem cells towards specific lineages and promote their differentiation into specialized cell types. Basic protocols or recipes have been established through years of experimentation for differentiating pluripotent stem cells into specific cell types.
- Laboratory Testing: Throughout the process of generating stem cell lines, scientists perform various tests to confirm the stem cell characteristics. These tests include:
- Verifying gene expression: Scientists check for the expression of multiple genes that are essential for stem cell function.
- Assessing proliferation rate: The rate of cell division is evaluated to ensure that the cells have the ability to self-renew.
- Examining genome integrity: Chromosome analysis is performed to examine the integrity of the stem cell genome.
- Demonstrating differentiation potential: Stem cells are tested to determine their ability to differentiate into specialized cell types. This can be achieved by removing signals that maintain pluripotency, causing pluripotent stem cells to spontaneously differentiate, or by adding specific signals that induce differentiation of adult stem cells into desired cell phenotypes.
By employing these techniques and tests, scientists can successfully culture and manipulate stem cells in the laboratory, enabling research and applications in various fields such as regenerative medicine, disease modeling, and drug development.
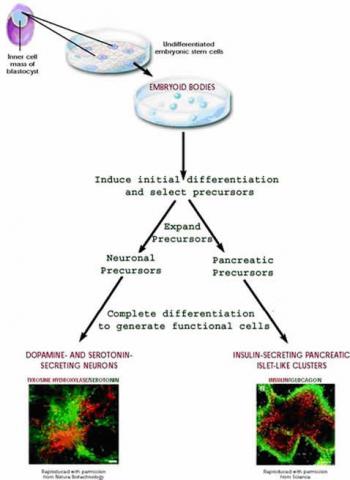
Feeder-free Stem Cell Culture
To culture stem cells in the laboratory, the following steps and materials are involved, as described in the provided content:
- Feeder-Free System: In a feeder-free system, fibroblast feeders are replaced with a biological matrix that provides a surface for the attachment of induced pluripotent stem cells (iPSCs). This eliminates the need for co-culturing stem cells with fibroblasts.
- Coating the Culture Dishes: Cell culture dishes are coated with Cell Basement Membrane, a biological matrix, to create a suitable surface for iPSC attachment. The dishes are incubated for one hour after coating to allow for proper preparation.
- Feeder-Free iPSC Culture Materials: The following materials are required for feeder-free iPSC culture:
- Pluripotent Stem Cell SFM XF/FF (500 mL, stored at -20°C): This is a specialized serum-free medium optimized for the growth and maintenance of iPSCs in a feeder-free system.
- Stem Cell Dissociation Reagent (250 mg, stored at 2°C to 8°C): This reagent is used for detaching and dissociating iPSCs from culture dishes when subculturing or passaging the cells.
- ROCK Inhibitor Y27632 (10 mg, stored at -20°C): This inhibitor helps to improve iPSC survival and attachment during the initial stages of culture.
- Cell Basement Membrane (5 mL, stored at -80°C): This biological matrix is used to coat the culture dishes and provide a suitable surface for iPSC attachment.
- Dulbecco’s Modified Eagle Medium (DMEM): F-12 Medium (500 mL, stored at 2°C to 8°C): This medium is a nutrient-rich culture medium commonly used for supporting the growth and maintenance of various cell types, including iPSCs.
- Dulbecco’s Phosphate Buffered Saline (D-PBS) (500 mL, stored at room temperature): This buffer solution is used for rinsing and washing iPSCs during the culture process.
- Starting the Culture: ATCC iPSCs, which are already adapted to the feeder-free system, can be directly cultured without the need for adaptation. The prepared culture dishes coated with Cell Basement Membrane are used to seed the iPSCs. The pluripotent stem cell SFM XF/FF medium, supplemented with appropriate growth factors and nutrients, is used to nourish and support the growth of iPSCs.
By following the procedures outlined above and utilizing the specific materials, stem cells can be successfully cultured in the laboratory using a feeder-free system. This allows for the growth, expansion, and manipulation of stem cells for various research and experimental purposes.
Preparation of Media Reagents
The preparation of media reagents for stem cell culture involves specific instructions and considerations. Here is a breakdown of the steps and information provided in the content:
- Pluripotent Stem Cell SFM XF/FF complete medium:
- Thaw the pluripotent Stem Cell SFM XF/FF complete medium at 2°C to 8°C overnight.
- The thawed medium is stable for up to two weeks at 2°C to 8°C.
- Prior to use, warm the medium for not more than 30 minutes in a 37°C water bath.
- It is recommended to prepare single-use aliquots of the medium to avoid repeated warming. If not used within two weeks, aliquots can be stored at -20°C.
- Cell Basement Membrane:
- Thaw the Cell Basement Membrane on ice and gently swirl to mix.
- Note that the Cell Basement Membrane will solidify in 15 to 30 minutes above 15°C.
