Designed to enlarge things too tiny for the human eye to see, a microscope is a tool. It provides a portal to the hidden world of minute structures, allowing medical practitioners, teachers, and researchers to examine materials, cells, and creatures in until unheard-of clarity.
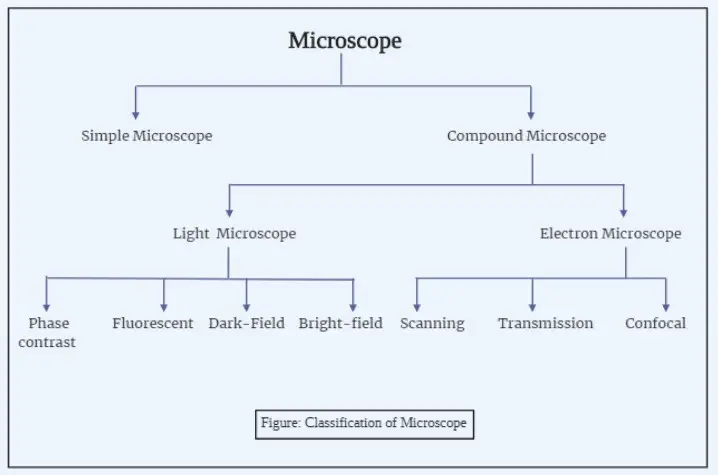
Microscopes range significantly in kind, from simple to very sophisticated models. Just a single lens, a basic microscope has low magnification yet is enough for rudimentary observation. Conversely, compound microscopes are quite beneficial in labs as they use several lenses to attain better clarity and magnificences. At the highest magnification, electron microscopes use beams of electrons rather than light to expose structures at levels ordinary optics could not equal.
The history of the microscope is closely entwined with scientific progress. It began modestly in the late 16th century, with crude devices that magnified pictures using simple lenses. Antonie van Leeuwenhoek unveiled increasingly complex compound microscopes by the 17th century, which let him explore the subtleties of live cells. His work created the framework for contemporary biology, altering our grasp of life’s fundamental elements.
Microscopy developed in great flourish as ages passed. The 19th century introduced specialist instruments like the polarising and fluorescence microscopes, which allowed researchers to probe deeper into light characteristics and cellular interactions. Then came the breakthrough electron microscope in the 20th century, enabling imaging of molecular and atomic-scale details—a leap that transformed science and medicine.
Today, microscopes are vital in domains ranging from medical diagnostics to materials research. They disclose tiny infections, explain cellular activities, and contribute in technological developments. Whether used by a student staring at a plant cell or a scientist researching quantum materials, microscopes bridge the visible and the invisible, giving doorways to realms previously unseen.
Different Types of Microscopes

Microscopes come in a fascinating variety, each built for unique applications and having distinct capabilities. They range from simple, easy forms to highly advanced tools capable of amazing detail. Here’s a dive into some of the many types of microscopes—each with its quirks and uses.
- Simple Microscope– Think of this as the starting type. With just one lens, it’s like a magnifying glass but a bit more accurate. Ideal for low-magnification jobs, its simplicity makes it suitable for newbies or simple observations.
- Compound Microscope- A choice in labs, this one boasts multiple lenses. It provides higher magnifications and better sharpness. Schools, medical facilities, and study labs stand by it. It’s the standard choice when tiny details matter.
- Phase Contrast Microscope – Designed for transparent objects, this microscope plays with light to show differences. No need to colour the sample. It’s great for studying live cells without altering them.
- Fluorescence Microscope- This high-tech device uses fluorescent dyes and special lights to reveal particular features within a sample. Researchers studying cellular components or tracking molecules depend on it greatly.
- Electron Microscopes (EM)- These are in a league of their own. Instead of light, they use electron beams for photography. Two main types exist:
- Scanning Electron Microscope (SEM)- Creates detailed 3D pictures of objects.
- Transmission Electron Microscope (TEM)– Goes deeper, revealing internal structures with incredible clarity.
- Dark Field Microscope -This unique type makes the background dark, letting only the subject shine brightly. It’s great for studying bacteria and live objects in motion.
- Dissecting Microscope (Stereo Microscope) – When depth sense is key, this one shines. It offers a three-dimensional view, ideal for dissections or working on complex items.
- Digital Microscope– Blending traditional optics with digital photography, this type allows you to show and record pictures on a screen. Great for sharing and reviewing results.
- Scanning Probe Microscope (SPM) – Used mainly in nanotechnology, it studies objects at the atomic level. The Atomic Force Microscope (AFM), a type of SPM, is highly exact, often mapping molecular surfaces.
- Inverted Microscope – Here, the light source and lenses are turned. It’s excellent for watching samples in containers like Petri plates.
- Acoustic Microscope- Using sound waves instead of light, it’s ideal for non-invasive study of internal structures in both biological and material studies.
- X-Ray Microscope – This advanced type uses X-rays to look into a sample’s inner workings. It’s an expert tool for complicated materials.
- Polarising Microscope – Designed for studying materials under polarised light, it’s highly useful in geology and science.
- Metallurgical Microscope – Tailored for metals and solid samples, this one studies surface structures and makeup in engineering and manufacturing.
