What is HPLC (High-performance liquid chromatography)?
- High-Performance Liquid Chromatography (HPLC) is a sophisticated analytical technique used extensively in various scientific fields to separate, identify, and quantify the components of a mixture. This technique, originally known as high-pressure liquid chromatography, has evolved significantly since its inception.
- HPLC operates on the principle of column chromatography but utilizes advanced technology to enhance its performance. Unlike traditional column chromatography, where gravity slowly drives the solvent through the column, HPLC employs high-pressure pumps to force the solvent, or mobile phase, through the system. This increased pressure allows for faster and more efficient separation of compounds.
- The core components of an HPLC system include the high-pressure pumps, the column, and the detector. The column, a key element in the system, is packed with adsorbent material—typically granular particles such as silica or polymer beads. The sample mixture, dissolved in a suitable solvent, is injected into the column where its components interact with the adsorbent material.
- Each component in the sample exhibits different affinities for the adsorbent, leading to varying migration rates through the column. This differential interaction results in the separation of the components as they elute from the column at different times. As the separated components pass through the detector, they generate a signal that is recorded as a chromatogram. This chromatogram displays peaks that correspond to the individual components of the sample, with each peak’s position (retention time) and area providing information about the component’s identity and concentration.
- HPLC is highly versatile and can be applied to a wide range of mixtures, including those found in pharmaceuticals, environmental samples, food products, and biological fluids. Its ability to perform precise and rapid analyses makes it invaluable in both research and industrial settings. For instance, in pharmaceutical manufacturing, HPLC ensures the purity of products, while in environmental science, it helps in detecting pollutants.
- The separation efficiency in HPLC is influenced by various factors, including the composition and temperature of the mobile phase, the nature of the adsorbent, and the applied pressure. The mobile phase, typically a mixture of solvents like water, acetonitrile, or methanol, interacts with the sample components, driving their separation based on physical properties such as hydrophobicity, ionic interactions, or dipole interactions.
- Overall, HPLC represents a significant advancement in chromatography, providing a powerful tool for analyzing complex mixtures with high precision and efficiency. Its development from basic column chromatography to its current high-performance form reflects ongoing improvements in analytical techniques and instrumentation.
Principle of HPLC (High-performance liquid chromatography)
- Initially, the mixture is introduced into the HPLC system via a sample loop connected to a valve. This valve directs the sample into the mobile phase, a solvent or a mixture of solvents that is propelled through the system under high pressure by the pumps. The role of the mobile phase is to carry the sample through the separation column.
- The separation column is packed with a stationary phase, which consists of granular materials with porous particles. These particles, typically made from substances such as silica, are designed to interact with the sample components to varying degrees. As the mobile phase flows through the column, different components of the sample are retained by the stationary phase to different extents, causing them to migrate at different rates.
- The varying retention times of the components result in their separation as they exit the column. Once the components have been separated, they are detected by an appropriate detector, such as a UV detector. The detector generates a signal that is processed by the HPLC software on a computer.
- The output of this process is a chromatogram, a graphical representation of the detector’s response over time. Each peak in the chromatogram corresponds to a different component of the sample, with the position and area of the peak providing information about the component’s identity and concentration.
- Therefore, the principle of HPLC involves the precise manipulation of the interactions between the sample, stationary phase, and mobile phase to achieve effective separation and analysis of the sample components. This process allows for detailed identification and quantification of substances within complex mixtures.