- Keep the Cell Basement Membrane, vials, and pipette tips on ice at all times to prevent solidification.
- If air bubbles form, centrifuge the Cell Basement Membrane at 300 x g for 10 minutes at 2°C to 8°C to eliminate them.
- Determine the appropriate volume per aliquot based on concentration and usage. The concentration information can be found on the certificate of analysis.
- Dilute the Cell Basement Membrane in DMEM:F12 to the desired working concentration.
- Dispense the aliquots into pre-cooled tubes on ice and immediately store them at -20°C or -80°C for long-term storage.
- Stem Cell Dissociation Reagent:
- Bring the vial of Stem Cell Dissociation Reagent to room temperature before opening.
- The lyophilized material should be stored desiccated once opened.
- Prepare a 0.5 U/mL working solution by dissolving the appropriate amount of Stem Cell Dissociation Reagent in DMEM:F12.
- Filter sterilize the solution through a 0.22 µm filter membrane.
- Aliquot the solution into working volumes according to routine usage.
- Store the aliquots at -20°C for up to three months. Avoid repeated freezing and thawing. Thawed aliquots can be kept at 2°C to 8°C for up to two weeks.
- ROCK Inhibitor Y27632:
- ROCK Inhibitor Y27632 is soluble to 100 mM in water or D-PBS.
- Prepare a 10 mM stock solution by adding 3 mL of sterile water or D-PBS to the 10 mg vial of ROCK Inhibitor Y27632 and mix thoroughly.
- Aliquot the solution into working volumes according to routine usage. It is used at a final concentration of 10 μM (1:1000 dilution) in the cell culture medium.
- Store the aliquots at -20°C. Avoid repeated freezing and thawing. Thawed aliquots can be kept at 2°C to 8°C for two weeks.
Following these instructions ensures the proper preparation and storage of media reagents used in stem cell culture.
Prepare Cell Basement Membrane Coated Culture Dishes
To prepare Cell Basement Membrane coated culture dishes, follow these steps:
- Determine the number of dishes and required volume:
- This protocol is designed for coating two 6 cm2 dishes.
- Two (2) mL of Cell Basement Membrane is required per 6 cm2 dish.
- Adjust the volumes according to the size and number of tissue culture vessels you are using.
- Handling Cell Basement Membrane:
- Cell Basement Membrane will solidify in 15-30 minutes above 15°C, so it’s crucial to keep it and labware (pipette tips, serological pipettes, conical tubes) on ice at all times to prevent premature gelation.
- If necessary, if the Cell Basement Membrane has solidified, you can return it to a liquid state by placing it on ice at 2°C to 8°C for 24-48 hours.
- Thawing and sterilizing:
- Remove one aliquot of Cell Basement Membrane from -20°C/-80°C storage and place it on ice.
- Thaw the Cell Basement Membrane in the refrigerator (2°C to 8°C) or on ice overnight.
- Rinse the vial with 70% ethanol to sterilize it.
- Ensure all subsequent steps are carried out under strict aseptic conditions.
- Diluting Cell Basement Membrane:
- Place 4 mL of cold DMEM: F-12 Medium in a cold 15 mL conical tube on ice.
- Add the thawed Cell Basement Membrane to the 4 mL of DMEM: F-12 Medium on ice.
- Mix the solution well. The final concentration of Cell Basement Membrane should be 150 μg/mL.
- Coating the culture dishes:
- Immediately coat the dishes with the diluted Cell Basement Membrane. Use 2 mL for each 6 cm2 dish.
- Gently swirl the dish to ensure that the entire surface is evenly covered.
- Note: If air bubbles form during the coating process, use a chilled pipette tip to break them up.
- Incubation:
- Leave the coated dishes at 37°C for one hour.
- Cell plating and storage:
- Aspirate the coating solution from the dishes.
- Immediately plate the cells onto the coated dishes, ensuring the coating does not dry out.
- If the dishes will not be used on the same day they are prepared, do not aspirate the coating solution. Instead, seal the coated dishes with Parafilm and store them at 2°C to 8°C for up to one week.
- Before use, warm the stored dishes to room temperature in a biological safety cabinet for at least one hour.
Following these steps will ensure the proper preparation and storage of Cell Basement Membrane coated culture dishes for subsequent cell culturing.
Thawing of Cryopreserved iPSCs
To thaw cryopreserved iPSCs, follow these steps:
- Pre-warm the Pluripotent Stem Cell SFM XF/FF medium:
- Place the required volume of medium in a sterile conical tube.
- Pre-warm it in a 37°C water bath.
- If using a small volume of medium (50 mL or less), warm only the necessary volume to avoid multiple warming cycles.
- Thawing the cells:
- Remove the cryovial containing the frozen cells from liquid nitrogen storage.
- Gently swirl the cryovial in a 37°C water bath, ensuring that the O-ring and cap stay out of the water.