- Pocket and USB Microscopes – These are the compact choices. Small, lightweight, and sometimes even digital, they’re handy for on-the-go notes.

1. Simple Microscope
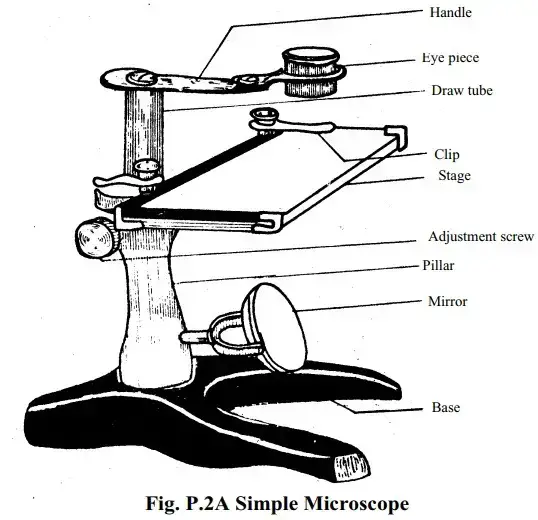
A simple microscope is the simplest straightforward sort of optical magnifier, relying on a single convex lens for its functioning. Its design is basic, yet its uses are surprisingly extensive. Often viewed as a rudimentary instrument, its simplicity makes it effective for magnifying items roughly ten times their true size.
The basic idea is easy yet interesting. When a sample is put at the lens’s focal point, a virtual, upright, and magnified picture appears. This picture forms at the least distance of distinct vision, allowing the observer to perceive minute details more clearly. Essential components of a simple microscope are a mirror to give light, a convex lens to magnify the sample, and a stage supported by a steel base.
Mathematical Insight
The magnifying power 𝑚 m is stated as:
m=1+D/F
Here, D represents the least distance of distinct vision, and F denotes the focal length of the convex lens.
Uses of Simple Microscopes
Despite its limitations, the basic microscope finds its way into different fields:
- Biological studies: Researchers use it to analyse the morphology of insects, algae, and fungus.
- Soil science: It helps assess soil composition and texture.
- Electronics repair: Delicate jobs like mending watches, mobiles, or microcomponents benefit from its clarity.
- Jewellery inspection: Jewellers rely on it to determine the quality of gemstones, such as rubies and diamonds.
- Script analysis: Historians and amateurs use it to analyse exquisite engravings or small inscriptions.
Limitations
- Magnification is limited—typically restricted at 10X.
- Illumination concerns emerge from its reliance on mirrors.
- It lacks a mechanical stage, making adjustments less accurate.
- Thin, well-prepared samples are needed for a good perspective.
- Low resolution and contrast restrict its ability to reveal detailed details.
2. Compound Microscope
A compound microscope is a powerful tool designed to magnify tiny specimens using visible light and a system of lenses. Unlike simpler designs, it relies on two primary lenses: the objective lens and the ocular lens (eyepiece). This combination allows it to achieve magnifications up to 1000X, offering detailed views of microscopic entities.
Mathematically, its magnification is represented as:
m = (D/f₀) × (L/fₑ)
Where:
- m: magnification
- D: least distance of distinct vision
- L: tube length
- f₀: focal length of the objective lens
- fₑ: focal length of the ocular lens
This relationship demonstrates how the interplay of lens properties governs magnification, ensuring precise imaging for detailed observations.
This device is important in several scientific areas, including microbiology, pathology, and life sciences. From assessing tissue architecture in histopathology to investigating cell shape in cytology, its applications are numerous.
How It Works
A compound microscope functions by a process of light focusing and magnification. Here’s a simplified explanation:
- Light from the illuminator goes through the condenser and is focused onto the specimen.
- The objective lens collects the transmitted light, generating a magnified main picture.
- This picture is then further expanded when light travels through the ocular lens, providing a secondary image visible to the spectator.
- The result? A highly detailed double-magnified representation of the specimen.
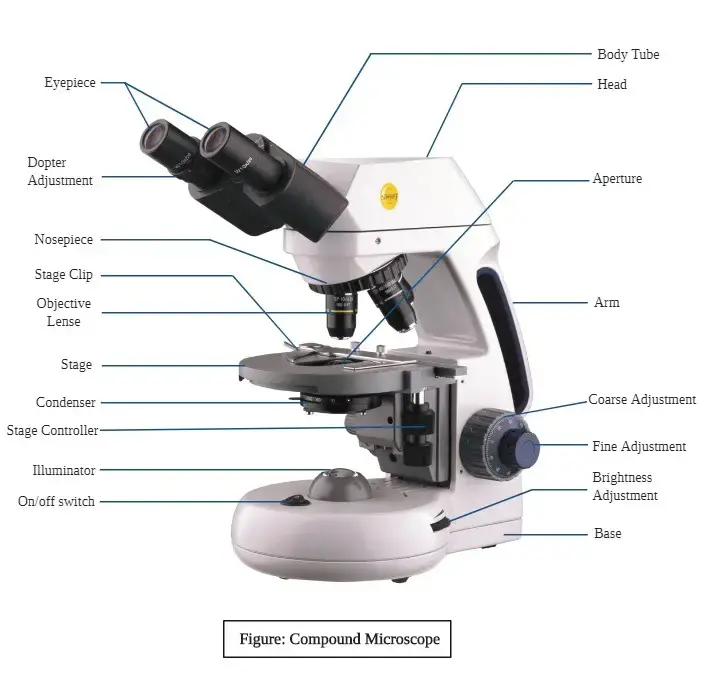
Key Components
The construction of a compound microscope involves multiple linked elements, each providing a specific function:
- Illuminator: Provides the light source.