Instrumentation of HPLC

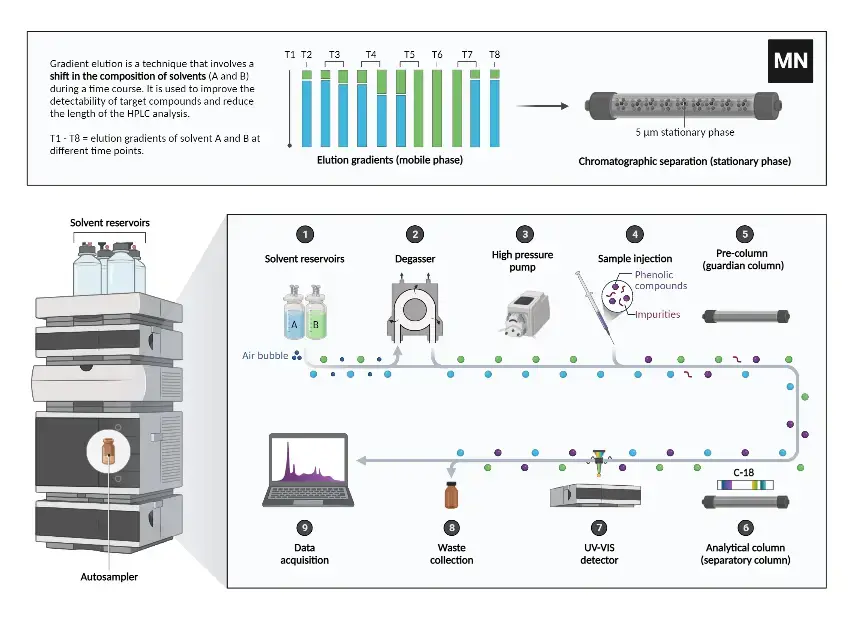
High-Performance Liquid Chromatography (HPLC) is a sophisticated analytical technique that relies on a precise set of instruments to achieve effective separation, detection, and analysis of chemical compounds. The key components of an HPLC system include the mobile phase reservoir, solvent delivery system, pump, injector, column, detector, computer, degasser, and column heater. Each plays a crucial role in the chromatography process. Here is a detailed examination of each component:
- Mobile Phase/Solvent Reservoir
- Function: The mobile phase or solvent reservoir holds the liquid that will be used to carry the sample through the chromatographic system.
- Description: Typically, this is a glass bottle that stores the HPLC solvent. Some advanced systems, such as those by Agilent, feature specialized compartments for multiple reservoirs. These compartments may include additional features for degassing the solvent and preventing exposure to air, which helps in maintaining the consistency of the mobile phase.
- Solvent Delivery System
- Function: This system is responsible for delivering the mobile phase to the chromatograph with a continuous, pulse-free flow.
- Description: It ensures a steady and precise flow rate of the mobile phase, despite variations in system back pressure. This consistent delivery is crucial for reproducibility and accuracy in chromatographic analyses.
- Pump
- Function: The pump propels the mobile phase through the chromatograph at a controlled flow rate, typically measured in milliliters per minute (mL/min).
- Description: Pumps in HPLC systems can generate pressures up to 6000-9000 psi (400-600 bar). They can be configured to deliver a constant mobile phase composition (isocratic mode) or to gradually change the composition (gradient mode). The pump’s ability to maintain consistent pressure and flow rate is critical for effective separation.
- Injector
- Function: The injector introduces the sample into the flow of the mobile phase.
- Description: Samples are generally introduced in volumes ranging from 5 to 20 microliters (µL). Injectors must withstand the high pressures present in the system. Automated versions, known as autosamplers, allow for high-throughput analysis by scheduling multiple injections.
- Column
- Function: The column is the central component where separation of the sample components occurs.
- Description: The stationary phase within the column interacts with the sample, leading to separation based on various physical and chemical properties. Columns are typically housed in stainless steel to withstand high pressures and chemical interactions. The packing material, often silica or polymer gels, contributes to the separation efficiency.
- Detector
- Function: The detector measures and quantifies the analytes as they elute from the column.
- Description: Detectors provide signals based on the presence and concentration of analytes. These signals are converted into chromatograms, which graphically represent the detector response over time. Various types of detectors are used depending on the nature of the sample and the analysis required.
- Computer/Data System
- Function: The computer controls the HPLC system and processes the data from the detector.
- Description: It manages all system modules and interprets the detector signals to determine both the retention times and quantities of sample components. Modern systems use sophisticated software for data acquisition, peak analysis, and quantitative calculations, enhancing the accuracy and efficiency of chromatographic analysis.
- Degasser
- Function: The degasser removes dissolved gases from the mobile phase to prevent interference and maintain baseline stability.
- Description: It employs polymer membrane tubing with tiny pores to allow gases to escape while retaining the liquid. This step is crucial for minimizing noise and ensuring a stable baseline during analysis.
- Column Heater
- Function: The column heater maintains the column at a consistent temperature, which is vital for reproducibility and optimal separation.
- Description: Temperature control helps in achieving repeatable results and can improve resolution for specific analyses. For instance, elevated temperatures are beneficial for separating sugars and organic acids. The column heater ensures that the temperature is maintained consistently, typically within a column oven.
Each of these components contributes to the overall performance of an HPLC system, ensuring accurate, reliable, and reproducible analysis of complex mixtures.