- Thaw the cells rapidly, usually taking approximately 1 to 2 minutes. Remove the cryovial from the water bath when only a few ice crystals remain.
- Sterilizing and transferring the cell suspension:
- Rinse the cryovial with 70% ethanol to sterilize it.
- Transfer the cell suspension from the cryovial to a 15 mL conical tube using a 1 mL or 5 mL pipette.
- Slowly add 4 mL of pre-warmed Pluripotent Stem Cell SFM XF/FF drop-wise to the conical tube.
- Use an additional 1 mL of medium to rinse the cryovial and transfer the liquid to the 15 mL tube.
- Gently shake the conical tube to mix the cells while adding the medium.
- Maintain the cells in clumps and avoid breaking them apart into a single-cell suspension.
- Centrifugation and resuspension:
- Centrifuge the cells at 200 x g for 5 minutes at room temperature.
- Aspirate and discard the supernatant.
- Tap the bottom of the tube gently to loosen the cell pellet.
- Add 1 mL of stem cell culture medium with ROCK Inhibitor Y27632.
- Gently resuspend the pellet by pipetting up and down 2 to 3 times with a 1 mL pipette tip. Take care not to over-pipette and create a single-cell suspension, as iPSCs should be maintained in clumps.
- Addition of ROCK Inhibitor and preparation of culture dishes:
- If necessary, aspirate the coating solution from the Cell Basement Membrane coated culture dishes prepared in the previous section.
- Add 4 mL of stem cell culture medium with ROCK Inhibitor Y27632 to each of the two 6 cm2 dishes.
- Seed 0.5 mL of the cell aggregates onto the prepared dishes.
- Incubation:
- Incubate the culture at 37°C in a suitable incubator.
- Maintain a 5% CO2 in air atmosphere for optimal growth conditions.
Following these steps will ensure the proper thawing and preparation of cryopreserved iPSCs for subsequent cell culture and expansion.
Maintenance of iPSC Cultures
To maintain iPSC cultures, follow these guidelines:
- Medium Change:
- On post-thaw day 1, perform a 100% medium change, removing all cells that did not attach.
- Perform a 100% medium change every day thereafter.
- Changing Media:
- Pre-warm the Pluripotent Stem Cell SFM XF/FF medium in a 37°C water bath.
- If using a small volume of medium (50 mL or less), warm only the required volume in a sterile conical tube to avoid multiple warming cycles.
- Remove the cells from the incubator and examine each dish under a microscope to assess cellular confluence and morphology of undifferentiated cells.
- Characteristics of differentiation include colonies with less defined edges, dark areas, or non-uniform morphology.
- Carefully aspirate the medium without disturbing the monolayer.
- Add 4 mL of fresh, pre-warmed Pluripotent Stem Cell SFM XF/FF to the 6 cm2 dish and return it to the incubator.
- Repeat the medium change every 24 hours, assessing cellular confluence under the microscope. If the cells are not ready for passage, repeat the medium change as described above.
- When cells are cultured for 5 days or have reached approximately 80% confluence with 90% undifferentiated cells, it is time to passage them.
- Identification and Removal of Differentiated Cells:
- Undifferentiated iPSCs form compact colonies with a high nucleus-to-cytoplasm ratio and prominent nucleoli. The center of the cells appears bright due to multilayering of colonies.
- During maintenance, differentiated cells may develop, which exhibit less-defined edges, dark areas, or non-uniform morphology.
- It is recommended to maintain less than 10% differentiation to ensure high-quality cultures. Differentiated cells must be removed from the culture.
- Observe the cultures under a microscope for the appearance of differentiated cells. Use a lens marker to mark areas of differentiation on the dish.
- Removal of Differentiated Cells:
- Attach a fine-tipped aspirating pipette to a vacuum source. The tip can be made smaller by attaching a 200 μL pipette tip to the end of the aspirating pipette.
- Suction away portions of colonies that appear differentiated, as marked earlier. Take care not to allow the cultures to dry out.
- Passage Cells:
- Cells are typically split at a 1:4 ratio when they are cultured for 5 days or reach 80% confluence.
- When colonies have grown to the point where adjacent colonies merge or differentiation occurs in the center of each colony, they are ready for passage.
Following these guidelines for the maintenance of iPSC cultures will help ensure the growth and preservation of undifferentiated cells while removing any differentiated cells to maintain a high-quality iPSC culture.
Passaging iPSCs
To passage iPSCs, follow these steps:
- Prepare:
- Ensure that culture dishes coated with Cell Basement Membrane are ready (refer to the section on Cell Basement Membrane).
- Remove an aliquot of 0.5 U/mL Stem Cell Dissociation Reagent working solution from the freezer and allow it to warm to room temperature (15°C to 25°C).
- Pre-warm Pluripotent Stem Cell SFM XF/FF in a 37°C water bath. If using a small volume of medium (50 mL or less), warm only the required volume in a sterile conical tube to avoid multiple warming cycles.