- Diaphragm: Regulates light intensity.
- Condenser: Focuses light onto the sample.
- Objective Lens: Magnifies the specimen initially.
- Ocular Lens (Eyepiece): Further enlarges the image.
- Adjustment Knobs: Fine and coarse knobs enable sharp focusing.
- Stage: Holds the specimen slide.
- Base and Arm: Provide stability and support.
Additional parts, such as the nosepiece, rack stop, and brightness control, enhance usability.
Applications
The compound microscope has a diverse range of uses:
- In microbiology, it reveals the structure of microorganisms.
- Histopathology involves its use to study tissues, tumours, and cellular changes.
- Cytologists depend on it for analysing cell types and structures.
- It’s a staple for biologists when observing slides of tissues or other biological elements.
Limitations
While versatile, the compound microscope has its constraints:
- Objects smaller than 0.4 μm, the wavelength of visible light, cannot be observed.
- Its resolution and contrast are relatively lower compared to advanced microscopy.
- It is unsuitable for imaging living internal structures.
- Thin, stained specimens are often required for effective observation.
Compound Microscope Diagram
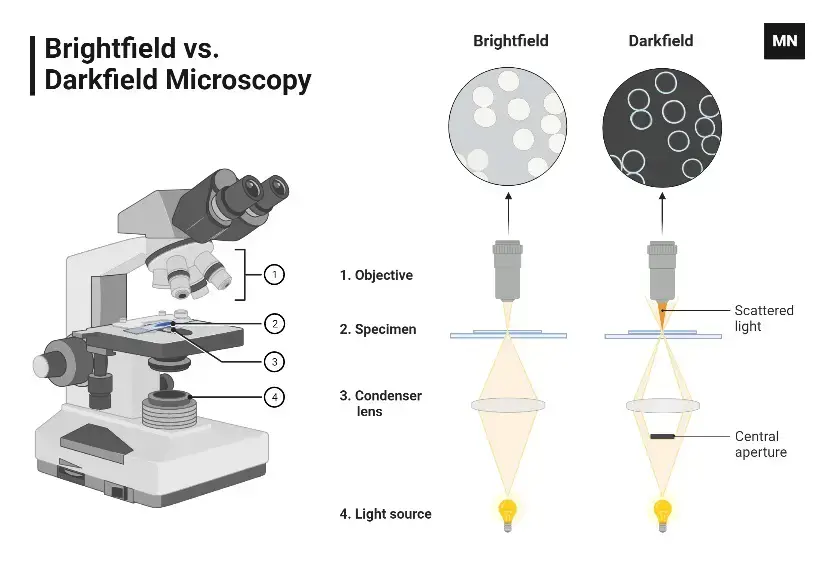
3. Bright-field Microscope
- In a bright-field microscope, the specimen appears as dark against the bright background.
- A bright-field microscope is a type of light microscope that uses visible light to illuminate a sample and a system of lenses to magnify the image. In a bright-field microscope, the light source is located below the stage, and the sample is illuminated from below.
- The light passes through the transparent or semi-transparent sample and is focused by the objective lens onto the eyepiece. This produces a bright image against a dark background, which makes it easy to see the details of the sample.
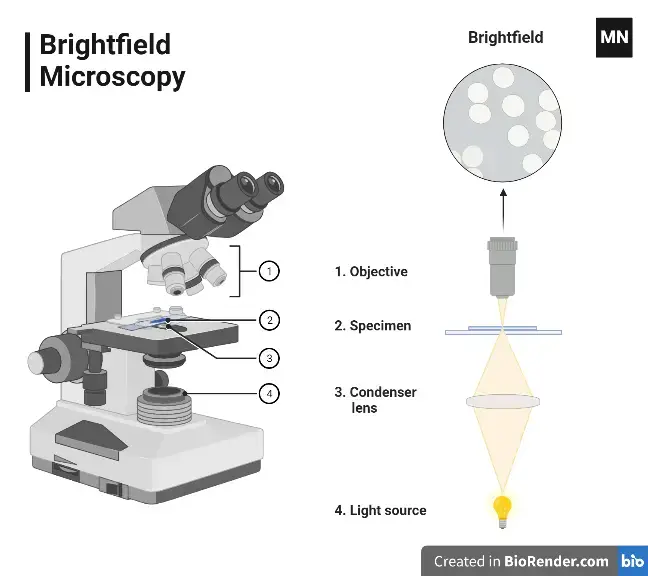
- Bright-field microscopes are the most common type of light microscope and are widely used in a variety of fields, including biology, medicine, and materials science. They are often used to study cells, tissues, and small structures within materials.
