What is Centrifuge?
- A centrifuge is a laboratory instrument that utilizes centrifugal force to separate mixtures based on their density. It is a valuable tool in science and medical research, as well as having applications in various aspects of daily life.
- The concept of centrifugation can be traced back to Benjamin Robins, a British military engineer, who developed an early device to measure drag on objects. This device consisted of an arm rotating around an axis. Building upon this concept, the Prandtl brothers created the first practical centrifuge by refining the design. They used it to separate milk from cream by rapidly rotating a container filled with milk. The centrifugal force generated during rotation caused the heavier cream to move towards the outer part of the container, facilitating separation.
- In a centrifuge, separation is achieved by spinning a mixture at high speeds, which generates a centrifugal force. The force exerted on the mixture causes the components with different densities to separate. The heavier particles or substances move towards the outer edges of the container, while the lighter ones remain closer to the center. This enables the separation of various materials based on their size, shape, density, and viscosity.
- Centrifuges find applications in diverse fields. In scientific and medical research, they are extensively used for the purification of cells, subcellular organelles, viruses, proteins, and nucleic acids. By spinning the mixture containing these components, centrifugation helps to isolate and concentrate the desired substance from the complex mixture. This purification process is crucial for studying and understanding the specific properties and functions of these biological materials.
- Beyond the realm of scientific research, centrifuges also have practical uses in everyday life. For example, they are employed in the food industry to separate cream from milk, as well as in the production of various beverages. In wastewater treatment plants, centrifuges are utilized to separate solid particles from liquids, facilitating the purification process. Additionally, they are employed in industrial processes to separate and extract valuable substances from mixtures.
- In summary, a centrifuge is a laboratory instrument that utilizes centrifugal force to separate mixtures based on their density. It has a rich history and has evolved from early designs to become an essential tool in scientific research, medical applications, and various industries. By harnessing the power of spinning and centrifugal force, centrifuges enable the purification and separation of different substances, playing a vital role in advancing knowledge and facilitating various processes in our daily lives.
Definition of Centrifuge
A centrifuge is a laboratory instrument that uses spinning and centrifugal force to separate mixtures based on their density.
Who first invented the Centrifuge?
Overall, the centrifuge is an important tool in a wide range of scientific and industrial applications, as it allows for the efficient separation and purification of various substances.
The concept of the centrifuge has been around for centuries, and various forms of the instrument have been developed and used for different purposes. However, the modern centrifuge as we know it today was first developed in the 19th century.
In 1864, the French engineer Antonin Prandtl designed and built a prototype centrifuge that was capable of generating a centrifugal force of up to 1,000 times the force of gravity. However, this early centrifuge was not widely adopted and was not used for any practical applications.
The first practical and widely used centrifuge was developed by the German engineer Gustav de Laval in 1885. De Laval’s centrifuge was a small, hand-powered device that was used to separate milk into cream and skim milk. This early centrifuge was the precursor to the modern milk-separating machine.
In the 20th century, the centrifuge was further developed and improved, and it became a key tool in a wide range of scientific and industrial applications. Today, centrifuges are used in a variety of fields, including biology, chemistry, medicine, agriculture, and environmental science.
Principle of a Centrifuge
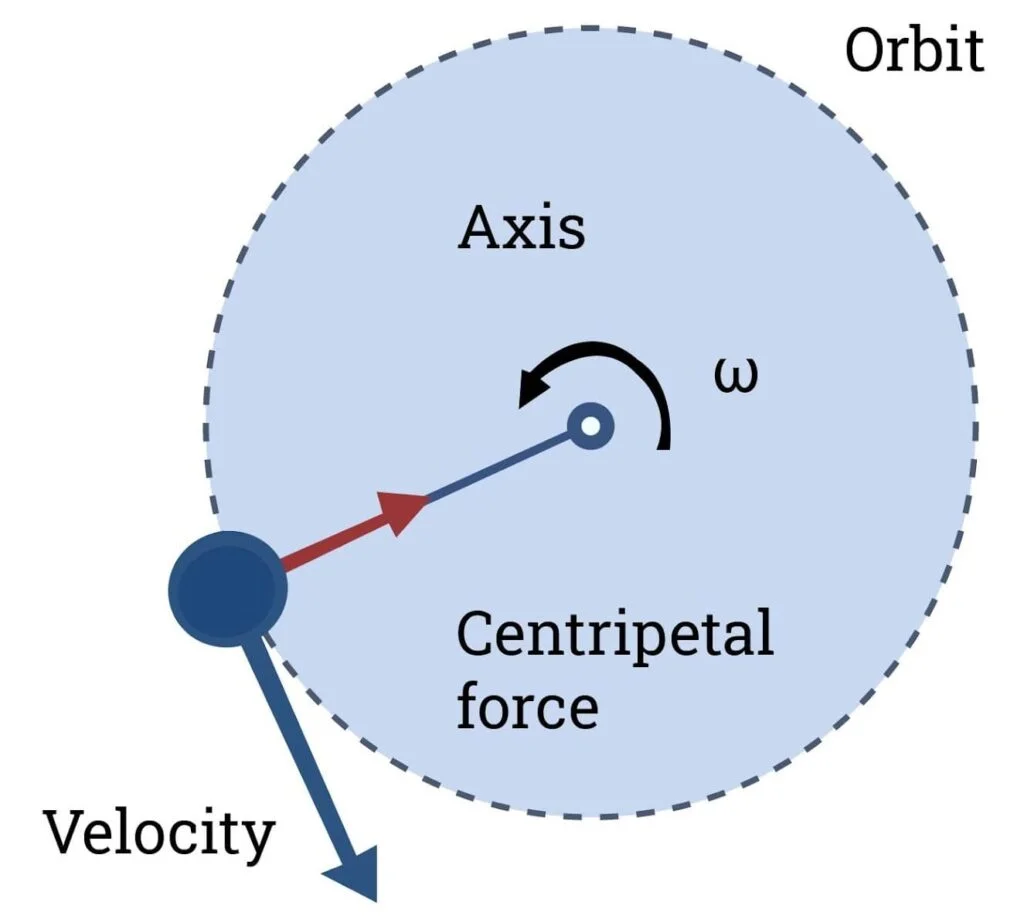
- The principle of a centrifuge is based on the sedimentation principle, which utilizes the force of gravity. When a sample is placed in a centrifuge and spun at high speeds, it creates a centrifugal force that separates the components based on their density.
- The sample, along with a liquid medium, is placed in bottles or tubes within the centrifuge’s rotor. As the rotor spins, the centrifugal force causes the denser particles or substances to settle towards the bottom of the rotor, while the lighter components remain closer to the top. This separation is known as sedimentation.
- By adjusting the speed of the rotor, the strength of the centrifugal force can be controlled. This allows for the separation and purification of substances with different densities. For example, cells, particles, or clay-like substances can be separated from a liquid medium using a centrifuge.
- The centrifuge’s principle is relatively simple: the force generated by spinning the sample causes the denser components to sediment out, while the lighter components remain suspended or rise to the top. This principle is applied in various scientific and industrial settings to purify and separate substances based on their density.
- Overall, the centrifuge plays a crucial role in many fields, including scientific research, medical diagnostics, and industrial processes. By harnessing the sedimentation principle and centrifugal force, it enables efficient separation and purification of samples, contributing to advancements in various disciplines.
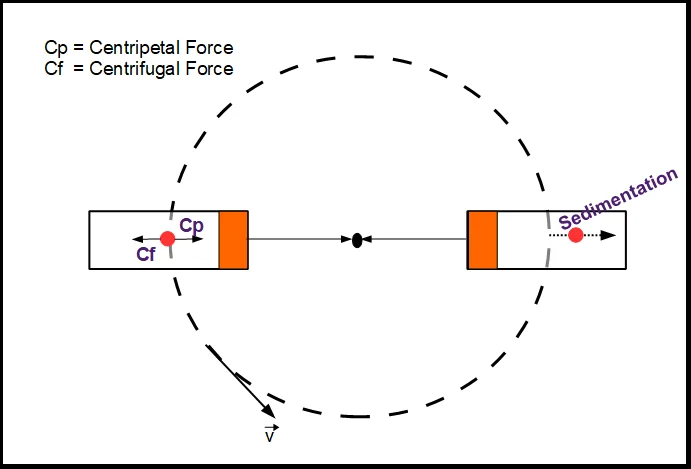
The principle of a centrifuge is based on the separation of substances by density. When a sample is placed in a centrifuge and spun at high speeds, a centrifugal force is created that causes the denser components of the sample to sediment out.
The magnitude of the centrifugal force depends on the speed of the centrifuge and the size of the rotor (the part of the centrifuge that holds the sample). The higher the speed and the larger the rotor, the greater the centrifugal force that is generated.
The separation of substances in a c entrifuge is based on the principle of sedimentation, which states that particles in a liquid will settle to the bottom of a container when the container is left undisturbed. In a centrifuge, the spinning motion of the rotor creates a gravitational force that is much stronger than the Earth’s gravitational force, causing the denser components of the sample to sediment out more quickly.
Overall, the principle of a centrifuge is based on the separation of substances by density, using a strong centrifugal force to accelerate the sedimentation process.
Sedimentation coefficient
- The sedimentation coefficient, also known as the Svedberg coefficient, is a measure of the rate at which a particle or molecule sediment (settles) in a centrifuge. It is expressed in Svedberg units (S), which are named after the Swedish scientist Theodor Svedberg, who developed the technique of ultracentrifugation.
- The sedimentation coefficient is determined by the size, shape, and mass of the particle or molecule. Larger, heavier particles have a higher sedimentation coefficient and will sediment more quickly in a centrifuge.
- The sedimentation coefficient is an important parameter in ultracentrifugation, a technique used to separate and purify large molecules, such as proteins and nucleic acids. It is also used to measure the size and shape of biological macromolecules, such as proteins and nucleic acids.
- In general, the sedimentation coefficient is inversely proportional to the size and mass of the particle or molecule. This means that smaller, lighter particles will have a lower sedimentation coefficient and will sediment more slowly in a centrifuge.
Factors Affecting Sedimentation coefficient
There are several factors that can affect the sedimentation coefficient of a particle or molecule in a centrifuge:
- Size and shape: The size and shape of the particle or molecule can significantly affect its sedimentation coefficient. Larger and more massive particles will have a higher sedimentation coefficient and will sediment more quickly, while smaller and lighter particles will have a lower sedimentation coefficient and will sediment more slowly.
- Molecular mass: The molecular mass of the particle or molecule can also affect its sedimentation coefficient. Higher molecular mass particles will have a higher sedimentation coefficient and will sediment more quickly, while lower molecular mass particles will have a lower sedimentation coefficient and will sediment more slowly.