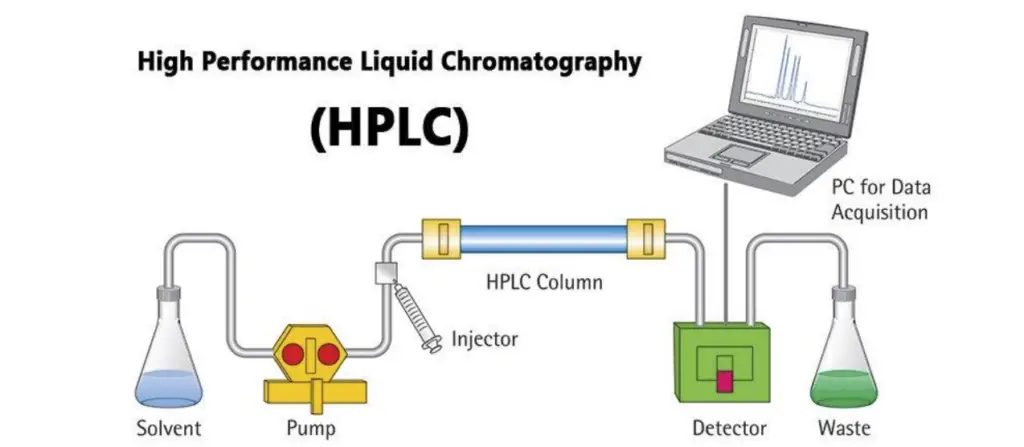
| Instrumentation | Function | Description |
|---|---|---|
| Mobile Phase/Solvent Reservoir | Holds the mobile phase/solvent. | Typically a glass bottle. Advanced systems may include features for degassing and air isolation. |
| Solvent Delivery System | Delivers mobile phase with a continuous, pulse-free flow. | Ensures steady flow rate despite system back pressure, crucial for reproducibility. |
| Pump | Forces the mobile phase through the chromatograph at a controlled flow rate. | Generates pressures up to 6000-9000 psi (400-600 bar). Can operate in isocratic or gradient modes. |
| Injector | Introduces the sample into the mobile phase. | Typically handles volumes of 5-20 µL. Must withstand high pressures; autosamplers automate the process. |
| Column | Separates sample components using the stationary phase. | Usually housed in stainless steel. Contains packing material like silica or polymer gels. |
| Detector | Measures and quantifies analytes as they elute from the column. | Converts analyte presence into chromatograms; various types available based on sample and analysis needs. |
| Computer/Data System | Controls the HPLC system and processes data from the detector. | Manages modules and interprets detector signals for retention times and quantities; uses advanced software. |
| Degasser | Removes dissolved gases from the mobile phase. | Uses polymer membrane tubing to prevent gas interference and maintain baseline stability. |
| Column Heater | Maintains consistent column temperature. | Ensures repeatable results and improved resolution for specific analyses; typically within a column oven. |
Types of HPLC
High-Performance Liquid Chromatography (HPLC) is a sophisticated analytical technique utilized to separate, identify, and quantify components within a mixture. The effectiveness of HPLC depends on the stationary phase used, which defines the method’s variant. Here are the primary types of HPLC, each employing a distinct approach for separation:
- Normal Phase HPLC (NP-HPLC)
- Principle: Normal Phase HPLC separates analytes based on their polarity. This method utilizes a polar stationary phase and a non-polar mobile phase.
- Stationary Phase: Typically silica or alumina, which possesses polar characteristics.
- Mobile Phase: Composed of non-polar solvents such as hexane, methylene chloride, chloroform, diethyl ether, or their mixtures.
- Mechanism: Polar analytes interact more strongly with the polar stationary phase, resulting in longer retention times compared to less polar substances. This selective retention facilitates the separation of compounds based on their polarity.
- Reversed Phase HPLC (RP-HPLC)
- Principle: In reversed phase chromatography, separation occurs through hydrophobic interactions. This variant employs a non-polar stationary phase and a moderately polar aqueous mobile phase.
- Stationary Phase: Non-polar materials such as octadecylsilane (C18) or octylsilane (C8) are commonly used.
- Mobile Phase: Typically consists of water mixed with organic solvents like methanol or acetonitrile.
- Mechanism: Analytes interact with the non-polar stationary phase through hydrophobic interactions. The extent of binding is proportional to the hydrophobic surface area of the analyte, resulting in separation based on the hydrophobicity of the components.