- One of the advantages of bright-field microscopy is that it is relatively simple and easy to use. It is also relatively inexpensive compared to other types of microscopes.
- However, bright-field microscopy has some limitations, including poor contrast for transparent or colorless samples, and the inability to distinguish between closely spaced objects or structures. To overcome these limitations, other types of microscopy, such as phase contrast microscopy or fluorescence microscopy, may be used.
Applications of Bright-field Microscope
- They are used in the laboratory for studying the outer structure of microorganisms.
Advantages and Disadvantages of Bright-field Microscope
Advantages:
- Widely available: Bright-field microscopes are widely available and are often used in educational settings and laboratories.
- Easy to use: Bright-field microscopes are relatively simple and easy to use, even for people who are new to microscopy.
- Inexpensive: Bright-field microscopes are generally less expensive compared to other types of microscopes.
- Good for opaque or semi-transparent samples: Bright-field microscopy is well-suited for studying opaque or semi-transparent samples, such as cells, tissues, and small structures within materials.
Disadvantages:
- Poor contrast for transparent or colorless samples: Bright-field microscopy may not provide sufficient contrast for transparent or colorless samples, such as bacteria or algae.
- Limited detail: Bright-field microscopy may not be able to distinguish between closely spaced objects or structures, which limits its ability to observe fine details.
- Limited lighting options: Bright-field microscopes often have a single light source, which may not be sufficient for observing some samples.
- Limited adjustments: Bright-field microscopes often have limited adjustments, such as the ability to focus or adjust the eyepieces, which may make it more difficult to obtain a clear image.
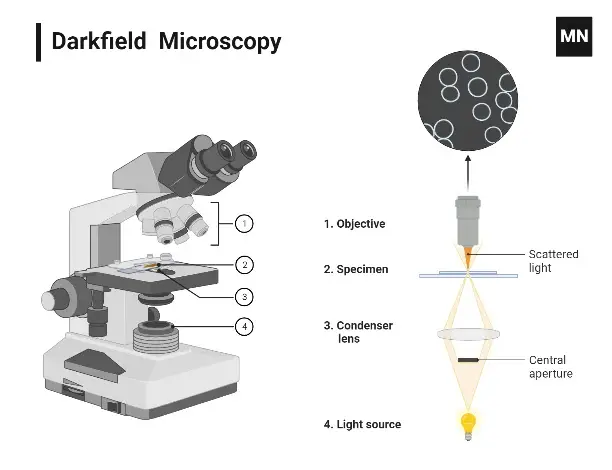
4. Dark-Field Microscope
A dark-field microscope is a specialist light microscope designed to showcase specimens against a contrasting dark backdrop. It does this by using just the light dispersed by the specimen, providing a brilliant picture with a black background surrounding it. This configuration is made feasible by an opaque disc placed below the condenser lens, which prohibits direct light from reaching the objective lens.
Unlike the bright-field microscope, it delivers better resolution and improved contrast. Another advantage? It removes the need for staining, which is particularly advantageous for monitoring living or fragile objects.
How It Works
The operating principle of a dark-field microscope relies around the scattering of light:
- The condenser channels light around the specimen, preventing direct rays from entering the objective lens.
- Light dispersed by the specimen travels into the objective lens, generating the picture.
- This dispersed light results in a bright specimen on a pitch-black backdrop.
- It’s a simple alteration, but the effects can be astounding, especially for specific biological or microscopic research.
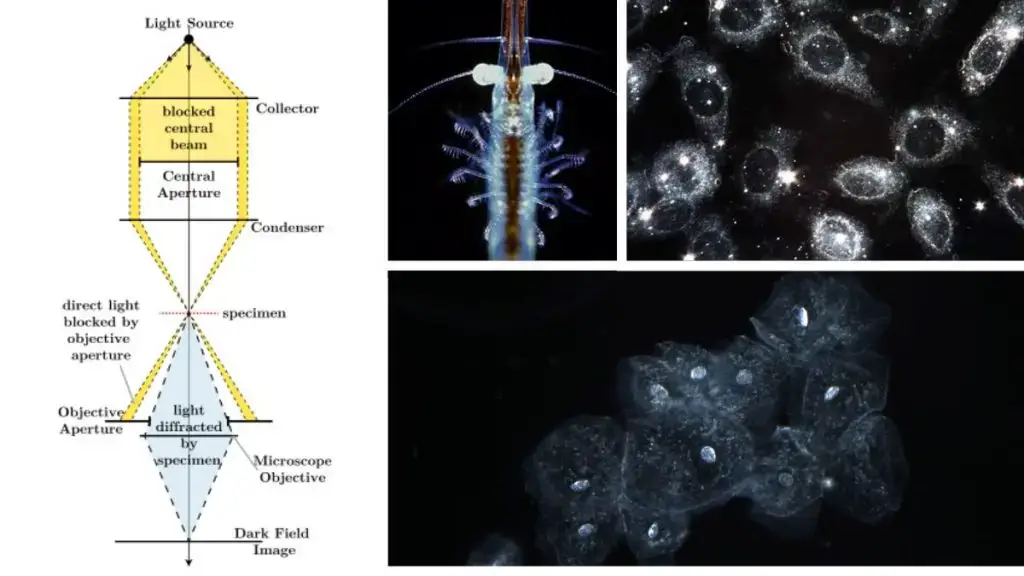
Applications
Dark-field microscopes find value in many disciplines due to their unique imaging capabilities:
- Microbiology: Ideal for investigating microbial movement, spirochetes, and other thin microorganisms.