- Density: The density of the particle or molecule can also affect its sedimentation coefficient. Denser particles will have a higher sedimentation coefficient and will sediment more quickly, while less dense particles will have a lower sedimentation coefficient and will sediment more slowly.
- Viscosity of the surrounding medium: The viscosity of the medium in which the particle or molecule is suspended can also affect its sedimentation coefficient. Higher viscosity media will result in a slower sedimentation rate, while lower viscosity media will result in a faster sedimentation rate.
- Temperature: The temperature of the surrounding medium can also affect the sedimentation coefficient. Higher temperatures can result in a faster sedimentation rate, while lower temperatures can result in a slower sedimentation rate.
- pH: The pH of the surrounding medium can also affect the sedimentation coefficient. Certain pH ranges can cause proteins to denature, which can affect their sedimentation coefficient.
- Concentration of particles: The concentration of particles in the surrounding medium can also affect the sedimentation coefficient. Higher concentrations of particles can result in a slower sedimentation rate, while lower concentrations of particles can result in a faster sedimentation rate.
Parts of a Centrifuge
The main parts of a centrifuge include:
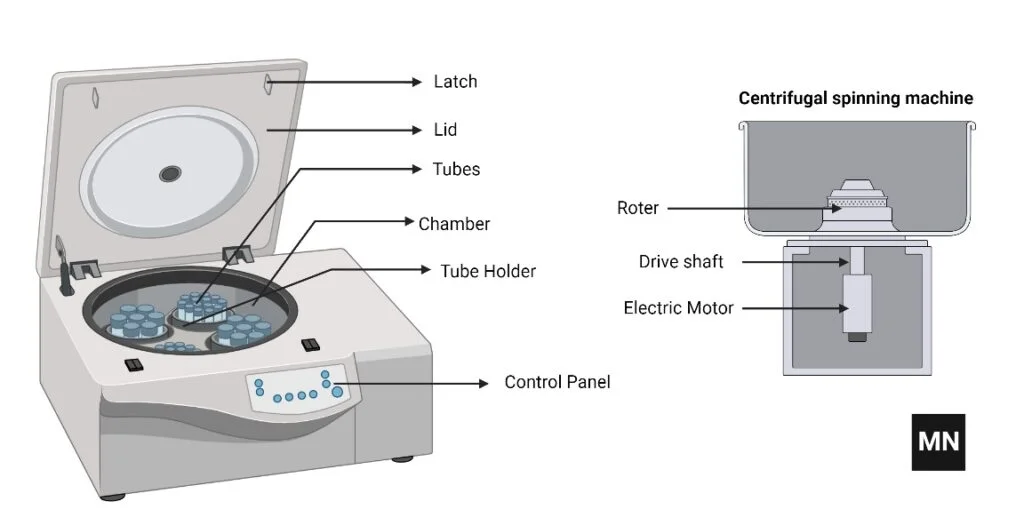
- Rotor: This is a spinning tube or container that holds the sample being processed. The rotor is typically made of metal or plastic and is mounted on a spindle, which allows it to rotate at high speeds.
- Driving System/Motor: This is the device that drives the rotation of the rotor. The motor is typically an electric motor, but some older or smaller centrifuges may be powered by a hand crank or a compressed air motor.
- Spindle: This is a shaft or rod that connects the rotor to the motor and allows the rotor to rotate. The spindle is typically made of metal and is mounted on bearings to reduce friction and wear.
- Containers: Several types of containers, such as test tubes, blood bags, cuvettes, centrifuge tubes, etc., are held in the rotors such that the sample rotates along as the rotor rotates.
- Control panel: This is the device that allows the user to set the speed and duration of the centrifugation process. The control panel may also include a timer and other features to allow for precise control of the process.
- Lid: This is a cover that is placed over the top of the rotor to contain the sample and protect the user from the spinning rotor. The lid may also include features such as a locking mechanism or a safety switch to prevent accidents.
- Latch: When a tube breaks, or there are other issues with the centrifuge while running, the latch keeps the lid closed.

There are many other parts that may be included in a centrifuge, depending on the specific model and its intended use. Some centrifuges may also include additional features such as temperature control, vibration isolation, or other specialized capabilities.
- Refrigeration System: The cooling system employs a completely sealed, air-tight cooled Copeland compressor unit with circuits for both refrigeration and heating control.
- Security Protection System: This security protection system includes main current protection, high temperature protection, high speed protection, balancing protection, and door cover protection.
Centrifuge Safety Features
There are several safety dangers associated with these gadgets, however they are typically fitted with safety mechanisms to protect people and the environment. Several characteristics include the following:
- Electric Lid Lock : This function prevents the lid from accidently opening while the centrifuge is in operation.
- Imbalance Sensor : The Imbalance Sensor detects when the centrifuge is not properly balanced and puts the centrifuge on pause if the vibration level increases.
- Sealed Rotors : These prevent biohazardous chemicals or substances from escaping or leaking throughout the process.
- Rotor Recognition Technology : The Rotor Recognition Technology determines which rotor is mounted and prevents the rotor from exceeding the limit operating speed.
Types of Centrifuge Rotors
There are many different types of centrifuge rotors available, and the appropriate rotor type depends on the specific application and the characteristics of the sample being processed. Some common types of centrifuge rotors include:
- Fixed-angle rotors: These rotors have a fixed angle of inclination, which is typically around 15-30 degrees. They are commonly used for routine separations and for samples that are relatively dense or viscous.
- Swing-bucket rotors: These rotors have buckets that swing out from the axis of rotation when the rotor is stopped, which makes it easier to load and unload the samples. They are commonly used for samples that are sensitive to shear forces or for samples that need to be quickly cooled after centrifugation.
- Puck rotors: These rotors have a circular shape and are typically used for small samples or samples that are sensitive to shear forces.
- Vertical rotors: These rotors have a vertical axis of rotation and are commonly used for high-speed separations or for samples that are sensitive to shear forces.
- Zonal rotors: These rotors have a series of concentric zones or rings, which allows for the separation of samples based on size or density. They are commonly used for separating cells or particles.
- Continuous flow rotors: These rotors are designed for continuous processing of large volumes of samples. They are commonly used in industrial applications or for large-scale separations.
Overall, the appropriate type of rotor depends on the specific characteristics of the sample being processed and the separation requirements of the application.
Characteristics of a Centrifuge
There are several characteristics that are important to consider when selecting a centrifuge for a particular application. These include:
- Speed: The speed of the centrifuge is an important factor to consider, as it determines the strength of the centrifugal force and the separation efficiency of the instrument. High-speed centrifuges are typically used for denser samples, while lower-speed centrifuges may be more suitable for lighter samples.
- Capacity: The capacity of the centrifuge refers to the size and number of samples that it can process at one time. This is an important consideration if you need to process large quantities of samples or if you need to process multiple samples simultaneously.
- Temperature control: Some centrifuges have the ability to control the temperature of the sample during the centrifugation process. This can be important for certain types of samples, such as proteins, which may be sensitive to temperature changes.
- Vibration isolation: Centrifuges can generate significant amounts of vibration during operation, which can affect the accuracy of the results. Some centrifuges have built-in vibration isolation systems to reduce the effects of vibration on the sample.
- Safety features: Centrifuges can be dangerous if they are not used properly, so it is important to consider the safety features of the instrument. These may include safety switches, emergency stop buttons, and locking lids to prevent accidents.
- Compatibility with different rotor types: Some centrifuges are compatible with a range of different rotor types, which can be useful if you need to process a variety of sample types.
- Ease of use: Consider the user-friendliness of the centrifuge, including the control panel, the sample loading and unloading process, and the maintenance requirements.
Types of centrifugation techniques
Centrifugation is a technique used to separate substances based on their density. There are several different types of centrifugation techniques, including:
1. Sedimentation centrifugation
This is the most basic form of centrifugation, in which a sample is placed in a rotor and spun at high speeds. The denser components of the sample will sediment out towards the bottom of the rotor due to the centrifugal force, while the lighter components will remain near the top.
- Sedimentation centrifugation is a technique used to separate substances based on their density. It is the most basic form of centrifugation, and it works by spinning a sample at high speeds in a rotor, which creates a centrifugal force that causes the denser components of the sample to sediment out towards the bottom of the rotor.
- Sedimentation centrifugation is commonly used to separate cells, particles, and other denser components from a sample. It can also be used to separate different types of cells or particles based on their density. For example, red blood cells and white blood cells can be separated using sedimentation centrifugation, as they have different densities.
- There are several factors that can affect the efficiency of sedimentation centrifugation, including the size and shape of the sample, the density of the sample, the speed of the rotor, and the duration of the centrifugation process. By carefully controlling these factors, it is possible to achieve high levels of separation efficiency with sedimentation centrifugation.
- Overall, sedimentation centrifugation is a widely used technique that is simple, efficient, and relatively inexpensive, making it a valuable tool in a wide range of scientific and industrial applications.
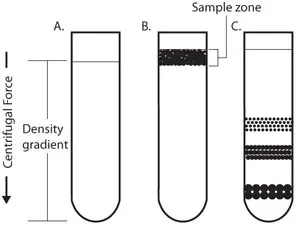
2. Density gradient centrifugation
This technique involves creating a density gradient in the sample by layering it on top of a gradient of denser solutions. The sample is then centrifuged, and the different components of the sample will sediment out at different positions along the gradient, depending on their density.
- Density gradient centrifugation is a technique used to separate substances based on their density. It involves creating a density gradient in the sample by layering it on top of a gradient of denser solutions. The sample is then centrifuged, and the different components of the sample will sediment out at different positions along the gradient, depending on their density.
- There are several types of density gradients that can be used in density gradient centrifugation, including continuous gradients and discontinuous gradients. Continuous gradients are created by gradually increasing the concentration of the denser solution from one end of the gradient to the other. Discontinuous gradients are created by layering discrete concentrations of the denser solution in the sample.
- Density gradient centrifugation is a powerful technique that allows for the separation of a wide range of substances, including cells, particles, proteins, and other biomolecules. It is commonly used in biological research and in the purification of biological samples.
- Overall, density gradient centrifugation is a useful technique for separating substances based on their density and for purifying biological samples. It is particularly useful for separating substances that are difficult to distinguish based on size or shape alone.
3. Rate-zonal centrifugation
This technique involves separating samples based on their size or shape. The sample is placed in a rotor that has a series of concentric zones or rings, and the rotor is spun at different speeds in each zone. Samples with different sizes or shapes will sediment out at different positions along the gradient.
- Rate-zonal centrifugation is a technique used to separate samples based on their size or shape. It involves placing the sample in a rotor that has a series of concentric zones or rings, and spinning the rotor at different speeds in each zone. Samples with different sizes or shapes will sediment out at different positions along the gradient.