- Aspirate Medium:
- Aspirate the medium from the cells.
- Rinse Cells:
- Rinse the cells twice by adding and removing 4 mL per dish of D-PBS.
- Add Stem Cell Dissociation Reagent:
- Add 2 mL of Stem Cell Dissociation Reagent working solution to each dish.
- Incubate:
- Incubate the dishes at 37°C for 10 to 15 minutes. Monitor the reaction under a microscope starting at 5 minutes, as the incubation time may vary depending on the cell line and colony size.
- The reaction is complete when the edges of the individual colonies start to loosen and fold back from the dish.
- Aspirate and Rinse:
- Carefully aspirate the Stem Cell Dissociation Reagent.
- Gently rinse the colonies by adding and removing 4 mL per dish of DMEM: F-12 Medium, taking care not to dislodge the cells during manipulation.
- Ensure complete removal of the Stem Cell Dissociation Reagent, as it is not inactivated by medium or serum.
- Add Pluripotent Stem Cell SFM XF/FF with ROCK Inhibitor:
- Add 2 mL of Pluripotent Stem Cell SFM XF/FF with ROCK Inhibitor Y27632 to the dish.
- Gently detach the cells by pipetting up and down 2 to 3 times with a 1-mL tip. Avoid over-pipetting to prevent the formation of single-cell suspensions, as these cells will not establish colonies after seeding. If necessary, use a cell scraper to detach the cells.
- Transfer and Rinse:
- Transfer the cell aggregates to a 15 mL conical tube.
- Add an additional 3 mL of Pluripotent Stem Cell SFM XF/FF with ROCK Inhibitor Y27632 to the surface of the dish to collect any remaining cells. Transfer this rinse to the 15 mL conical tube containing the cell aggregates.
- Centrifuge and Aspirate:
- Centrifuge the cells at 200 x g for 5 minutes at room temperature to pellet the cells.
- Aspirate the supernatant and discard it.
- Resuspend:
- Add 1 mL of Pluripotent Stem Cell SFM XF/FF with ROCK Inhibitor Y27632.
- Gently resuspend the pellet by pipetting up and down 2 to 3 times with a 1 mL tip, maintaining the small cell aggregates. Avoid over-pipetting to maintain the desired colony morphology and prevent the formation of single-cell suspensions.
- Plate and Incubate:
- Plate the cells as desired on feeder or feeder-free cultures.
- Incubate the culture at 37°C in a humidified 5% CO2/95% air incubator.
- Perform a 100% medium change every day.
- Passage the cells every 4 to 5 days when they reach 80% confluence.
Following these steps for passaging iPSCs will help maintain their growth and ensure successful expansion for further experimentation or use.
Feeder-dependent iPSC Culture
Feeder-dependent iPSC culture involves the use of fibroblast-seeded plates as a supportive layer for iPSC growth. Here are the key points to consider:
- Preparation of Fibroblast-Seeded Plates:
- Fibroblast-seeded plates need to be prepared in advance. These plates serve as a feeder layer for iPSC culture.
- iPSC colonies can be easily distinguished from the fibroblasts. iPSC colonies exhibit well-defined and compact morphology.
- Individual iPSCs within the colony are tightly packed and have a high nucleus-to-cytoplasm volume ratio.
- It is important to ensure that the fibroblast-seeded plates are in good condition and capable of supporting iPSC growth.
- Materials Required:
- Pluripotent Stem Cell SFM XF/FF (500 mL), stored at -20°C.
- ROCK Inhibitor Y27632 (10 mg), stored at -20°C.
- DMEM (500 mL), stored at 2°C to 8°C.
- DMEM: F-12 Medium (500 mL), stored at 2°C to 8°C.
- Fetal Bovine Serum (FBS) (500 mL), stored at -20°C.
- D-PBS (500 mL), stored at room temperature.
Note: These materials are necessary for the feeder-dependent iPSC culture system.
- Morphology of iPSC Colonies:
- iPSC colonies can vary in morphology. Some may exhibit loose colony morphology with less-defined edges.
- However, undifferentiated iPSC colonies ready for passage typically display tightly packed iPSCs with well-defined sharp edges.
- Individual cells within the colony show prominent nucleoli and have a high nucleus-to-cytoplasm volume ratio.
These characteristics aid in identifying and maintaining the quality of iPSC cultures grown on fibroblast-seeded plates using Pluripotent Stem Cell SFM XF.
Feeder-dependent iPSC culture provides a supportive environment for the growth and expansion of iPSCs, allowing for the maintenance of their pluripotent state and the retention of their stem cell properties.
Preparation of Media Reagents
The preparation of media reagents involves the following steps:
- Pluripotent Stem Cell SFM XF/FF:
- Pluripotent Stem Cell SFM XF/FF medium is ready to use and does not require any additional supplements.