- Cytology: Used for visualising interior organelles without staining.
- Nanotechnology: When paired with hyperspectral imaging, it assists in characterising nanoparticles.
- Capsulated organisms: Effective in viewing features that may otherwise stay inconspicuous.
- Everyday tech: Plays a part in enabling computer mice to work across transparent surfaces.
Limitations
While strong, the dark-field microscope has its set of constraints:
- Even minor pollutants, like dust, can scatter light and give false pictures.
- Samples must be thinly dispersed and suitably lit.
- The specimen has to be damp or wet to guarantee clarity and efficacy.
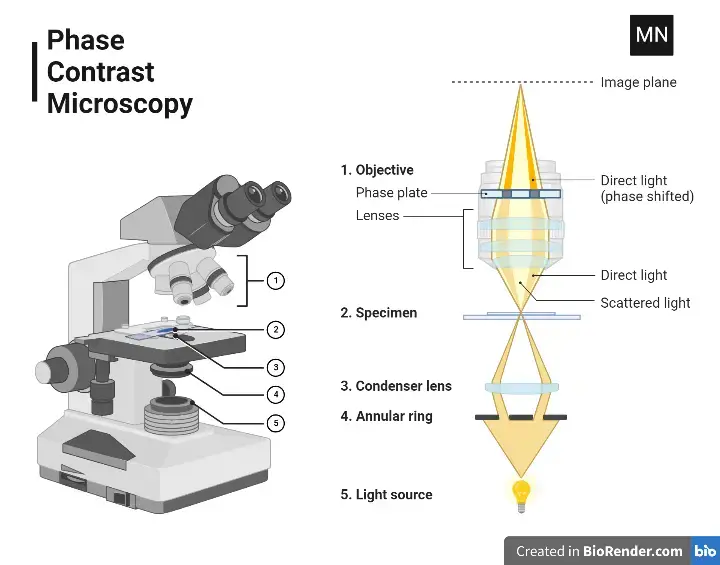
5. Phase-contrast Microscope
A phase-contrast microscope is a refined optical microscope that transforms minute phase shifts in light into observable differences in intensity. This enhancement provides greater contrast, making otherwise invisible details stand out to the human eye. When light passes through transparent specimens, slight phase shifts occur, which our eyes cannot naturally detect. By employing specialised components, these shifts are converted into changes in brightness, producing clear and detailed images.
Key Principle
The microscope’s functioning revolves around how light interacts with specimens:
- Light from the source is focused onto the specimen using a condenser annulus.
- As the light travels through the sample, it encounters varying refractive indices and thicknesses.
- These variations cause phase shifts in the light, which are imperceptible to human vision.
- A phase plate, located in the optical path, converts these phase differences into intensity variations, creating visible contrast.
This principle makes it possible to observe living cells and transparent structures without needing staining or fixing, preserving their natural state.
Unique Components
The phase-contrast microscope has all the standard parts of a compound microscope, along with two essential optical components:
1. Condenser Annulus:
- Also called the sub-stage annular diaphragm.
- It directs a hollow cone of light onto the specimen.
- Positioned beneath the condenser, it consists of a light-absorbing circular plate with a transparent ring that shapes the beam.
2. Phase Plate:
- Found above the objective’s rear focal plane.
- It alters the phase and amplitude of light emerging from the specimen.
- Divided into two regions: the conjugate area (aligned with the annulus) and the complementary area, which is coated with light-retarding materials like magnesium fluoride.
- Available in two types: positive phase plates (with thinner conjugate areas) and negative phase plates (with thicker ones).
Applications
The phase-contrast microscope is widely used across disciplines, offering a non-invasive way to study living and transparent samples:
- Viewing living cells in their natural state.
- Observing protozoans, diatoms, plankton, cysts, helminths, and larvae.
- Examining subcellular structures and cellular processes.
- Analysing thin tissue slices.
- Investigating materials like glass fragments, crystals, lithographic patterns, and latex dispersions.
Limitations
Despite its advantages, this microscope is not without drawbacks:
- Thick specimens are unsuitable as they obscure light transmission.
- A halo effect and shade-off are common artefacts, which may obscure fine details.
- The condenser annulus reduces aperture size, leading to decreased resolution.
4. Fluorescent Microscope
A wonder of optical engineering, the fluorescence microscope elevates light microscopy. It’s not your ordinary microscope. No; it reveals information hidden to the unaided eye by amplifying a picture via fluorescence or even phosphorescence. This instrument allows us to view the tiny world with a clarity difficult to match by means of a mix of technology and biological intuition.
What therefore is the magic underlying it? All of it begins with a very elegant idea known as fluorescence. Something wonderful occurs when light—usually monochromatic—is shined on a properly stained fluorophore specimen. The light the specimen absorbs excites its electrons to a higher energy level. The enjoyable process starts here. Longer wavelength light is emitted by the excited electrons returning to their resting condition. That’s the fluorescence. After then, the produced light is gathered to create a very detailed, sharper, brighter image.