- Rate-zonal centrifugation is a powerful technique that allows for the separation of a wide range of substances, including cells, particles, proteins, and other biomolecules. It is commonly used in biological research and in the purification of biological samples.
- There are several factors that can affect the efficiency of rate-zonal centrifugation, including the size and shape of the sample, the density of the sample, the speed of the rotor, and the duration of the centrifugation process. By carefully controlling these factors, it is possible to achieve high levels of separation efficiency with rate-zonal centrifugation.
- Overall, rate-zonal centrifugation is a useful technique for separating substances based on their size or shape and for purifying biological samples. It is particularly useful for separating substances that are difficult to distinguish based on density alone.
4. Continuous flow centrifugation
This technique involves continuously processing large volumes of samples in a specialized rotor. The samples are introduced at one end of the rotor, and the separated components are collected at the other end.
- Continuous flow centrifugation is a technique used to continuously process large volumes of samples. It involves introducing the samples at one end of a specialized rotor and collecting the separated components at the other end.
- Continuous flow centrifugation is a powerful technique that allows for the efficient separation and purification of a wide range of substances, including cells, particles, proteins, and other biomolecules. It is commonly used in industrial and environmental applications, such as the purification of water and other fluids, as well as in biological research.
- There are several factors that can affect the efficiency of continuous flow centrifugation, including the size and shape of the sample, the density of the sample, the speed of the rotor, and the duration of the centrifugation process. By carefully controlling these factors, it is possible to achieve high levels of separation efficiency with continuous flow centrifugation.
- Overall, continuous flow centrifugation is a useful technique for continuously processing large volumes of samples and for purifying a wide range of substances. It is particularly useful in industrial and environmental applications where large quantities of samples need to be processed efficiently.
5. Precipitation centrifugation
This technique involves adding a precipitating agent to a sample, which causes the components of the sample to form a precipitate. The sample is then centrifuged, and the precipitate will sediment out due to the centrifugal force.
- Precipitation centrifugation is a technique used to separate substances based on their ability to form a precipitate when exposed to certain conditions. It involves adding a precipitating agent to a sample, which causes the components of the sample to form a precipitate. The sample is then centrifuged, and the precipitate will sediment out due to the centrifugal force.
- Precipitation centrifugation is a powerful technique that allows for the separation and purification of a wide range of substances, including proteins, nucleic acids, and other biomolecules. It is commonly used in biological research and in the purification of biological samples.
- There are several factors that can affect the efficiency of precipitation centrifugation, including the nature of the sample, the type of precipitating agent used, the concentration of the precipitating agent, and the conditions under which the precipitate is formed. By carefully controlling these factors, it is possible to achieve high levels of separation efficiency with precipitation centrifugation.
- Overall, precipitation centrifugation is a useful technique for separating and purifying a wide range of substances, particularly proteins and other biomolecules. It is particularly useful for separating substances that are difficult to distinguish based on size, shape, or density alone.
Overall, these are just a few examples of the many different types of centrifugation techniques that are used in various scientific and industrial applications.
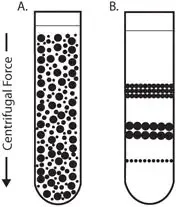
6. Isopycnic centrifugation
Isopycnic centrifugation is a technique used in biochemistry and molecular biology to separate molecules based on their density. It is often used to separate DNA or RNA molecules based on size and/or shape.
The technique involves spinning a solution containing the molecules to be separated in a special centrifuge rotor that has a gradient of density. The molecules will sediment through the gradient based on their density, with the denser molecules sedimenting further down the gradient. By carefully controlling the speed and duration of the centrifugation, it is possible to separate the molecules based on their density.
Isopycnic centrifugation is a useful tool for purifying and separating DNA and RNA molecules, and it is often used in conjunction with other techniques, such as gel electrophoresis, to further purify and characterize the molecules.
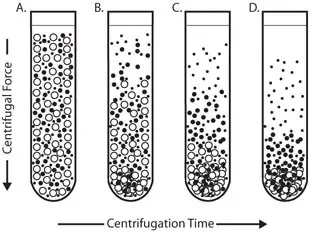
What is Differential centrifugation?
Differential centrifugation is a technique used in biochemistry and molecular biology to separate different components of a mixture based on their size, shape, and density. It is often used to purify and isolate different cellular components, such as organelles or subcellular particles.
The technique involves spinning a sample in a centrifuge at high speeds to sediment the different components based on their physical properties. The most dense and heaviest components will sediment to the bottom of the tube, while the less dense and lighter components will remain in the supernatant. By carefully controlling the speed and duration of the centrifugation, it is possible to separate the different components of the mixture.
Differential centrifugation is often used in conjunction with other techniques, such as filtration or precipitation, to further purify and isolate the components of interest. It is a useful tool for studying the structure and function of different cellular components and for preparing samples for further analysis.
Types of Centrifuges
Centrifugation is a widely used method for separating mixtures in scientific and industrial applications, based on the density differences of the components. The technique employs centrifugal force to segregate substances of varying densities. There are numerous centrifugation techniques, each tailored to specific applications and sample characteristics. Understanding these various types can help in selecting the most appropriate method for a given task.
- Benchtop Centrifuges are compact devices ideal for routine laboratory work, including the separation of blood components and the purification of cellular materials. Their small size and ease of use make them suitable for tasks requiring small sample volumes.
- Floor-standing Centrifuges cater to larger sample sizes or higher-throughput demands. They are often found in research and clinical labs where the volume and frequency of samples necessitate more substantial equipment.
- High-speed Centrifuges are designed to achieve extremely high rotational speeds, enabling the efficient separation of smaller particles that may not be effectively processed by lower-speed centrifuges. They are crucial in separating nucleic acids and proteins for molecular biology applications.
- Refrigerated Centrifuges maintain a controlled temperature environment during operation, essential for temperature-sensitive samples such as live cells or enzymes that could be denatured by heat generated during centrifugation.
- Microcentrifuges are specialized for very small volumes, often used in molecular biology for DNA, RNA, and protein extraction and purification. Their compact size makes them a staple on laboratory benches.
- Industrial Centrifuges are robust, built to handle large-scale separations such as those in wastewater treatment, food processing, and the chemical industry. Their design is optimized for continuous, heavy-duty use.
- Haematocrit Centrifuges focus on blood analysis, specifically measuring the ratio of red blood cells in blood. This type is widely used in medical laboratories for diagnostic purposes.
- Low-speed Centrifuges are suitable for gentle separations where high speeds might damage the components, such as in the isolation of large cells or organelles.
- Continuous Flow Centrifuges allow for the uninterrupted processing of samples, ideal for high-throughput environments. They can continuously feed in the sample, separate it, and discharge the components without stopping, making them suitable for industrial applications.
- Ultracentrifuges achieve exceptionally high speeds and are used in scientific research to study the properties and functions of macromolecules like proteins and nucleic acids.
- Basket Centrifuges and Decanter Centrifuges are variants often used in industrial settings for solid-liquid separations. Their designs facilitate the efficient processing of materials by spinning at high speeds, forcing solids to the periphery.
- Other specialized centrifuges, such as Inverting Filter Centrifuges, Vertical Solid Bowl Centrifuges, Pusher Centrifuges, and Horizontal Peeler Centrifuges, cater to specific industrial needs. For example, Oil Centrifuges are critical in applications requiring oil purification, such as in automotive or manufacturing industries.
Each centrifugation technique offers unique advantages, making it crucial to select the most appropriate type based on the specific requirements of the application, including the nature of the sample, the desired outcome, and the operational environment.
| Type of Centrifuge | Description | Common Applications | Advantages | Limitations |
|---|---|---|---|---|
| Benchtop Centrifuges | Compact, portable units designed for small-scale separations, typically used on laboratory benches. | Routine lab separations, cell and particle pelleting, DNA/RNA extractions. | Compact and cost-effective, suitable for small samples. | Limited capacity and speed, less versatile for larger or more complex separations. |
| Floor-Standing Centrifuges | Larger units placed on the floor, suitable for higher-throughput applications and larger samples. | Blood component separations, protein purification, industrial processing. | Higher capacity and speed, more rotor options. | Larger footprint, higher cost, and more complex operation. |
| High-Speed Centrifuges | Designed for very high speeds, facilitating efficient separations of denser samples. | Subcellular fractionation, protein isolation, high-resolution separations. | High separation efficiency, capable of handling dense samples. | High cost, more complex maintenance, and specific safety precautions required. |
| Refrigerated Centrifuges | Equipped with cooling systems to maintain temperature during operation, ideal for temperature-sensitive samples. | Enzyme and cell separations, sedimentation of fine particles, molecular biology applications. | Temperature control preserves sample integrity, increased separation efficiency for certain samples. | Higher cost due to refrigeration system, larger size, and energy consumption. |
| Microcentrifuges | Small, high-speed units for microvolume samples, commonly used in molecular biology and biochemistry labs. | DNA/RNA isolation, pelleting of small particles, enzyme assays. | Compact and fast, designed for small volumes. | Limited capacity, not suitable for large-scale separations. |
| Industrial Centrifuges | Heavy-duty centrifuges designed for large-scale and continuous processing in industrial settings. | Wastewater treatment, oil and gas, food and beverage production. | High throughput, robust design for demanding applications. | High initial and operating costs, requires skilled operators, large footprint. |
| Haematocrit Centrifuges | Specialized for measuring the ratio of the volume of red blood cells to the total volume of blood. | Blood analysis, medical diagnostics, research on blood disorders. | Precise measurement of haematocrit levels. | Limited to blood sample analysis, requires careful handling of capillary tubes. |
| Low-Speed Centrifuges | Operate at lower speeds, suitable for gentle separations where high centrifugal force might damage the sample. | Cell culture, sedimentation of bulky particles, some bioseparations. | Gentle on samples, reducing potential for damage. | Limited separation capabilities for particles with small size or density differences. |
| Continuous Flow Centrifuges | Allow continuous introduction and separation of material without stopping the machine, ideal for large volumes. | Industrial processing, chemical manufacturing, high-throughput bioseparations. | High processing capacity, efficient for large-scale operations. | Complex setup and operation, higher maintenance requirements, may not be suitable for all types of samples. |
| Ultracentrifuges | Ultra-high-speed centrifuges for separating molecules of very small size and high density. | Molecular biology research, virology, protein structure analysis. | Very high resolution and separation efficiency. | Expensive, requires specialized training to operate, potential risk with ultra-high speeds. |
| Basket Centrifuges | Utilize a spinning basket to separate solid from liquids, often used in the pharmaceutical and chemical industries. | Solid-liquid separations, sludge dewatering, crystal harvesting. | Effective for solid-liquid separations, can handle a range of particle sizes. | Periodic cleaning required, not continuous flow, may require manual unloading. |
| Decanter Centrifuges | Feature a rotating drum and are used for the continuous separation of solids from liquids in suspension. | Wastewater treatment, sludge processing, food industry separations. | Continuous operation, suitable for a variety of slurry types. | Complex machinery, high capital and maintenance costs, requires skilled operation. |
| Other Types | Including specialized centrifuges like inverting filter, vertical solid bowl, pusher, peeler, and oil centrifuges, each designed for specific applications and separation challenges. | Diverse applications across industries depending on the centrifuge type. | Tailored to specific separation needs, offering solutions for challenging separations. | Each has its own set of limitations, often related to the specific design and intended application, such as maintenance requirements or operational complexity. |
Centrifuge Operating Procedure
There are a few general steps that should be followed when operating a centrifuge:
- Pre-use preparation: Before using the centrifuge, be sure to properly prepare the samples and any necessary equipment, such as centrifuge tubes or bottles. Follow the manufacturer’s instructions for the proper preparation and handling of the samples.