- If you have a 500 mL bottle of medium that will not be consumed within two weeks, it is recommended to aliquot the medium into desired volumes (e.g., 100 mL) and store them at -20°C.
- DMEM + 15% FBS (Feeder medium):
- Thaw the Fetal Bovine Serum (FBS) at room temperature.
- Add FBS to DMEM to achieve a final concentration of 15%.
- The complete feeder medium can be stored at 2°C to 8°C for up to two weeks.
- Reconstitution of Stem Cell Dissociation Reagent:
- Prepare a working solution of Stem Cell Dissociation Reagent with a concentration of 0.5 U/mL.
- Reconstitute the reagent in DMEM: F-12 Medium.
- Aliquot the working solution into appropriate volumes based on your usage requirements. Refer to the specific instructions in the Feeder-free iPSC Culture section for detailed information on reconstitution.
- Reconstitution of ROCK Inhibitor Y27632:
- Prepare a stock solution of ROCK Inhibitor Y27632 with a concentration of 10 mM.
- Reconstitute the inhibitor in D-PBS.
- Follow the instructions provided in the Preparation of Media Reagents step 4 for specific details on reconstitution.
These steps ensure the proper preparation of media reagents required for iPSC culture, including the Pluripotent Stem Cell SFM XF/FF medium, feeder medium (DMEM + 15% FBS), Stem Cell Dissociation Reagent, and ROCK Inhibitor Y27632. Following these instructions will help maintain the quality and effectiveness of the reagents used in iPSC culture.
Preparation of Feeder Cell Coated Dishes
The preparation of feeder cell coated dishes involves the following steps:
- Pre-warm DMEM + 15% FBS (Feeder Medium) in a 37°C water bath. If using a small volume of medium (50 mL or less), warm only the required volume in a sterile conical tube. Avoid multiple warming cycles of the complete medium.
- Pipette 5 mL of DMEM + 15% FBS into a 15 mL conical tube.
- Thaw one vial of Mouse Embryonic Fibroblasts (MEFs) or Human Foreskin Fibroblasts (HFFs) by gently swirling it in a 37°C water bath. Keep the O-ring and cap out of the water to prevent contamination. Thaw rapidly for approximately 1 to 2 minutes, removing the cryovial when a few ice crystals remain.
- Sterilize the vial by rinsing it with 70% ethanol. Ensure strict aseptic conditions from this point onward.
- Use a 1 mL or 5 mL pipette to gently transfer the cell suspension to the 15 mL conical tube containing DMEM + 15% FBS. Use an additional 1 mL of medium to rinse the vial and transfer its contents to the 15 mL tube.
- Add 4 mL of DMEM + 15% FBS to the tube, bringing the total volume to 10 mL.
- Gently mix and pellet the cells by centrifugation at 200 x g for 5 minutes.
- Discard the supernatant and resuspend the cells with 10 mL of fresh, pre-warmed DMEM + 15% FBS.
- Count the viable cells using a method such as erythrosin B exclusion. The cell viability should be above 85%.
- Add the appropriate amount of fresh, pre-warmed DMEM + 15% FBS to plate the cells at a seeding density of 1.2 x 104 cells/cm2. Refer to Table 2 for suggested plating volumes based on the growth area of the culture dish.
- Incubate the feeder cells at 37°C and 5% CO2. They should be plated 24 hours before use and stored under these conditions for no more than 7 days.
- When storing the plates, change the DMEM + 15% FBS medium twice during the week or when the pH decreases. Avoid excessive alkalinity of the medium during the cell recovery process.
Table 2 provides suggested seeding densities and plating volumes for MEFs/HFFs in different culture dishes, based on a calculated density of 12,000 cells/cm2.
| Cell Culture Vessel | Growth Area (cm2) | Total Number of Feeder Cells per Dish | Optimal Volume for Plating (mL) |
|---|---|---|---|
| 24 well | 2.0 | 2.4 x 104 | 0.5 |
| 12 well | 4.0 | 4.8 x 104 | 1.0 |
| 6 well / 35 mm2 | 9.5 | 1.2 x 105 | 2.0 |
| 6 cm2 | 21 | 3.4 x 105 | 4.0 |
| 10 cm2 | 56 | 9.4 x 105 | 10 |
Following these steps will help in the proper preparation of feeder cell coated dishes, which are essential for maintaining the typical morphology and growth of induced pluripotent stem cells (iPSCs) in a feeder-dependent cell culture system.
Thawing of Cryopreserved iPSCs
Thawing of cryopreserved induced pluripotent stem cells (iPSCs) involves the following steps:
- Pre-warm Pluripotent Stem Cell SFM XF/FF medium in a 37°C water bath. If using a small volume of medium (50 mL or less), warm only the required volume in a sterile conical tube. Avoid multiple warming cycles of the complete medium.