Here’s a basic step-by-step process: On the microscope stage the fluorescent dye-covered specimen is positioned. High-energy light then comes in. Usually from the UV range, this light passes through an excitation filter that lets the sample glow on a limited band of short wavelengths. For all other wavelengths? Obstructed. Brilliant, right?
But that is not all. The light then passes via a dichroic filter, which points it onto the specimen. Excited by this energy, the fluorophore absorbs it and drives electrons into a higher state. These electrons release light with a far longer wavelength when they return to their normal state. Now shining with fluorescence, this released light goes via the dichroic mirror and strikes an emission filter to guarantee only the longer wavelengths—those magnificent fluorescent ones—make it through.
At last, this filtered light passes via the ocular lenses and finds the detector. Here the contrast is amazing and an expanded picture results. The dark and silent background provides the ideal stage for the shining specimen, which stands out in all its great brilliance. It’s like witnessing the unseen rendered clear.
For scientists, fluorescence microscopes open secrets in the chemical and biological domains like treasure chests. Whether you’re studying living cells, pinpointing specific proteins, or mapping out the structures of cell organelles, this tool is indispensable. Beyond just clarity, its sensitivity is astonishing. The fluorophore-stained samples reveal hidden patterns—ones that could easily be missed with conventional microscopy.
In the hands of biologists, pathologists, and researchers, it’s not just about zooming in on cells. It’s about noticing minute details, studying deep biological processes, and discovering molecular participants in ways that were formerly considered inconceivable.
Here’s the interesting part: these microscopes have become crucial in illness diagnosis, notably in cancer research, immunology, and neurology. By use of them, scientists have discovered markers and more precisely followed illnesses than ever before. For research, the capacity to examine DNA, proteins, and biological components at such great clarity changes everything.
At the junction of biology and technology, the fluorescence microscope is a shining illustration of what results from curiosity combined with invention.
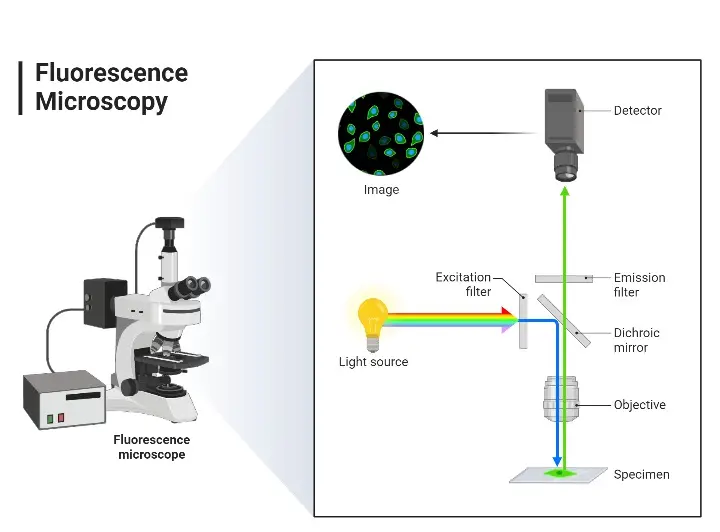
Fluorescence Microscope Parts
The main parts are:
- Fluorophore (Fluorescent Dye)- Fluorophores (fluorescent dyes) are chemicals that give off light when they are energized. Examples are fluorescein, rhodamine, and acridine orange. These organic molecules color living structures.
- Light Source– Light sources mainly include mercury vapor lamps, but high-energy UV light can also be produced using xenon lamps, LEDs, and lasers.
- Excitation Filter – A band-pass filter positioned before the specimen that specifically lets short-wavelength light through to excite the fluorophore.
- Emission Filter – This filter is placed after the specimen. It only lets the light from the fluorophore through and blocks the light used for excitation, providing clear and high-quality images.
- Dichroic Mirror – This special mirror is placed at a 45° angle between the filters. It reflects the light that activates the fluorophore and allows the released light to pass through to the emission filter.