- Load the samples: Carefully load the samples into the centrifuge rotor or carrier, following the manufacturer’s instructions for the proper loading technique. Make sure the samples are balanced evenly in the rotor to avoid vibration or imbalance.
- Select the appropriate settings: Set the centrifuge to the appropriate speed, time, and temperature settings for the separation task being performed, following the manufacturer’s instructions.
- Start the centrifuge: Turn on the centrifuge and start the separation process.
- Monitor the process: Keep an eye on the centrifuge during the separation process to ensure that it is running smoothly and that there are no problems with the samples or the equipment.
- Unload the samples: When the separation process is complete, carefully unload the samples from the centrifuge, following the manufacturer’s instructions for the proper handling of the samples.
- Clean and maintain the centrifuge: After use, be sure to properly clean and maintain the centrifuge according to the manufacturer’s instructions. This may include cleaning the rotor and other components, replacing worn parts, and performing regular maintenance tasks.
Overall, it is important to follow the manufacturer’s instructions and safety guidelines when operating a centrifuge, as well as to use proper technique and caution when handling samples and equipment.
Centrifuge Balancing – How to balance a centrifuge?
Centrifuge is a machine that rotates a sample at high speeds to separate substances of different densities. Balancing a centrifuge involves ensuring that the sample is evenly distributed in the rotor so that the centrifuge runs smoothly and safely. Here are some tips for balancing a centrifuge:
- Check the rotor capacity: Make sure the sample size is within the capacity of the rotor. Overloading the rotor can cause imbalance and damage the centrifuge.
- Distribute the sample evenly: Use a pipette to distribute the sample evenly in the tubes or tubes in the rotor.
- Balance the rotor: Place tubes of similar size and weight on opposite sides of the rotor. This helps balance the rotor and ensures that it spins smoothly.
- Check the balance indicator: Most centrifuges have a balance indicator that shows whether the rotor is balanced. If the indicator shows an imbalance, adjust the placement of the tubes until the rotor is balanced.
- Follow the manufacturer’s instructions: Consult the manual for specific instructions on how to balance the rotor for your particular centrifuge.
It is important to balance a centrifuge properly to ensure that it runs smoothly and safely. If you have any doubts or are unsure about how to balance your centrifuge, it is best to consult the manual or seek assistance from a qualified technician.
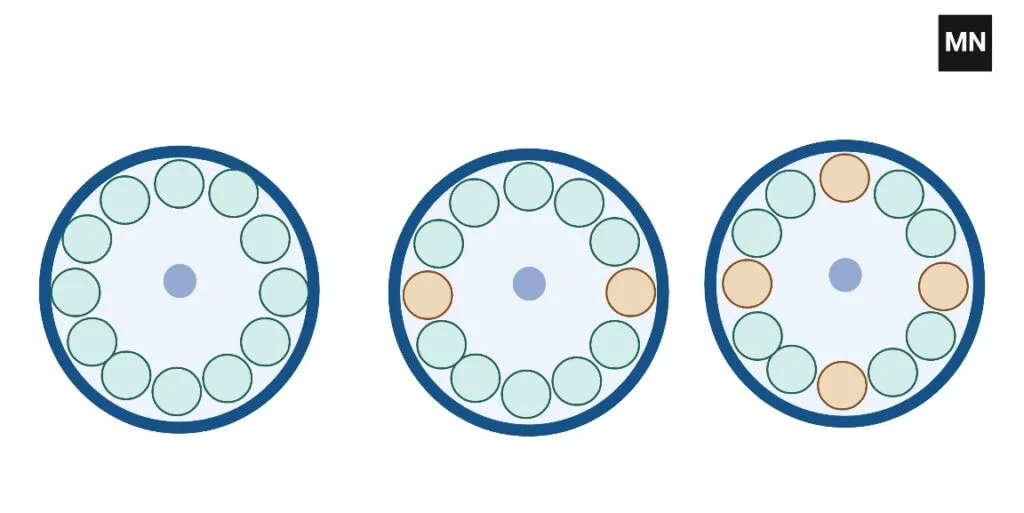
How to balance 3 tubes In Centrifuge
Two methods exist for balancing three tubes. The first alternative is to place three sample tubes side-by-side and three balance tubes directly across from them.
Optionally, three sample tubes can be evenly distributed around the rotor.
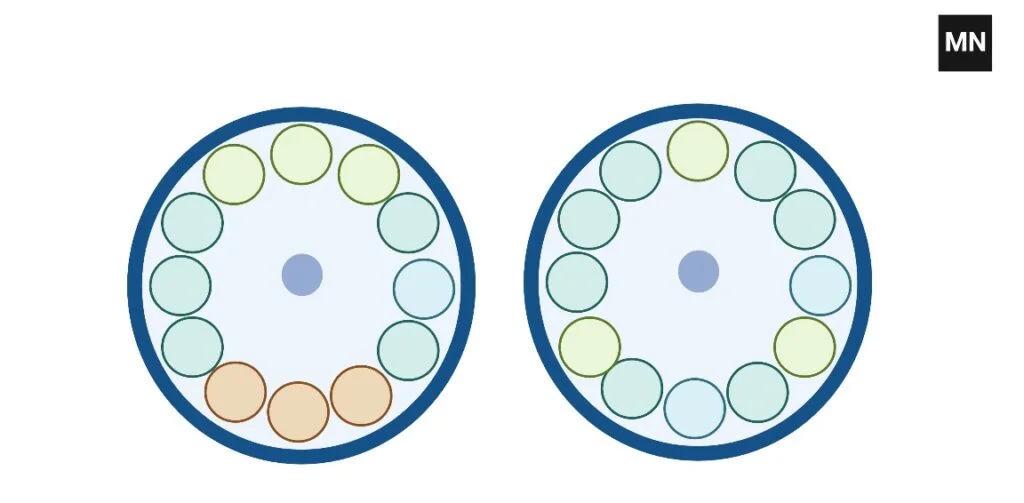
How to balance 5 tubes In Centrifuge
To balance five tubes, construct one balance tube and position two sets of three tubes across from one another.
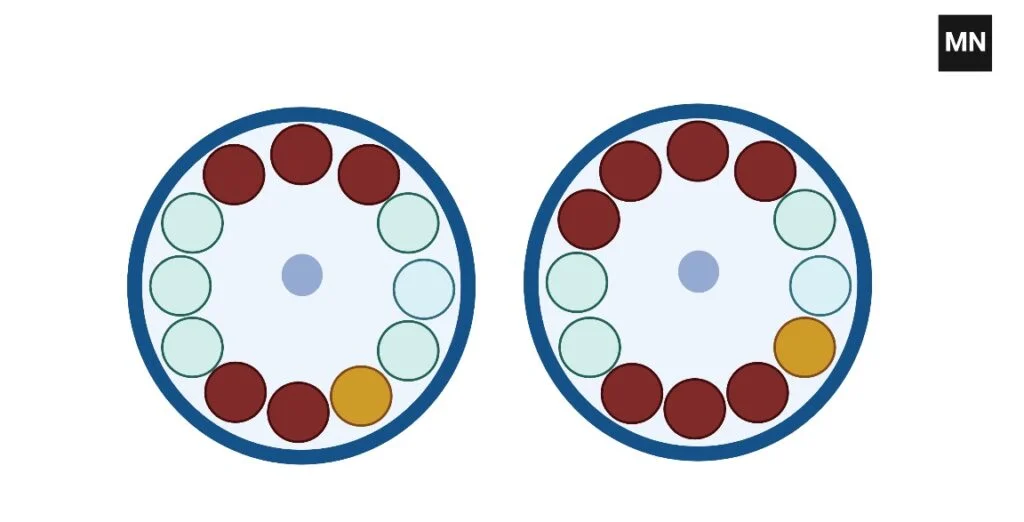
How to balance 7 tubes In Centrifuge
To balance seven tubes, you must construct one balancing tube and put two sets of four tubes across from one another.
Care and Maintenance of Centrifuge
Here are some tips for caring for and maintaining a centrifuge:
- Follow the manufacturer’s instructions: Always follow the manufacturer’s instructions for operating and maintaining the centrifuge. This will help ensure that the equipment is used safely and properly, and that it is maintained in good working condition.
- Regularly inspect the equipment: Regularly inspect the centrifuge for signs of wear or damage, such as cracks, dents, or other defects. If any problems are detected, repair or replace the equipment as necessary.
- Keep the centrifuge clean: Regularly clean the centrifuge to prevent contamination and ensure optimal performance. Use a clean, dry cloth to wipe down the exterior of the equipment, and clean the interior as needed.
- Perform regular maintenance: Follow the manufacturer’s recommended maintenance schedule to keep the centrifuge in good working condition. This may include lubricating moving parts, replacing worn or damaged parts, and performing other maintenance tasks.
- Use the correct samples and tubes: Use the correct samples and tubes for the centrifuge to prevent damage to the equipment. Follow the manufacturer’s guidelines for maximum load limits and tube sizes.
- Use caution when handling hazardous materials: If you are working with hazardous materials, take appropriate precautions to avoid accidental spills or releases.
- Follow safety guidelines: Follow all safety guidelines and procedures when using the centrifuge, and be aware of potential hazards.
- Store the centrifuge properly: When not in use, store the centrifuge in a clean, dry place to prevent damage. Follow the manufacturer’s guidelines for proper storage.
Applications of Centrifuge
Centrifuges are widely used in a variety of industries and applications for tasks such as separating and purifying materials, clarifying liquids, and sedimenting particles. Some specific examples of the applications of centrifuges include:
- Medicine: Centrifuges are used in medicine for tasks such as the separation of blood cells and plasma, the preparation of vaccines, and the purification of proteins and other biomolecules.