- Before thawing the cells, ensure that the culture dishes seeded with Mouse Embryonic Fibroblasts (MEFs) or Human Foreskin Fibroblasts (HFFs) are prepared. Refer to the “Preparation of Feeder Cell Coated Dishes” section for instructions.
- Replace the medium in the MEF or HFF feeder dishes (DMEM + 15% FBS) with 4 mL of Pluripotent Stem Cell SFM XF/FF containing 10 μM ROCK Inhibitor Y27632. Place the dish in the incubator for 15 minutes to allow the medium to reach its normal pH range of 7.0-7.6. You will need two 6 cm2 plates for each vial of cells being thawed.
- Remove the cryovial containing the frozen cells from the liquid nitrogen storage.
- Thaw the cells by gently swirling the cryovial in a 37°C water bath. Keep the O-ring and cap out of the water to prevent contamination. Thaw rapidly for approximately 1 to 2 minutes, removing the cryovial from the water bath when only a few ice crystals remain.
- Sterilize the cryovial with 70% ethanol. Ensure strict aseptic conditions for all subsequent operations.
- Use a 1 mL or 5 mL pipette to gently transfer the cell suspension to a 15 mL conical tube.
- Slowly add 4 mL of Pluripotent Stem Cell SFM XF/FF drop-wise to the conical tube. Use an additional 1 mL of medium to rinse the cryovial and transfer the liquid to the 15 mL tube. Gently shake the conical tube to mix the cells while adding the medium.
- Gently pipette the cells up and down 2 or 3 times to ensure thorough mixing. It is important to maintain the cells in clumps and not break apart the aggregates into a single-cell suspension.
- Centrifuge the cells at 200 x g for 5 minutes at room temperature.
- Aspirate and discard the supernatant. Gently tap the bottom of the tube to loosen the cell pellet.
- Add 1 mL of Pluripotent Stem Cell SFM XF/FF containing 10 μM ROCK Inhibitor Y27632 to the tube. Gently resuspend the pellet by pipetting up and down 2 or 3 times with a 1 mL pipette tip, maintaining the cell aggregates. Again, do not break apart the aggregates into a single-cell suspension.
- Seed 0.5 mL of the cell aggregates onto two 6 cm2 MEF or HFF feeder dishes prepared in step 2.
- Change the medium the next day and daily thereafter until the cells have been cultured for 5 days or the colonies reach 80% confluency. Subsequent medium changes do not require the addition of ROCK Inhibitor Y27632.
Following these steps will ensure proper thawing of cryopreserved iPSCs and their subsequent seeding onto feeder dishes for further culture and growth.
Maintenance of Feeder-dependent iPSC Cultures
Maintenance of feeder-dependent iPSC cultures involves regular media changes, identification and removal of differentiated cells, and determining the appropriate time for cell passage. Here are the steps involved:
Changing Media:
- Pre-warm Pluripotent Stem Cell SFM XF/FF in a 37°C water bath. If using a small volume of medium, warm only the necessary amount in a sterile conical tube. Avoid multiple warming cycles of the medium.
- Remove the iPSC cultures from the incubator and examine each dish under a microscope to assess cellular confluence and the morphology of undifferentiated cells. Differentiated cells can be identified by characteristics such as less defined edges, dark areas, or non-uniform morphology.
- Carefully aspirate the medium from the dish without disturbing the monolayer of cells.
- Add 4 mL of fresh, pre-warmed Pluripotent Stem Cell SFM XF/FF to the 6 cm2 dish and return the dish to the incubator.
- Every 24 hours, examine each dish under the microscope to determine the percentage of cellular confluence. If the cells are not ready for passage, repeat steps 3 and 4. When the cells have been cultured for 5 days or have reached approximately 80% confluence with 90% undifferentiated cells, it is time to perform a cell passage.
Identification and Removal of Differentiated Cells:
- To maintain high-quality cultures, it is recommended to have less than 10% differentiation. Differentiated cells need to be removed from the iPSC culture.
- Undifferentiated iPSCs grow as compact colonies with a high nucleus-to-cytoplasm ratio and prominent nucleoli. In Pluripotent Stem Cell SFM XF/FF, small iPSC colonies may initially exhibit loose colony morphology, but they will become more compact as the colony grows larger. The colonies should maintain a distinct border on the feeder cells. However, during expansion and maintenance, differentiated cells or other cell types may develop. Differentiating colonies will have less defined edges, dark areas, or a non-uniform morphology that is not typical of pluripotent stem cells.
- Observe the cultures under the microscope to identify areas of differentiated cells. Use a lens marker to mark these areas on the dish.
- To remove clusters of differentiated cells, attach a fine-tipped aspirating pipette to a vacuum source. The tip can be made smaller by attaching a 200 μL pipette tip to the end of the aspirating pipette. Aspirate the portions of colonies that appear differentiated, as marked in step 3. Take care not to let the cultures dry out.