Types of Fluorescence Microscope
- Epifluorescence Microscope
- Most common type
- Uses same light path for excitation and detection through objective lens
- Confocal Microscope
- Uses spatial pinhole to block out-of-focus light
- Produces high-resolution 2D/3D images of thick specimens
- Uses laser light and oscillating mirror for precise spot focusing
- Applications: Eye disease detection, pharmaceutical QC, 3D scanning
- Limitations: Limited wavelength options, high cost
- Multiphoton Microscope
- Uses multiple photons for fluorophore excitation
- Produces high-resolution 3D images
- Common variants: two-photon and three-photon excitation
- Total Internal Reflection Fluorescence (TIRF) Microscope
- Images fluorophores near solid surfaces in aqueous environments
- Provides high resolution, better contrast, and clearer images with reduced background
Uses of Fluorescence Microscope
- Study cellular structures and components by detecting fluorescent molecules, enabling visualization of specific proteins, organelles, and DNA
- Track molecular interactions and cellular processes in real-time, particularly useful for:
- Protein trafficking
- Cell division
- Gene expression
- Signal transduction
- Analyze biological samples in medical diagnostics:
- Cancer cell detection
- Pathogen identification
- Tissue analysis
- Immunofluorescence testing
- Research applications:
- Drug development and screening
- Neuroscience studies
- Developmental biology
- Cell biology investigations
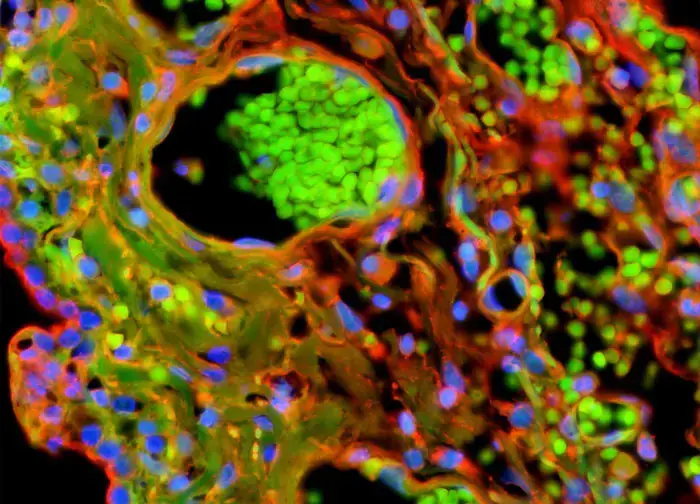
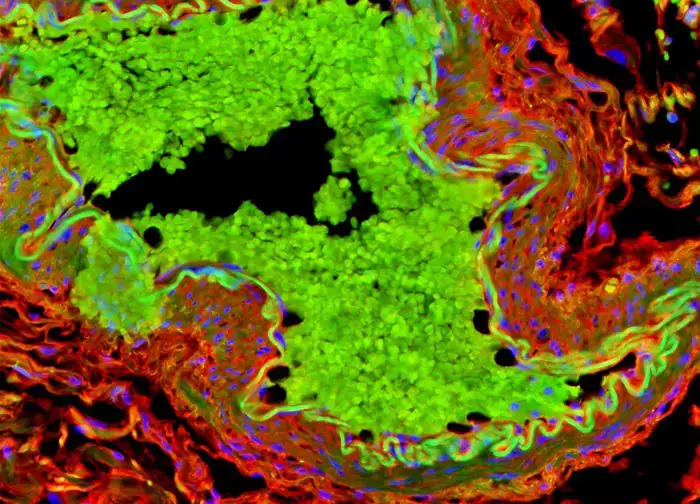
Advantages of Fluorescent Microscope
- High Contrast and Resolution
- Superior visualization of cellular structures
- Better detail discrimination compared to brightfield microscopy
- Enhanced ability to observe nanoscale features
- Specific Molecular Detection
- Selective labeling of target molecules
- Multiple target visualization simultaneously
- Precise tracking of cellular processes
- Live Cell Imaging
- Real-time observation of biological processes
- Non-destructive analysis
- Dynamic cellular interaction studies
- Versatile Applications
- Cell biology research
- Medical diagnostics
- Drug screening
- Materials science analysis
- Quantitative Analysis
- Molecule concentration measurements
- Protein interaction studies
- Gene expression analysis
Disadvantages of Fluorescent Microscope
- Equipment Limitations
- High initial cost and maintenance expenses
- Regular replacement of expensive components
- Complex setup requirements
- Technical Challenges
- Photobleaching of fluorescent markers
- Phototoxicity affecting live specimens
- Autofluorescence interference
- Limited depth penetration
- Operational Requirements
- Need for specialized training
- Complex sample preparation
- Dark room or controlled lighting environment
- Time-consuming alignment and calibration
- Sample Constraints
- Limited observation time for live specimens
- Potential artifact introduction during preparation
- Fluorophore availability and compatibility
- Storage and stability issues of fluorescent samples
- Image Quality Issues
- Background noise
- Signal fading over time
- Cross-talk between fluorophores
- Resolution limitations in thick samples
5. Electron Microscope
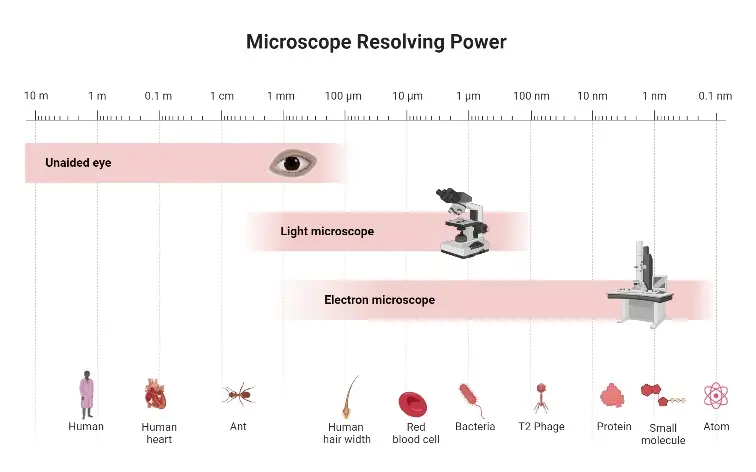
An electron microscope (EM) is a specialist tool for high magnifications achieved by accelerating electron beams instead of light beams. electron microscopes use electromagnetic lenses to concentrate the electron stream, unlike optical microscopes which depend on glass lenses. This lets researchers see features as fine as 0.2 nanometres, hence greatly increasing resolution. With magnifications reaching up to 10 million times, this equipment has transformed imaging in several scientific domains.