- Biotechnology: Centrifuges are widely used in biotechnology for tasks such as the isolation and purification of proteins, nucleic acids, and cells, as well as the characterization of biomolecules.
- Chemical industry: Centrifuges are used in the chemical industry for tasks such as the separation of pigments, dyes, catalysts, and other chemical compounds.
- Food and beverage industry: Centrifuges are used in the food and beverage industry for tasks such as the separation of solids from liquids in the production of milk, juices, and beer, as well as the clarification and purification of various types of liquids, such as wine and vegetable oils.
- Environmental industry: Centrifuges are used in the environmental industry for tasks such as the separation and purification of water and wastewater, as well as the removal of solids and contaminants from water and other liquids.
- Oil and gas industry: Centrifuges are used in the oil and gas industry for tasks such as the separation and purification of crude oil, diesel fuel, and natural gas liquids.
Overall, centrifuges are a valuable tool for a wide range of separation and purification tasks, and are widely used in various industries for a variety of applications.
Use of Centrifuge in laboratory
entrifuges are commonly used in laboratories for a variety of tasks, including:
- Separating and purifying biomolecules: Centrifuges are used to separate and purify proteins, nucleic acids, and other biomolecules, as well as to isolate cells and viruses.
- Clarifying liquids: Centrifuges are used to clarify and purify various types of liquids, such as water, chemical solutions, and biological samples.
- Sedimenting particles: Centrifuges are used to sediment particles, such as cells, out of a liquid sample, allowing for their easy collection and analysis.
- Studying the properties of solutions and mixtures: Centrifuges are used to study the properties of solutions and mixtures, including the size, shape, and molecular weight of small particles.
- Preparing samples for analysis: Centrifuges are used to prepare samples for various types of analysis, such as spectroscopy, chromatography, and microscopy.
Overall, centrifuges are an important tool for many tasks in the laboratory, and are widely used in a variety of scientific and industrial applications.
Advantages of Centrifuge
- High separation efficiency: Centrifuges can separate substances with a high degree of accuracy and efficiency, making them useful for a wide range of applications.
- Fast processing times: Centrifuges can process large volumes of material in a short amount of time, making them useful for applications that require rapid processing.
- Versatility: Centrifuges can be used to separate a wide range of substances, including liquids, gases, and solids.
- Ease of use: Centrifuges are relatively simple to operate, making them accessible to a wide range of users.
- Multiple separation techniques: Centrifuges can use various techniques to separate substances, including sedimentation, filtration, and decantation.
- Gentle on sensitive samples: Centrifuges can be used to separate delicate or sensitive materials without damaging them.
- Ability to handle large volumes: Centrifuges can process large volumes of material, making them useful for industrial and large-scale applications.
- Ability to handle a wide range of temperatures: Centrifuges can operate over a wide range of temperatures, making them useful for a wide range of applications.
- Ability to handle a wide range of pressures: Centrifuges can operate over a wide range of pressures, making them useful for a wide range of applications.
- Safety: Centrifuges are generally safe to use, as they are equipped with safety features to prevent accidents or injuries.
Limitations of Centrifuge
- High cost: Centrifuges can be expensive to purchase and maintain, which may be a disadvantage for some users.
- Specialized training: Operating a centrifuge may require specialized training, which may not be readily available to all users.
- Space requirements: Centrifuges can be large and bulky, requiring a significant amount of space for storage and operation.
- Noise: Centrifuges can produce significant levels of noise, which may be a problem in certain environments.
- Risk of sample contamination: There is a risk of sample contamination during the centrifugation process, which can affect the accuracy and reliability of results.
- Risk of sample damage: There is a risk of sample damage during the centrifugation process, especially if the samples are delicate or sensitive.
- Limited compatibility: Centrifuges may not be compatible with certain types of samples or materials, limiting their use in certain applications.
- Limited separation capabilities: Centrifuges may not be able to separate substances with a high degree of accuracy or efficiency in certain cases.
- Risk of injury: There is a risk of injury when operating a centrifuge, especially if the equipment is not used correctly or is not properly maintained.
- Risk of equipment failure: There is a risk of equipment failure when using a centrifuge, which can lead to lost time and resources.
Precautions
There are several precautions that should be taken when using a centrifuge:
- Wear protective clothing: Centrifuges can produce significant levels of noise, vibration, and heat, so it is important to wear appropriate protective clothing, such as earplugs, safety goggles, and gloves, when operating the equipment.
- Follow proper operating procedures: It is important to follow the manufacturer’s instructions and established operating procedures when using a centrifuge. This includes proper sample preparation, loading and unloading techniques, and maintenance procedures.
- Use caution when handling samples: Be careful when handling samples to avoid contamination or damage. Use proper handling techniques and protective equipment as needed.
- Monitor equipment for signs of wear or damage: Regularly inspect the centrifuge for signs of wear or damage, and perform maintenance as needed to ensure proper operation.
- Use caution when working with hazardous materials: If you are working with hazardous materials, take appropriate precautions to avoid accidental spills or releases.
- Follow safety guidelines: Follow all safety guidelines and procedures when using a centrifuge, and be aware of potential hazards.
- Use caution when working with high-speed equipment: High-speed equipment, such as centrifuges, can be dangerous if not used properly. Use caution when operating the equipment and be aware of potential hazards.
- Follow proper disposal procedures: Properly dispose of any hazardous materials or waste generated during the centrifugation process to prevent contamination or harm to the environment.
What does RCF stand for centrifuge?
RCF stands for “relative centrifugal force.” In centrifugation, the relative centrifugal force (RCF) is a measure of the strength of the centrifugal force applied to a sample. It is calculated by multiplying the acceleration due to the centrifugal force (g-force) by the mass of the sample.
The RCF is expressed in units of force per unit mass (e.g., g or N/kg). It is used to determine the separation efficiency of a centrifuge and to optimize the conditions for a specific separation.
To calculate the RCF for a sample in a centrifuge, you can use the following formula:
RCF = (g-force) × (mass of sample)
For example, if a sample with a mass of 1 gram is spun at a g-force of 10,000 G, the RCF would be:
RCF = (10,000 G) × (1 gram) = 10,000 g
It is important to consider the RCF when selecting a centrifuge and to follow the manufacturer’s guidelines and any relevant protocols or procedures to ensure the safety of the operator and the accuracy of the results.
What is RPM in Centrifuge?
In a centrifuge, RPM (revolutions per minute) refers to the speed at which the rotor is spinning. The rotor is the part of the centrifuge that holds the sample to be separated, and it is typically made of metal or other materials that can withstand high speeds.
The RPM of a centrifuge is an important factor in determining the strength of the centrifugal force applied to the sample. The higher the RPM, the stronger the centrifugal force, which can affect the separation of substances or particles in the sample. The RPM of a centrifuge can be adjusted to meet the specific needs of a given application.
In general, the RPM of a centrifuge will range from a few hundred to several thousand, depending on the type of centrifuge and the materials being separated. For example, a high-speed centrifuge used to separate small particles or molecules may have an RPM of several thousand, while a low-speed centrifuge used to separate larger particles or cells may have an RPM of a few hundred.
How RPM is calculated?
Revolutions per minute (RPM) is a measure of the speed at which an object is rotating or spinning. It is calculated by dividing the number of revolutions that an object completes in one minute by the number of minutes it takes to complete those revolutions.
The formula for calculating RPM is:
RPM = (number of revolutions) / (time in minutes)
For example, if an object completes 10 revolutions in 2 minutes, its RPM would be:
RPM = (10 revolutions) / (2 minutes) = 5 RPM
To convert RPM to other units of measurement, you can use the following conversions:
1 RPM = 1/60 Hz (hertz) 1 RPM = 2π/60 rad/s (radians per second) 1 RPM = 1/9.549296 × 10^-4 rad/s (radians per second) 1 RPM = 1/60 rev/s (revolutions per second)
To convert from other units of measurement to RPM, you can use the following conversions:
1 Hz = 60 RPM 1 rad/s = 60/(2π) RPM 1 rev/s = 60 RPM
Relationship between RPM and RCF
The relationship between RPM and relative centrifugal force (RCF) in a centrifuge is determined by the size and shape of the sample and the rotor being used, as well as the specific application.
In general, increasing the RPM of a centrifuge will increase the RCF applied to the sample, resulting in a stronger separation force. However, the relationship between RPM and RCF is not linear, and the actual RCF achieved for a given RPM will depend on the specific circumstances.
To determine the RCF for a given RPM, you can use the following formula:
RCF = (RPM² × radius of rotor) / (1,000 × radius of Earth)
Where the radius of the rotor is expressed in millimeters and the radius of the Earth is 6,371 km.
For example, if a sample is spun at an RPM of 5,000 in a centrifuge with a rotor radius of 10 cm, the RCF would be:
RCF = (5,000² × 100) / (1,000 × 6,371) = 3,906 g
It is important to consider the RCF when selecting a centrifuge and to follow the manufacturer’s guidelines and any relevant protocols or procedures to ensure the safety of the operator and the accuracy of the results.
What is the difference between RPM and RCF?
RPM (revolutions per minute) is a measure of the speed at which an object is rotating or spinning, while RCF (relative centrifugal force) is a measure of the strength of the centrifugal force applied to a sample.
RPM is calculated by dividing the number of revolutions that an object completes in one minute by the number of minutes it takes to complete those revolutions. RCF is calculated by multiplying the acceleration due to the centrifugal force (g-force) by the mass of the sample.
The relationship between RPM and RCF in a centrifuge is determined by the size and shape of the sample and the rotor being used, as well as the specific application. In general, increasing the RPM of a centrifuge will increase the RCF applied to the sample, resulting in a stronger separation force. However, the relationship between RPM and RCF is not linear, and the actual RCF achieved for a given RPM will depend on the specific circumstances.
It is important to consider both RPM and RCF when selecting a centrifuge and to follow the manufacturer’s guidelines and any relevant protocols or procedures to ensure the safety of the operator and the accuracy of the results.