- Ion Exchange Chromatography
- Principle: Ion exchange chromatography separates analytes based on electrostatic interactions between ions in the sample and charged sites on the stationary phase.
- Stationary Phase: Contains charged functional groups, either positively (cation exchange) or negatively (anion exchange) charged.
- Mechanism: Ions in the sample are attracted to oppositely charged sites on the stationary phase. The strength of these interactions determines the retention time. Ions with similar charges are repelled and excluded from the interaction. This method is frequently used for water purification, protein separation, and analysis of carbohydrates and oligosaccharides.
- Size Exclusion Chromatography (SEC)
- Principle: SEC, also known as gel permeation chromatography or gel filtration chromatography, separates molecules based on their size.
- Stationary Phase: Composed of porous gel beads that create a sieve-like structure.
- Mechanism: Larger molecules are excluded from entering the pores and thus elute first. Smaller molecules penetrate the pores and travel a longer path, eluting later. This technique is especially useful for determining the molecular weight of polymers and assessing the structural properties of proteins.
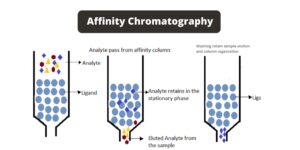
- Bioaffinity Chromatography
- Principle: This method is based on the specific, reversible interactions between biomolecules (e.g., proteins) and ligands attached to the stationary phase.
- Stationary Phase: Ligands are covalently bonded to a solid support, creating a bioaffinity matrix.
- Mechanism: Proteins or other biomolecules bind specifically to the ligands. The bound proteins can be eluted by:
- Bio-specific Elution: Adding a free ligand to the elution buffer, which competes with the column-bound ligand.
- Non-specific Elution: Altering conditions such as pH or ionic strength to weaken the interaction between the protein and the stationary phase.
- Applications: This method allows for high-purity separation of biomolecules in a single step, often achieving 10-1000-fold purification.
Each HPLC variant provides distinct advantages depending on the specific separation needs, from polarity-based to size-based separations and highly selective biomolecular interactions.
Applications of HPLC
High-Performance Liquid Chromatography (HPLC) has become an indispensable analytical technique across various scientific fields due to its versatility and precision. The following outlines the diverse applications of HPLC, emphasizing its role in different sectors:
- Pharmaceutical Industry
- Drug Analysis:
- Identification and Quantification: HPLC is utilized to identify and quantify active pharmaceutical ingredients and metabolites in drug formulations. This ensures that the correct dosage is administered and that the drug is effective.
- Shelf-Life Determination: It helps in assessing the stability and shelf-life of pharmaceutical products by monitoring the degradation of compounds over time.
- Tablet Dissolution Studies: HPLC measures the dissolution rate of tablets, which is crucial for evaluating the drug release profile.
- Quality Control:
- Product Purity: HPLC assesses the purity of pharmaceutical products, detecting any impurities or contaminants that may affect the efficacy and safety of the drug.
- Drug Analysis:
- Environmental Analytics
- Pollutant Detection:
- Analysis of Contaminants: HPLC is employed to identify and quantify pollutants in environmental samples, such as water and soil, helping in the monitoring of environmental quality.
- Phenolic Compounds in Water: It detects phenolic compounds in drinking water, which are important indicators of water pollution.
- Bio-monitoring:
- Detection of Pollutants: HPLC is used in bio-monitoring to assess pollutant levels in sedimented samples and track their impact on the environment.
- Pollutant Detection:
- Biochemical and Clinical Research
- Biopolymer Separation:
- Purification of Proteins and Nucleic Acids: HPLC separates and purifies biopolymers, such as enzymes and nucleic acids, which is essential for research and therapeutic applications.
- Clinical Analysis:
- Quantification of Biomolecules: It quantifies ions, antibiotics, bilirubin, and other biomarkers in biological fluids, aiding in disease diagnosis and monitoring.
- Neuropeptide Detection: HPLC detects endogenous neuropeptides in brain extracellular fluids, contributing to neuroscience research.
- Biopolymer Separation:
- Food and Flavor Industry
- Quality Assurance:
- Analysis of Beverages and Food Products: HPLC ensures the quality and safety of food products, such as soft drinks, juices, and beer, by analyzing sugar content, polycyclic compounds, and other additives.