When to Passage Cells:
- iPSC cultures are typically split at a 1:4 ratio when cells reach approximately 80% confluence, usually after 4 to 5 days. The colonies are ready for passage when adjacent colonies merge or differentiation occurs in the center of each colony.
- The morphology of undifferentiated iPSCs 2 days post-passage will appear different from the morphology of undifferentiated iPSCs 5 days post-passage, indicating that they are ready to be split.
By following these steps, feeder-dependent iPSC cultures can be maintained, ensuring the removal of differentiated cells and timely passage of cells for continued growth and expansion.
Passaging Feeder-dependent iPSC Cultures
Passaging feeder-dependent iPSC cultures involves dissociating and expanding the cells on a 6 cm2 dish. Here is a step-by-step guide for the process:
- Adjust the volumes according to the size and number of tissue culture vessels being used. Ensure that the culture dishes are coated with feeder cells as per the Preparation of Dishes with Feeder Cells protocol.
- Remove an aliquot of 0.5 U/mL Stem Cell Dissociation Reagent working solution from the freezer and allow it to warm to room temperature.
- Pre-warm Pluripotent Stem Cell SFM XF/FF in a 37°C water bath. If using a small volume of medium, warm only the necessary amount in a sterile conical tube. Avoid multiple warming cycles of the medium.
- Aspirate and discard the stem cell culture medium from the dish.
- Rinse the cells twice by adding and removing 4 mL of D-PBS per dish.
- Add 2 mL of Stem Cell Dissociation Reagent working solution to each dish.
- Incubate at 37°C for 10-15 minutes. Monitor the reaction under a microscope, starting at 5 minutes, as incubation time may vary depending on the cell line and colony size. The reaction is complete when the edges of the individual colonies begin to loosen and fold back from the dish.
- Carefully aspirate the Stem Cell Dissociation Reagent and gently rinse the colonies by adding and removing 4 mL of DMEM: F-12 Medium. Take care not to dislodge the cells during this step.
- Add 2 mL of Pluripotent Stem Cell SFM XF/FF to each dish. Gently detach the cells by pipetting up and down 2 to 3 times with a 1 mL tip. It is crucial to maintain the cells in clumps and not break them apart into a single-cell suspension.
- Transfer the cell aggregates to a 15 mL conical tube.
- Add an additional 4 mL of Pluripotent Stem Cell SFM XF/FF to the dish to collect any remaining cells on the surface. Transfer this rinse to the 15 mL conical tube containing the cell aggregates.
- Centrifuge the cells at 200 x g for 5 minutes at room temperature to pellet them.
- Aspirate the supernatant and gently tap the bottom of the tube to loosen the cell pellet.
- For each dish processed, add 2 mL of Pluripotent Stem Cell SFM XF/FF in the presence of 10 μM ROCK Inhibitor Y27632 to the 15 mL conical tube. Prepare the medium with ROCK inhibitor by adding 20 μL of 10 mM ROCK Inhibitor Y27632 to 20 mL of medium.
- Gently resuspend the pellet by pipetting up and down 2 to 3 times with a 1 mL tip, maintaining the small cell aggregates. Do not break apart the aggregates into a single-cell suspension.
- If desired, to deplete fibroblasts from the cultures, add the entire suspension to an uncoated 6 cm2 tissue culture dish with 4 mL of Pluripotent Stem Cell SFM XF/FF. Incubate for one hour at 37°C and 5% CO2. The fibroblasts will adhere while the iPSC clusters remain in suspension.
- Collect the medium into a 15 mL conical tube and centrifuge at 200 x g for 5 minutes at room temperature to pellet the cells.
- Aspirate the supernatant and gently tap the bottom of the tube to loosen the cell pellet.
- For each dish processed, add 2 mL of Pluripotent Stem Cell SFM XF/FF in the presence of 10 μM ROCK Inhibitor Y27632 to the 15 mL conical tube.
- Transfer 0.5 mL of the cell aggregates onto MEF- or HFF-coated dishes containing 4 mL Pluripotent Stem Cell SFM XF/FF in the presence of 10 μM ROCK Inhibitor Y27632.
- Swiftly move the dishes in a forward-to-backward, then left-to-right pattern once to gently disperse the cells evenly across the surface. Incubate the dishes overnight at 37°C and 5% CO2.
- Change the medium daily until the colonies are large enough for further passaging. Subsequent cell culture medium changes do not require the addition of ROCK Inhibitor Y27632.
Following these steps will allow for the successful passaging of feeder-dependent iPSC cultures, promoting their growth and expansion.
Protocol for Cryopreservation
The following is a protocol for cryopreservation of cells:
- Remove an aliquot of 0.5 U/mL Stem Cell Dissociation Reagent working solution from the freezer and allow it to warm to room temperature.
- Aspirate and discard the stem cell medium from the dish.