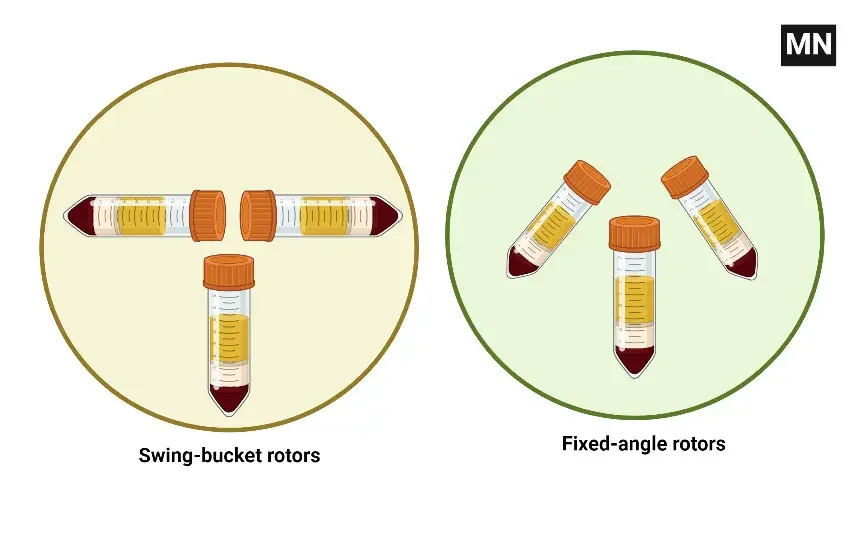
Differences between swing bucket rotor and fixed angle rotor
Swing bucket rotors and fixed angle rotors are two types of rotors that are commonly used in centrifuges to hold and spin samples. There are several key differences between these two types of rotors:
- Shape: Swing bucket rotors have a flexible, bucket-like shape that allows the tubes or containers holding the sample to swing out to a horizontal position during the centrifugation process. Fixed angle rotors have a more rigid, angled shape that holds the tubes or containers at a fixed angle (typically 45° or 90°) during the centrifugation process.
- Sample capacity: Swing bucket rotors are typically larger and have a higher sample capacity than fixed angle rotors, as the flexible buckets can hold a larger volume of samples. Fixed angle rotors are typically smaller and have a lower sample capacity, as the tubes or containers are held in a fixed position.
- Applications: Swing bucket rotors are typically used for large-volume samples or for samples that are sensitive to the forces generated by centrifugation. Fixed angle rotors are typically used for smaller-volume samples or for samples that are more resistant to the forces generated by centrifugation.
- Separation efficiency: Fixed angle rotors typically have a higher separation efficiency than swing bucket rotors, as the fixed angle of the tubes or containers allows for better separation of the components in the sample.
It is important to consider the specific needs of the application and the characteristics of the sample when selecting a rotor for a centrifuge. It is also important to follow the manufacturer’s guidelines and any relevant protocols or procedures to ensure the safety of the operator and the accuracy of the results.
How to use Eppendorf centrifuge?
Eppendorf centrifuges are commonly used in laboratories to separate biological materials such as cells, proteins, and DNA. To use an Eppendorf centrifuge, follow these steps:
- Make sure the centrifuge is properly calibrated and in good working order.
- Place the sample to be separated in a suitable tube or container, such as an Eppendorf microcentrifuge tube.
- Close the tube or container securely to prevent leakage.
- Select the appropriate rotor for the sample and application.
- Place the tubes or containers in the rotor, making sure that they are properly balanced and secure.
- Close the lid of the centrifuge and make sure that it is locked in place.
- Select the appropriate speed and time settings for the sample and application.
- Start the centrifuge by pressing the appropriate button or switch.
- Allow the centrifuge to run for the desired time, then turn it off and allow the rotor to come to a complete stop.
- Carefully remove the tubes or containers from the rotor and use caution when handling the sample, as it may be hot or slippery.
It is important to follow the manufacturer’s guidelines and any relevant safety regulations when operating an Eppendorf centrifuge to ensure the safety of the operator and the integrity of the sample.
Centrifuge Examples
1. ONiLAB’s Scientific Mini Centrifuge 7000RPM, 2680 x g RCF, Lab Benchtop Centrifuge

- Quite and stable Running: Low noise level ≤45dB, Max up to 7000rpm
- Quick and Easy Rotor Change: Click-on design allows for quick and easy rotor change without tools
- 2 Replaceable Rotors: 0.2/0.5/1.5/2.0ml x 8 and 0.2mL×32 PCR strips or 0.2mL×4 PCR 8 strips
- Accelerates and Brakes in seconds: Auto braking on door opening and stop quickly when the lid is opened or powered off
- Safe, endurable and robust rotor with clamp locking design
2. LABFISH PRP Lab Benchtop Centrifuge Machine

- 【POWERFUL CENTRIFUGAL】Powerful brushless motor with high performance.Wide range electronic speed control 500-5000 rpm, maximum speed up to 5000RPM, maximum relative centrifugal force 3074 xg. Widely used in laboratory, medical, clinical and industrial applications.
- 【EASY TO USE】LCD digital display can intuitively adjust the experiment time and speed. Timing range 10s-99min59s, electronic speed control 500-5000r/ min. Instantaneous centrifugation function, press the shot button, the centrifuge starts running, release the button, stop running.
- 【ALUMINUM ALLOY ROTOR】LABFISH low-speed centrifuge with built-in fixed-angle rotor can accommodate 6*15ml centrifuge tubes or 6*5/10ml. low-speed brake, fast and effective separation.
- 【OPERATION SAFETY】Secure intelligent electronic lock, the lid will open automatically after the machine ends operation. The instrument will automatically alarm when there is a malfunction.
- 【EXCELLENT SATISFACTION】1 year Limited Warranty. If you have any issue, we’re always here to help you.Labfish enables to continually make improvement of products based on suggestion.
3. HFS (R) Desktop Electric Centrifuge Lab (Timer 0-30min)

- [Parameters]: 110V, 60Hz; Stepless Control; Capacity: 20ml × 6pcs (total six tube); Speed: ≥3000r/min, ≤4000r/min±10%; Maximum Speed: 4000r/min±10%; Adjustable Speed control from 0-4000RPM; Maximum Relative Centrifugal Force:1790*g;
- [Using Conditions]: Ambient temperature: 0~30℃;Relative moisture: <80%; No electroconductive dust, explosive and corrosive gas in the surrounding air. Don’t put anything on the lid;
- [Advantages]: With safety switch when open the lid, the centrifuge stops automatically; Scope of supply includes the rotor; High speed for short centrifugation timers; Small footprint; Simple to operate; Step speed adjustment; Classical metal case;
- [APPLICATION]: It is mainly used for the appraisal of radioactivity immunity and separation of cell or particle. It is an ideal instrument for qualitative analysis of serum, plasma and radioimmunoassay in hospitals and laboratories;
- [Package]: 1 centrifuge; three prong plug, 6 pcs Plastic centrifuge tubes Diameter:20mm Length: 105mm.
4. JIAWANSHUN 8x20ml PRP Centrifuge Machine for Blood Lab Centrifuge Machine for Serum

- 1. Note: the capacity of this centrifuge is 20x8ml. Please note that the capacity in the rotor tube cannot be 20ml. The capacity of the rotor tube shall not exceed 80% of the centrifugal tube capacity. Otherwise, the centrifugal effect will be very poor. It’s the same with other types of centrifuges. The capacity of the centrifuge tube itself is not the capacity of the actual test.
- 2. The speed and time can be adjusted according to the need. It is very convenient and easy to use. The adjustable speed range is 0-4000r/min and the timing range is 0-999mins. The maximum centrifugal force is 1920 xg.
- 3. With a digital display screen, the speed and time are clear at a glance, and the reading is clear.
- 4. Brushless DC motor drive, eliminating the trouble of replacement, high efficiency and stability, long service life
- 5. It can be used in medical cosmetology industry, PRP, serum and fat separation, etc.
5. ORILAO Lab Centrifugal Machine Lower-Speed with Timer 0-60min (0-4000 RPM Cap:20ml X 6 Tube)

- Made of high quality metal material, separating the liquid from the solid particles or the liquid mixture of different density.
- Maximum Speed: 4000r/min; Maximum Relative Centrifugal Force: 1790×g.
- Equipped with speed control and timer to meet your requirement and the timer also functions as on/off switch; Timer Range: 0-60min.
- Portable; Small size and light weight, ideal for lab, chemistry, hospital and colleges so on, mainly used to analyse in plasma, serum, urea, vaccine, etc.
- Has a maximum capacity of 6 x 20ml and a maximum speed of 4000rpm, making it a great choice for low volume sample separation.
FAQ
What is a centrifuge?
A centrifuge is a device that uses centrifugal force to separate different components of a mixture based on their size, shape, and density. It consists of a rotating drum or rotor that spins at high speeds, causing the components of the mixture to sediment based on their physical properties.
Centrifuges are commonly used in biochemistry and molecular biology to separate different cellular components, such as organelles or subcellular particles, or to purify and isolate biological molecules, such as DNA, RNA, or proteins. They are also used in a variety of other applications, such as blood banking, where they are used to separate different components of blood, and in industrial processes, where they are used to separate different materials.
There are many different types of centrifuges available, each designed for a specific application. Some centrifuges are designed to spin small samples at high speeds, while others are designed to handle large samples or high volumes of material. Some centrifuges are also equipped with special rotors that allow them to spin samples in different directions or at different angles, which can be useful for separating different components of a mixture.
What does a centrifuge do?
A centrifuge is a device that uses centrifugal force to separate different components of a mixture based on their size, shape, and density. It consists of a rotating drum or rotor that spins at high speeds, causing the components of the mixture to sediment based on their physical properties.
Centrifuges are commonly used in biochemistry and molecular biology to separate different cellular components, such as organelles or subcellular particles, or to purify and isolate biological molecules, such as DNA, RNA, or proteins. They are also used in a variety of other applications, such as blood banking, where they are used to separate different components of blood, and in industrial processes, where they are used to separate different materials.
There are many different types of centrifuges available, each designed for a specific application. Some centrifuges are designed to spin small samples at high speeds, while others are designed to handle large samples or high volumes of material. Some centrifuges are also equipped with special rotors that allow them to spin samples in different directions or at different angles, which can be useful for separating different components of a mixture.
Why is it important to have a balanced centrifuge?
It is important to have a balanced centrifuge because an unbalanced centrifuge can cause serious problems and potentially be dangerous. When a centrifuge is unbalanced, the rotor will not be evenly distributed and will be heavier on one side. This can cause the centrifuge to vibrate excessively, which can damage the equipment and potentially lead to accidents or injuries.
A balanced centrifuge is essential for ensuring the safety of the equipment and the people who use it. It also helps to ensure the accuracy and reliability of the results obtained from the centrifugation process. An unbalanced centrifuge can cause the sample to sediment unevenly, leading to incorrect results or loss of sample material.
To balance a centrifuge, it is important to ensure that the load is evenly distributed in the rotor and that the centrifuge is properly balanced before each use. This can be done by adding or removing samples or using balance tubes to ensure that the rotor is evenly balanced. Proper maintenance and handling of the centrifuge can help to prevent unbalancing and ensure that the equipment is safe and reliable.
How long does a centrifuge spin?
The length of time that a centrifuge spins depends on the specific application and the type of centrifuge being used. Some centrifuges may spin for just a few minutes, while others may spin for several hours. In general, the length of time that a centrifuge spins is determined by the desired separation of the components in the sample, the size and shape of the sample, and the density of the components being separated.