- Trace Analysis:
- Detection of Contaminants: It performs trace analysis of contaminants, such as military explosives, in agricultural crops, ensuring food safety.
- Quality Assurance:
- Forensic Analysis
- Drug Testing:
- Quantification in Biological Samples: HPLC quantifies drugs in biological samples, such as blood and urine, which is vital for forensic investigations and toxicology.
- Identification of Substances:
- Detection of Anabolic Steroids: It identifies anabolic steroids and other substances in various biological samples, aiding in doping control and forensic analysis.
- Drug Testing:
- Industrial Applications
- Product Purity and Quality Control:
- Analysis of Synthetic Polymers: HPLC is used to analyze synthetic polymers, ensuring their purity and quality in industrial applications.
- Isolation of Valuable Products: It isolates and purifies valuable chemical products, contributing to efficient production processes.
- Product Purity and Quality Control:
- Specialized Chromatographic Techniques
- Ligand-Exchange Chromatography: This technique is used for the separation of metal ions based on their interaction with ligands.
- Ion-Exchange Chromatography: Applied in the separation of proteins and carbohydrates, it relies on the ionic interactions between the sample and the stationary phase.
- High-pH Anion-Exchange Chromatography: This method specifically separates carbohydrates and oligosaccharides, providing detailed analysis of complex carbohydrate mixtures.
Uses of HPLC
High-Performance Liquid Chromatography (HPLC) is a versatile analytical technique employed across a variety of scientific and industrial fields. Its applications extend from fundamental research to quality control and regulatory compliance. Below are the primary uses of HPLC:
- Chemistry and Biochemistry Research
- Analyzing Complex Mixtures:
- Separation of Components: HPLC is used to analyze complex chemical mixtures by separating individual components based on their chemical properties. This allows for detailed study of each substance within a mixture.
- Purifying Chemical Compounds:
- Isolation of Pure Substances: The technique isolates and purifies specific chemical compounds from mixtures, which is essential for further research or product development.
- Developing Synthesis Processes:
- Optimization of Reactions: HPLC helps in developing and optimizing processes for synthesizing chemical compounds by monitoring reaction progress and purity.
- Isolating Natural Products:
- Extraction and Purification: Natural products, such as plant extracts and bioactive compounds, are isolated and purified using HPLC, facilitating their study and potential application.
- Predicting Physical Properties:
- Characterization of Substances: HPLC can be employed to predict and assess the physical properties of compounds based on their chromatographic behavior.
- Analyzing Complex Mixtures:
- Quality Control
- Ensuring Purity of Raw Materials:
- Testing Raw Materials: HPLC is used to verify the purity of raw materials before they enter production processes, ensuring that they meet required standards.
- Controlling and Improving Process Yields:
- Process Monitoring: The technique monitors and controls the yield of chemical processes by analyzing intermediate and final products, aiding in process optimization.
- Quantifying Final Product Assays:
- Final Product Analysis: HPLC quantifies the concentration of active ingredients and contaminants in final products, ensuring they meet quality specifications.
- Evaluating Product Stability:
- Stability Testing: The technique assesses the stability of products over time by monitoring degradation products, which helps in determining shelf life and storage conditions.
- Ensuring Purity of Raw Materials:
- Environmental Analysis
- Analyzing Air and Water Pollutants:
- Pollutant Detection: HPLC is used to analyze pollutants in environmental samples, such as air and water, enabling the detection of trace contaminants and ensuring environmental safety.
- Analyzing Air and Water Pollutants:
- Regulatory Compliance
- Food and Drug Surveillance:
- Regulatory Testing: Federal and state regulatory agencies utilize HPLC to survey and test food and drug products. This ensures compliance with safety and quality standards, protecting public health.
- Food and Drug Surveillance:
Advantages of HPLC
High-Performance Liquid Chromatography (HPLC) is renowned for its numerous advantages, which contribute to its widespread use in various scientific and industrial applications. The key benefits of HPLC include:
- Speed
- Rapid Analysis:
- Short Analysis Time: HPLC provides rapid separation and analysis of compounds. This efficiency allows for high-throughput analysis, which is crucial in both research and production environments.
- Increased Productivity: The quick turnaround time enhances laboratory productivity, enabling the analysis of a larger number of samples in a shorter period.
- Rapid Analysis:
- Efficiency
- High Resolution:
- Effective Separation: The technique offers high-resolution separation of complex mixtures, allowing for the effective separation of closely related compounds.