- Rinse the cells twice by adding and removing 4 mL of D-PBS per 6 cm2 dish.
- Note that for optimal results, cells should be cultured to 80% confluency before freezing.
- Add 2 mL of Stem Cell Dissociation Reagent working solution to each dish.
- Incubate at 37°C for 10-15 minutes or until the edges of the individual colonies begin to loosen and fold back. Monitor the dish under a microscope, starting at 5 minutes, as incubation time may vary depending on the cell line and colony size.
- Aspirate the Stem Cell Dissociation Reagent and gently rinse the colonies by adding and removing 4 mL of DMEM: F-12 Medium. Take care not to dislodge the cells during this step.
- Add 2 mL of stem cell culture medium to each dish and detach the cells by pipetting up and down 2 to 3 times with a 1 mL tip. It is important not to over-pipette the culture into a single-cell suspension. The cells should remain aggregated.
- Note that one 6 cm2 dish yields 2 mL of frozen cells, which will be frozen in 1 mL aliquots.
- Transfer the cell aggregates to a 15 mL conical tube.
- Add an additional 3 mL of stem cell culture medium to the dish to collect any remaining cells. Transfer this rinse to the 15 mL conical tube containing the cell aggregates.
- Centrifuge the cells at 200 x g for 5 minutes at room temperature to pellet them. While the cells are spinning, remove the Stem Cell Freezing Media from storage and swirl it to mix. Keep it cold and decontaminate it by dipping it in or spraying it with 70% alcohol.
- Aspirate the supernatant and discard it. Gently tap the bottom of the tube to loosen the cell pellet.
- Add 2 mL of cold Stem Cell Freezing Media to the tube. Gently resuspend the pellet by pipetting up and down 2 to 3 times with a 1 mL tip, maintaining the small cell aggregates. Cells recover more effectively from the freezing/thawing process if they are frozen in clumps.
- Immediately transfer 1 mL of the cell suspension into each of the two cryovials.
- Freeze the cells gradually at a rate of -1°C/min until the temperature reaches -70°C to -80°C. You can use a cryopreservation container such as a CoolCell freezing container or ATCC ACS-6000.
- Note that the cells should not be left at -80°C for more than 24 to 48 hours. Once at -80°C, transfer the frozen cryovials to the vapor phase of liquid nitrogen for long-term storage.
Following this protocol will allow for the cryopreservation of cells, ensuring their viability and long-term storage for future use.
FAQ
What are stem cells?
Stem cells are unique cells that have the ability to differentiate into various specialized cell types in the body. They possess the potential to self-renew and proliferate, making them valuable for regenerative medicine and research purposes.
What is stem cell culture?
Stem cell culture refers to the process of growing and maintaining stem cells in a controlled laboratory environment. It involves providing the cells with appropriate culture conditions, growth factors, and nutrients to support their survival, growth, and differentiation.
Why is stem cell culture important?
Stem cell culture is crucial for studying stem cell biology, understanding their properties and behavior, and exploring their potential applications in medicine. It provides researchers with a controlled environment to manipulate and study stem cells, allowing for advancements in stem cell-based therapies and regenerative medicine.
What types of stem cells are commonly cultured?
Various types of stem cells can be cultured, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells such as mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs).
What are the key components of stem cell culture media?
Stem cell culture media typically contain a basal medium supplemented with growth factors, cytokines, and other additives to support stem cell growth and maintenance. The specific components vary depending on the type of stem cells being cultured and their specific requirements.
How are stem cells cultured in the laboratory?
Stem cells are typically cultured in dishes or flasks coated with a layer of supportive material, such as feeder cells or extracellular matrix proteins. The culture media are carefully prepared and provided to the cells, and the cultures are maintained in controlled incubators with specific temperature, humidity, and gas conditions.
What are feeder cells in stem cell culture?
Feeder cells are typically mouse embryonic fibroblast cells (MEFs) or human feeder cells that provide a supportive environment for the growth and maintenance of pluripotent stem cells. They secrete factors that help stem cells self-renew and prevent them from differentiating.
How do you maintain the pluripotency of stem cells in culture?
To maintain pluripotency, stem cells are cultured in conditions that inhibit their spontaneous differentiation. This includes providing them with specific growth factors and signaling molecules, as well as optimizing the culture media and culture techniques to promote self-renewal and prevent differentiation.
What challenges are associated with stem cell culture?
Stem cell culture can be challenging due to the delicate nature of stem cells and their specific requirements. Maintaining their pluripotency, preventing contamination, and controlling the balance between self-renewal and differentiation are some of the key challenges faced in stem cell culture.
Can stem cells be cryopreserved for long-term storage?
Yes, stem cells can be cryopreserved for long-term storage. Cryopreservation involves freezing the cells at low temperatures using cryoprotective agents and storing them in liquid nitrogen. This allows stem cells to be stored for extended periods and thawed when needed for experiments or clinical applications.