In biochemistry and molecular biology, centrifuges are often used to separate different cellular components or to purify and isolate biological molecules. The length of time that the centrifuge spins will depend on the specific separation being performed and the characteristics of the sample being separated. For example, a sample being separated by isopycnic centrifugation may spin for several hours, while a sample being separated by differential centrifugation may spin for just a few minutes.
It is important to follow the manufacturer’s guidelines for the specific centrifuge being used and to carefully consider the characteristics of the sample being separated when determining the appropriate length of time for the centrifuge to spin.
How much does a centrifuge cost?
The cost of a centrifuge can vary widely depending on the specific model and its features. Centrifuges can range in price from a few hundred dollars for a basic tabletop model to tens of thousands of dollars for a high-end, specialized research-grade centrifuge.
Factors that can affect the cost of a centrifuge include the size and capacity of the rotor, the maximum speed at which the rotor can spin, the type of rotor (e.g. fixed-angle, swinging-bucket), and any additional features or accessories that may be included.
In general, tabletop centrifuges are less expensive than larger, floor-standing models, but they may also have lower capacities and lower maximum speeds. Research-grade centrifuges are typically more expensive than basic models, but they may offer higher performance and greater versatility.
It is important to carefully consider the specific needs and requirements of your application when choosing a centrifuge to ensure that you select a model that is appropriate and cost-effective for your needs.
When do you centrifuge the specimen?
The decision to centrifuge a specimen depends on the specific goals of the experiment or analysis and the characteristics of the specimen being tested. Centrifugation is often used to separate different components of a mixture based on their size, shape, and density, so it may be used when it is necessary to purify or isolate specific components of the specimen.
In general, centrifugation is typically performed after the specimen has been collected and processed, and after any necessary preparatory steps have been completed. For example, in a clinical laboratory, a blood sample may be collected and then processed to remove any clots or debris before being centrifuged to separate the different components of the blood.
It is important to carefully consider the specific goals of the experiment or analysis and the characteristics of the specimen when deciding whether or not to centrifuge the specimen. In some cases, centrifugation may not be necessary or may not be the most appropriate method for separating the components of interest.
Why is blood separated in a centrifuge?
Blood is separated in a centrifuge to separate the different components of the blood based on their size, shape, and density. Blood is made up of several different components, including red blood cells, white blood cells, and plasma. Each of these components has a different density and will sediment at a different rate when the blood is spun in a centrifuge.
By separating the different components of the blood, it is possible to study and analyze them individually or to prepare them for further use. For example, red blood cells can be used for transfusions, while plasma can be used for a variety of purposes, including blood clotting and protein analysis.
Centrifugation is a quick and efficient way to separate the different components of the blood and is commonly used in clinical laboratories and research settings. It is important to follow the manufacturer’s guidelines for the specific centrifuge being used and to carefully consider the characteristics of the sample being separated when determining the appropriate speed and duration for the centrifuge to spin.
What is the purpose of a centrifuge?
The purpose of a centrifuge is to separate different components of a mixture based on their size, shape, and density. It uses centrifugal force to sediment the components of the mixture based on their physical properties, allowing them to be separated and isolated.
Centrifuges are commonly used in biochemistry and molecular biology to purify and isolate biological molecules, such as DNA, RNA, or proteins, or to separate different cellular components, such as organelles or subcellular particles. They are also used in a variety of other applications, such as blood banking, where they are used to separate different components of blood, and in industrial processes, where they are used to separate different materials.
There are many different types of centrifuges available, each designed for a specific application. Some centrifuges are designed to spin small samples at high speeds, while others are designed to handle large samples or high volumes of material. Some centrifuges are also equipped with special rotors that allow them to spin samples in different directions or at different angles, which can be useful for separating different components of a mixture.
How long to centrifuge blood?
The length of time to centrifuge blood depends on the specific separation being performed and the characteristics of the sample. In general, blood samples are typically centrifuged for 10-15 minutes at a relatively low speed, such as around 500-1000 x g, to separate the different components of the blood.
During centrifugation, the red blood cells, which are the heaviest and densest component of the blood, will sediment to the bottom of the tube, while the lighter plasma and white blood cells will remain in the supernatant. By carefully controlling the speed and duration of the centrifugation, it is possible to separate the different components of the blood and prepare them for further analysis or use.
It is important to follow the manufacturer’s guidelines for the specific centrifuge being used and to carefully consider the characteristics of the sample being separated when determining the appropriate length of time for the centrifuge to spin.
How to balance a centrifuge with an odd number of tubes?
To balance a centrifuge with an odd number of tubes, you can follow these steps:
Begin by filling the tubes with the sample and closing the caps securely.
Place the tubes in the rotor, taking care to evenly distribute them around the rotor.
If necessary, use balance tubes to help evenly distribute the weight of the samples in the rotor. Balance tubes are empty tubes that can be added to the rotor to help balance the load.
Check the balance of the rotor by gently tilting it back and forth. If the rotor is evenly balanced, it should remain level and not tilt to one side.
If the rotor is not balanced, adjust the position of the tubes or add or remove balance tubes until the rotor is balanced.
Once the rotor is balanced, close the centrifuge lid and start the centrifuge according to the manufacturer’s instructions.
It is important to ensure that the centrifuge is properly balanced before each use to prevent vibration and damage to the equipment and to ensure the accuracy and reliability of the results obtained from the centrifugation process.
How many revolutions did the centrifuge complete after being turned off?
It is not possible to determine how many revolutions a centrifuge has completed after it has been turned off without additional information. The number of revolutions a centrifuge completes will depend on a variety of factors, including the speed at which it is spinning, the length of time it has been running, and the characteristics of the sample being separated.
To determine the number of revolutions a centrifuge has completed, you would need to know the speed at which the centrifuge was spinning, the length of time it was running, and the size and shape of the rotor. You would also need to know the specific type of centrifuge and its characteristics, as different models may have different rotor sizes and shapes, and may spin at different speeds.
Without this information, it is not possible to determine the number of revolutions a centrifuge has completed after it has been turned off.
What is centrifugation process?
Centrifugation is a process that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture. It is commonly used in laboratories to separate biological materials such as cells, proteins, and DNA.
The process involves placing a sample in a tube or other container and spinning it at high speeds in a machine called a centrifuge. The centrifugal force generated by the spinning motion causes the heavier or denser materials to settle to the bottom of the tube, while lighter materials remain at the top.
There are several different types of centrifugation, including differential centrifugation, which is used to separate cells and organelles based on their size and density, and sedimentation centrifugation, which is used to separate large molecules such as proteins and DNA based on their size.
Centrifugation is a useful technique for purifying and separating biological materials, and it is an important tool in many areas of research and biotechnology.
What is centrifugation used for?
Centrifugation is a widely used technique in a variety of fields, including biology, chemistry, and medicine. Some common applications of centrifugation include:
Separating cells and organelles: Centrifugation can be used to separate different types of cells or organelles based on their size and density. For example, it can be used to purify red blood cells, white blood cells, or other cell types from a mixed sample.
Separating molecules: Centrifugation can be used to separate molecules such as proteins, DNA, and RNA based on their size. This is often done using a technique called sedimentation centrifugation, which separates molecules based on their sedimentation coefficient, a measure of how fast they move through a liquid when subjected to a centrifugal force.
Purifying proteins: Centrifugation can be used to purify proteins from complex mixtures such as cell lysates or tissue homogenates. This is often done using a technique called ultracentrifugation, which separates molecules based on their size and shape.
Clarifying solutions: Centrifugation can be used to remove solid particles or other contaminants from solutions. For example, it can be used to clarify beer or wine by removing yeast or other solid particles that may be present in the liquid.
Separating mixtures: Centrifugation can be used to separate mixtures of substances based on their density. For example, it can be used to separate heavy and light materials in a sample, or to separate different types of particles based on their size.
Analyzing particles: Centrifugation can be used to analyze the size and shape of particles in a sample, such as bacteria or cells. This can be done by measuring the sedimentation rate of the particles, which is a measure of how fast they settle to the bottom of a tube when subjected to a centrifugal force.
What is centrifugation and examples?
Centrifugation is a process that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture. It is commonly used in laboratories to separate biological materials such as cells, proteins, and DNA.
Here are a few examples of how centrifugation is used in different fields:
In biology: Centrifugation is often used to separate cells and organelles from tissue homogenates or cell lysates. For example, a researcher might use differential centrifugation to separate cells from a mixed sample, or sedimentation centrifugation to separate proteins or DNA based on their size.
In chemistry: Centrifugation can be used to separate different types of molecules based on their size and density. For example, it can be used to purify proteins from complex mixtures, or to separate different types of particles based on their size.
In medicine: Centrifugation is used in many medical applications, including the separation of red and white blood cells, the purification of proteins for use in therapies, and the analysis of particles such as bacteria or viruses.
In industrial processes: Centrifugation is used in a variety of industrial processes, including the separation of oil and water, the clarification of liquids such as beer and wine, and the purification of products such as drugs and food additives.
In environmental science: Centrifugation is used to analyze and separate particles in environmental samples, such as water or air, to determine the presence of contaminants or other substances of interest.
What is centrifugation also called?
Centrifugation is also known as centrifugal separation. It is a process that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture.
There are several different types of centrifugation, including differential centrifugation, which is used to separate cells and organelles based on their size and density, and sedimentation centrifugation, which is used to separate large molecules such as proteins and DNA based on their size.
Other terms that are often used in reference to centrifugation include centrifugal filtration, which refers to the use of centrifugation to filter particles from a liquid, and ultracentrifugation, which refers to the use of high-speed centrifugation to separate and purify large molecules such as proteins
What is centrifuge in simple terms?
A centrifuge is a machine that uses centrifugal force to separate substances of different densities or to separate particles of different sizes in a mixture. It consists of a spinning rotor that is placed inside a container or tube holding the sample to be separated. When the rotor is spun at high speeds, the centrifugal force generated by the spinning motion causes the heavier or denser materials to settle to the bottom of the container, while lighter materials remain at the top.
Centrifuges are commonly used in laboratories to separate biological materials such as cells, proteins, and DNA. They are also used in a variety of industrial and medical applications, including the separation of oil and water, the clarification of liquids such as beer and wine, and the purification of products such as drugs and food additives.
What causes centrifugation?
Centrifugation is caused by the application of a centrifugal force to a sample that is placed in a container or tube and spun at high speeds. The centrifugal force is generated by the spinning motion of the sample and acts on the particles in the sample, causing them to move away from the center of the container.
The magnitude of the centrifugal force is determined by the speed of the spinning motion and the radius of the sample. As the speed increases or the radius decreases, the centrifugal force increases, causing the particles in the sample to move further away from the center.