- Minimized Solvent Usage: HPLC systems use solvents efficiently, often requiring smaller volumes compared to other techniques, which can reduce operational costs and environmental impact.
- Optimal Use of Resources:
- Cost-Effective: Despite the high initial investment, the efficient use of resources and reduced need for extensive sample preparation make HPLC a cost-effective choice in the long run.
- High Resolution:
- Accuracy
- Precise Quantification:
- Reliable Measurements: HPLC provides accurate and reliable quantification of compounds, essential for analytical precision in various fields such as pharmaceuticals and environmental analysis.
- Consistent Results: The technique ensures consistent and reproducible results, which is vital for quality control and regulatory compliance.
- Detailed Analysis:
- High Sensitivity: HPLC can detect and quantify trace levels of compounds, making it suitable for applications requiring high sensitivity and accuracy.
- Precise Quantification:
- Versatility
- Wide Range of Applications:
- Adaptable to Different Samples: HPLC is versatile and can analyze a broad spectrum of sample types, including pharmaceuticals, environmental samples, and food products.
- Multiple Detection Methods: The technique supports various detection methods, such as UV, fluorescence, and mass spectrometry, enhancing its applicability for different analytical needs.
- Flexible Method Development:
- Customizable Conditions: HPLC allows for the adjustment of various parameters, such as column type and mobile phase composition, to optimize separation for specific applications.
- Wide Range of Applications:
- Precision
- High Precision in Analysis:
- Accurate Separations: HPLC delivers precise and accurate separations, which is critical for the identification and quantification of complex mixtures.
- Consistency: The technique ensures reproducibility in separations and quantifications, which is essential for scientific research and quality control.
- High Precision in Analysis:
- Powerful and Adaptable
- Robust Performance:
- Handling Complex Samples: HPLC can handle complex and challenging samples, such as those with high matrix effects or requiring high-resolution separation.
- Adaptability to Various Fields:
- Robust Performance:
Limitations of HPLC
While High-Performance Liquid Chromatography (HPLC) is a powerful and widely used analytical technique, it does have several limitations. These constraints can affect its suitability and efficiency depending on the specific application. The primary limitations of HPLC include:
- Cost
- Expensive Operation:
- High Consumable Costs: The use of large quantities of high-purity organic solvents can be costly. These solvents are essential for the mobile phase in HPLC, and their continuous use contributes significantly to operational expenses.
- Initial Investment: The cost of acquiring and maintaining HPLC equipment is substantial, including expenses for pumps, detectors, columns, and other components.
- Maintenance Expenses:
- Regular Upkeep: Maintenance of HPLC systems requires periodic replacement of components such as columns and seals, which adds to the overall cost.
- Expensive Operation:
- Complexity
- Operational Complexity:
- Complex Instrumentation: The setup and operation of HPLC systems are complex. This complexity includes adjusting various parameters such as flow rate, temperature, and mobile phase composition to achieve optimal separation.
- Requirement for Skilled Personnel: Operating HPLC systems effectively necessitates highly trained and skilled personnel. Proper training is required to handle the equipment and interpret results accurately.
- Technical Expertise:
- Advanced Knowledge Needed: Understanding the principles of chromatographic separation and method development requires a high level of technical expertise.
- Operational Complexity:
- Sensitivity Issues
- Low Sensitivity for Certain Compounds:
- Detection Challenges: HPLC can exhibit low sensitivity for certain compounds, particularly those that are present in very low concentrations or have low UV absorbance.
- Irreversible Adsorption: Some substances may irreversibly adsorb to the column, resulting in incomplete detection or loss of analyte.
- Inadequate for Volatile Compounds:
- Volatility Limitations: Volatile substances are not effectively separated by HPLC. Gas chromatography is generally more suitable for analyzing volatile compounds due to its ability to handle gases and low-boiling-point substances.
- Low Sensitivity for Certain Compounds:
- Maintenance and Operation
- High Maintenance Costs:
- Frequent Replacements: Routine maintenance involves replacing worn-out parts such as pumps, columns, and detectors, which can be expensive and time-consuming.
- Operational Complexity:
- Time-Consuming Setup: The setup and calibration of HPLC systems can be time-consuming. This includes preparing mobile phases, equilibrating columns, and optimizing conditions for each specific analysis.