Centrifugation is used to separate substances or particles of different densities or sizes in a mixture by exploiting the differences in the way that they respond to the centrifugal force. Heavier or denser materials will tend to settle to the bottom of the container, while lighter materials will remain at the top. This allows the substances or particles to be separated based on their density or size.
Who is the father of centrifugation?
The credit for the development of centrifugation is often given to the French scientist Antoine César Becquerel, who first described the use of centrifugal force to separate substances in 1827. However, the first practical application of centrifugation was developed by the English chemist and industrialist James Small, who used a spinning motion to separate cream from milk in the early 19th century.
The modern centrifuge, which uses a spinning rotor to generate centrifugal force, was developed in the early 20th century by the German chemist and inventor Theodor Svedberg. Svedberg’s work laid the foundation for the development of many different types of centrifuges, which are now widely used in a variety of fields including biology, chemistry, and medicine.
What is the maximum speed of centrifuge?
The maximum speed of a centrifuge is determined by the design of the centrifuge and the materials used in its construction. In general, the maximum speed of a centrifuge will depend on the size and type of rotor being used, the size and shape of the sample being spun, and the materials that the rotor and sample are made of.
Some high-speed centrifuges are capable of spinning at speeds of up to 100,000 RPM or more, while others are designed to spin at more moderate speeds of a few thousand RPM. The maximum speed of a centrifuge is typically limited by factors such as the strength and durability of the materials used in its construction, the stability of the rotor, and the safety of the operator.
It is important to use caution when operating a centrifuge at high speeds, as the forces generated by the spinning motion can be dangerous if the centrifuge is not properly designed or maintained. It is also important to follow the manufacturer’s guidelines for the safe use of a centrifuge, as well as any relevant safety regulations.
Why do we centrifuge at 4 degrees?
It is common to store biological samples, such as cells or proteins, at low temperatures, such as 4°C (39°F), to preserve their integrity and prevent degradation. Centrifugation is often used to separate or purify these samples, and it is important to maintain the sample at a low temperature during the centrifugation process to avoid damaging the sample.
Maintaining the sample at a low temperature during centrifugation can also help to reduce the risk of sample contamination, as some contaminants may be less stable at low temperatures. Additionally, low temperature can help to prevent the formation of ice crystals or other forms of damage that may occur if the sample is allowed to warm up during the centrifugation process.
In general, it is recommended to store biological samples at 4°C or lower to preserve their integrity and to minimize the risk of degradation or contamination. This is especially important for samples that are sensitive or prone to damage, such as proteins or DNA.
What happens if you centrifuge too fast?
Spinning a sample too fast in a centrifuge can cause a number of problems, including:
Damage to the sample: The high speeds and forces generated by centrifugation can cause mechanical damage to the sample, leading to the denaturation or degradation of proteins, DNA, or other molecules in the sample. This can affect the accuracy and reliability of the results of any experiments or analyses performed on the sample.
Risk of contamination: Operating a centrifuge at high speeds can generate aerosols or other forms of contamination, which can affect the integrity of the sample and the accuracy of the results.
Risk of accidents: Centrifugation at high speeds can be dangerous if the centrifuge is not properly designed or maintained, as the forces generated by the spinning motion can be hazardous to the operator or bystanders.
Inaccurate results: Spinning a sample too fast in a centrifuge can cause the sample to become too concentrated, leading to inaccurate results. This can be especially problematic if the sample contains particles or molecules of different sizes, as the separation may not be as effective at high speeds.
It is important to follow the manufacturer’s guidelines and any relevant safety regulations when operating a centrifuge to ensure that the sample is spun at an appropriate speed for the specific application.
What happens if you centrifuge for too long?
Spinning a sample for too long in a centrifuge can cause a number of problems, including:
Damage to the sample: The high speeds and forces generated by centrifugation can cause mechanical damage to the sample, leading to the denaturation or degradation of proteins, DNA, or other molecules in the sample. This can affect the accuracy and reliability of the results of any experiments or analyses performed on the sample.
Risk of contamination: Operating a centrifuge for a long period of time can increase the risk of contamination, as the sample may be exposed to the environment for an extended period.
Risk of accidents: Centrifugation for a long period of time can be hazardous if the centrifuge is not properly designed or maintained, as the forces generated by the spinning motion can be dangerous to the operator or bystanders.
Inaccurate results: Spinning a sample for too long in a centrifuge can cause the sample to become too concentrated, leading to inaccurate results. This can be especially problematic if the sample contains particles or molecules of different sizes, as the separation may not be as effective after an extended period of time.
It is important to follow the manufacturer’s guidelines and any relevant safety regulations when operating a centrifuge to ensure that the sample is spun for an appropriate amount of time for the specific application.
What is the most common error when using a centrifuge?
One of the most common errors when using a centrifuge is not following the manufacturer’s guidelines or relevant safety regulations. It is important to carefully read and understand the instructions for operating the centrifuge and to follow all recommended procedures to ensure the safety of the operator and the integrity of the sample.
Other common errors when using a centrifuge include:
Overloading the centrifuge: Putting too much weight or volume in the centrifuge can cause the rotor to become unbalanced, leading to vibrations and potentially damaging the centrifuge or the sample.
Using the wrong type of rotor: Using a rotor that is not designed for the specific sample or application can lead to inaccurate results or damage to the sample.
Using the wrong speed or time: Spinning the sample at an inappropriate speed or for too long can cause damage to the sample or lead to inaccurate results.
Improperly sealing the tubes or containers: If the tubes or containers holding the sample are not properly sealed, the sample may become contaminated or the results of the separation may be inaccurate.
Not maintaining the centrifuge: Failing to properly maintain the centrifuge, including cleaning it regularly and checking for wear and damage, can lead to problems with the performance of the centrifuge and the accuracy of the results.
How long do you centrifuge for serum?
The optimal time for centrifuging a sample of serum (the clear, straw-colored liquid component of blood) will depend on a number of factors, including the size and type of rotor being used, the volume and density of the sample, and the specific goals of the separation.
In general, it is recommended to spin serum samples at a moderate speed (e.g., 1,500-2,000 RPM) for a period of 10-15 minutes. This should be sufficient to separate the serum from any solid components present in the sample, such as cells or clotting factors.
It is important to follow the manufacturer’s guidelines for the specific centrifuge being used, as well as any relevant protocols or procedures, to ensure the safety of the operator and the integrity of the sample. In some cases, it may be necessary to adjust the speed or time of the centrifugation based on the specific needs of the application.
Can you centrifuge blood twice?
It is generally not recommended to centrifuge blood more than once, as the high speeds and forces generated by centrifugation can cause damage to the cells and other components of the blood. Additionally, repeated centrifugation can increase the risk of contamination and may lead to inaccurate results.
However, there are some specific situations in which it may be necessary to centrifuge blood more than once. For example, if the sample is very large or if it contains a high concentration of cells or other solid components, it may be necessary to perform multiple rounds of centrifugation to effectively separate the components of the blood.
If it is necessary to centrifuge blood more than once, it is important to follow the manufacturer’s guidelines for the specific centrifuge being used, as well as any relevant protocols or procedures, to ensure the safety of the operator and the integrity of the sample. In addition, it may be necessary to use protective measures, such as wearing gloves and a face mask, to minimize the risk of contamination.
What does G stand for in centrifugation?
In centrifugation, “G” stands for “gravity.” Centrifugation is a process that uses centrifugal force to separate substances or particles in a mixture based on their size, density, or other characteristics. Centrifugal force is generated by the spinning motion of the sample and acts in a direction that is perpendicular to the axis of rotation.
The strength of the centrifugal force is typically measured in units of “G” or “g-force,” which represents the acceleration of the sample due to the centrifugal force. The g-force is equal to the acceleration of gravity (9.81 m/s^2) multiplied by the radius of the sample divided by the radius of the Earth (6,371 km).
For example, if a sample is spinning at a speed of 1,000 RPM in a centrifuge with a radius of 10 cm, the g-force would be approximately 1,000 G. This is much stronger than the force of gravity that we experience on Earth, which is typically around 1 G.
The g-force applied to a sample during centrifugation can affect the separation of the components in the sample, as well as the stability and integrity of the sample itself. It is important to carefully consider the g-force when selecting a centrifuge and to follow the manufacturer’s guidelines for the specific centrifuge being used to ensure the safety of the operator and the accuracy of the results.
What is maximum speed of high speed centrifuge?
The maximum speed of a high-speed centrifuge will depend on the specific design and capabilities of the centrifuge. Some high-speed centrifuges are capable of spinning at speeds of up to 100,000 RPM or more, while others are designed to spin at more moderate speeds of a few thousand RPM.
High-speed centrifuges are typically used to separate small particles or molecules, such as proteins or DNA, or to purify samples for analysis. They are often used in research and industrial applications where high speed and high separation efficiency are required.
It is important to carefully consider the specific needs of the application and the capabilities of the centrifuge when selecting a high-speed centrifuge. It is also important to follow the manufacturer’s guidelines and any relevant safety regulations when operating the centrifuge to ensure the safety of the operator and the integrity of the sample.
References
- Moran, S. (2017). Centrifuges. Process Plant Layout, 389–397. doi:10.1016/b978-0-12-803355-5.00027-5
- Bridges, S., & Robinson, L. (2020). Centrifuges. A Practical Handbook for Drilling Fluids Processing, 475–488. doi:10.1016/b978-0-12-821341-4.00021-x
- Berk, Z. (2009). Centrifugation. Food Process Engineering and Technology, 217–232. doi:10.1016/b978-0-12-373660-4.00009-0
- https://www.ehs.uci.edu/sop/hazardous-operations/centrifuge-sop.pdf
- https://www.mls.be/en/p/lab-apparatus/centrifuges/centrifuges-hettich/hematocrit-centrifuges/
- https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/protein-biology/protein-pulldown/centrifugation-separations
- https://www.iqsdirectory.com/articles/centrifuge.html
- https://www.tec2med.com/centrifuge-rotor-rcf-rpm/
- https://www.toppr.com/guides/chemistry/is-matter-around-us-pure/centrifugation/
- https://www.biocompare.com/Lab-Equipment/Laboratory-Centrifuges/
- https://druckerdiagnostics.com/knowledge/how-a-centrifuge-works/
- https://study.com/academy/lesson/what-is-centrifugation-definition-process-uses.html
- https://www.genfollower.com/centrifuges-types-uses-in-laboratories/
- https://www.labmanager.com/product-focus/the-basics-of-centrifuge-operation-and-maintenance-1433
- https://www.coleparmer.in/c/centrifuges
- https://microbenotes.com/centrifuge-principle-parts-types-uses-examples
- https://www.labtron.com/centrifuge
- https://www.vedantu.com/chemistry/uses-of-centrifuge