- High Maintenance Costs:
- Limited Applicability
- Not Ideal for All Compounds:
- Volatile Substances: As mentioned, HPLC is not ideal for volatile compounds, which are better analyzed using gas chromatography techniques.
- Sensitivity Variability: The technique may not be suitable for compounds that are either very low in concentration or exhibit poor interaction with the stationary phase used in HPLC.
- Not Ideal for All Compounds:
FAQ
What is High-Performance Liquid Chromatography (HPLC)?
High-Performance Liquid Chromatography (HPLC) is an analytical technique used to separate, identify, and quantify components in a mixture. It involves the passage of a sample mixture through a column containing a stationary phase and a mobile phase, with different components in the sample interacting differently with the stationary phase, resulting in their separation.
How does HPLC differ from other chromatographic techniques?
HPLC differs from other chromatographic techniques, such as Gas Chromatography (GC), in that it utilizes a liquid mobile phase instead of a gas. This makes HPLC suitable for the separation and analysis of a wider range of compounds, including non-volatile and thermally labile substances.
What are the common applications of HPLC?
HPLC has diverse applications in various fields, including pharmaceutical analysis, environmental monitoring, food and beverage analysis, forensic science, clinical diagnostics, and more. It is used for analyzing drugs, separating complex mixtures, determining impurities, quantifying analytes in biological samples, and quality control of industrial products, among other applications.
What are the different types of HPLC columns?
There are various types of HPLC columns, including normal phase, reverse phase, ion exchange, and size exclusion columns. Normal phase columns use a polar stationary phase and a non-polar mobile phase, while reverse phase columns use a non-polar stationary phase and a polar mobile phase. Ion exchange columns contain ionic groups for separating anions and cations, while size exclusion columns separate compounds based on their size.
Which detector is commonly used in HPLC?
The most commonly used detector in HPLC is the UV-Vis detector, which measures the absorbance of analytes at specific wavelengths. Other detectors used in HPLC include fluorescence detectors, refractive index detectors, and mass spectrometers, which offer additional selectivity and sensitivity for specific applications.
How can HPLC analysis be optimized for better results?
To optimize HPLC analysis, several factors can be adjusted, including the choice of column, mobile phase composition, flow rate, temperature, and detector settings. Method development experiments can be performed to find the most suitable conditions for achieving efficient separations and desired analytical results.
What is the significance of sample preparation in HPLC?
Sample preparation is crucial in HPLC to ensure accurate and reliable results. It involves the extraction, purification, and concentration of analytes from the sample matrix, removing interferences, and preparing the sample for injection into the HPLC system. Proper sample preparation techniques contribute to enhanced sensitivity, reduced matrix effects, and improved separation efficiency.
What are the advantages of using HPLC over other analytical techniques?
HPLC offers several advantages, including high separation efficiency, versatility in analyzing different compound types, the ability to handle complex sample matrices, and accurate quantification of analytes. It is also suitable for both qualitative and quantitative analysis, and it can be automated for high-throughput applications.
Can HPLC be used for chiral separations?
Yes, HPLC can be used for chiral separations. Chiral compounds have molecules that are non-superimposable mirror images of each other, and HPLC with chiral stationary phases can selectively separate and quantify these enantiomers.
What are the key parameters to consider for HPLC method validation?
HPLC method validation involves assessing various parameters, including specificity, linearity, accuracy, precision, robustness, limit of detection (LOD), and limit of quantitation (LOQ). Method validation ensures that the developed HPLC method is reliable, reproducible, and fit for its intended purpose.
References
- https://www.shodex.com/en/kouza/a.html
- https://www.alphacrom.com/en/hplc-basics
- https://laboratoryinfo.com/hplc/
- https://sciencing.com/disadvantages-advantages-hplc-5911530.html
- https://www.slideshare.net/krakeshguptha/hplc-26970638
- https://www.chemguide.co.uk/analysis/chromatography/hplc.html
- https://learnaboutpharma.com/hplc-definition-principle-diagram-instrumentation-types-and-applications/
- https://www.ijres.org/papers/Volume-9/Issue-8/Series-4/D09082328.pdf
- https://www.slideshare.net/slideshow/hplcintroduction-theory-instrumentation-advantage-limitationapplications-pptx/255621204
- https://www.slideshare.net/slideshow/instrumentation-of-hplc-69314042/69314042
- https://www.shimadzu.com/an/service-support/technical-support/analysis-basics/basic/what_is_hplc.html