The somewhat small wavelength of electrons—roughly 100,000 times shorter than visible light—allows one to create such high-resolution pictures. This reveals complex molecules and provides unmatched clarity and contrast. The clarity of the pictures obtained is amazing, with crisp features that are impossible with standard light microscopy.
Operational Principle
The operating principle of an electron microscope is based on the interaction between accelerated electrons and the sample. Usually ranging from 100 kV to 1000 kV, electrons produced by an electron gun—commonly utilising a heated tungsten filament or field emission source—are accelerated in a vacuum chamber using high-voltage potentials. The electron beam is curved and focussed onto the sample using a series of apertures and electromagnetic lenses.
When the electron beam impacts the specimen, it interacts with the material in complicated ways. The density of the sample or the refractive characteristics will determine how far electrons scatter. Magnetic lenses gather the dispersed electrons and turns these interactions into a picture.
Why Electrons?
The usage of electrons instead of light originates from their substantially shorter wavelength. Visible light has limits in discerning objects below 200 nanometres, while electron wavelengths enable for visualising atomic-scale features.

Electron Microscope Parts
- Electron Gun:
- Generates electron beams using tungsten filament cathode
- Negative cap focuses electrons, anode accelerates beam
- Electromagnetic Lenses:
- Uses magnetic fields instead of glass
- Three types: condenser (focuses beam), objective (collects/magnifies), projector (further magnification)
- Aperture System:
- Pinholes (2-100 μm) filter unwanted electrons
- Two locations: below condenser lens and between objective/projector lenses
- Sample Holder:
- Platform with mechanical arm
- Uses thin carbon film on metal grid
- Vacuum System:
- Maintains vacuum in closed column
- Enables unimpeded electron travel
- Imaging System:
- Electromagnetic lenses refocus and enlarge
- Phosphorescent screen displays electron micrograph
- Camera records final image
Application of Electron Microscope
- Used in microbiology to investigate viruses, bacteria, flagella, and pili—providing insights into the ultrastructure of microorganisms.
- In cell biology, it helps research cellular organelles like mitochondria and ribosomes, assisting in understanding their form and function.
- Widely employed in crystallography to examine the arrangement of atoms within crystals and nanomaterials.
- Applied in forensic science—specifically for ballistic analysis, where the careful inspection of gunshot residue or bullet markings can benefit investigations.
- Geologists use these microscopes to analyse the composition and structure of minerals, rocks, and even gemstones, exposing minutiae that cannot be detected otherwise.
Limitations of Electron Microscope
- Costly system – Electron microscopes are notoriously pricey. Their installation, maintenance, and operation involve considerable cost, making them unattainable for many smaller labs or institutions.
- Lack of colour – The pictures generated by electron microscopes are generally black and white. While modern software may create artificial colour, the absence of genuine colour in the imaging process might restrict some study conclusions.
- Thin samples required — Transmission electron microscopes (TEM) requires very thin samples for imaging. The sample must be no thicker than a few nanometres, which can make preparation arduous and occasionally unfeasible for bigger or more complicated specimens.
- Vacuum environment needed — A vacuum system is important to operate electron microscopes. This need means that biological samples, for example, must often be fixed or dehydrated beforehand. The lack of a native environment could distort or harm sensitive structures.
- Resolution constraints – Although electron microscopes can attain very high resolution, there are still limitations in terms of what they can resolve. Some finer details may remain unclear, especially in regions where sample preparation effects the structure.
- Potential for harm – Electron beams, although strong, may potentially damage specimens. High-energy electrons can knock atoms off the sample, leading to structural alterations or even deterioration, especially in biological tissues.
Types of Electron Microscope
Electron microscopes are the devices that changed the world of microscopy. They expose humanity to the realities of life’s building blocks on previously inaccessible levels. Many types of electron microscopes exist, but the most common are the Transmission Electron Microscope (TEM) and the Scanning Electron Microscope (SEM), each with specific pros and cons.
- Transmission Electron Microscope (TEM)– The TEM takes images of thin slices via electron transmission. Therefore, the samples must be exceedingly thin—under 100 nm—which is about 200 less than a compound microscope requires. The electrons run through the sample, and after being scattered by the different internal aspects of the sample, exit out the opposite end to create an image on the fluorescent screen located on that end. The images are 2D and black and white, with magnification capabilities of 2X to 50,000X. The TEM is one of the more standard variants of an electron microscope used in laboratories and academia because it provides the highest resolution and therefore, the best images of internal structures. However, it does not allow for comparison with other samples since it cannot render 3D images like the other varieties.
- Scanning Electron Microscope (SEM) – However, the SEM is a variant of the TEM; instead of electrons being transmitted through the particle, the SEM focuses a high-energy beam of electrons across the surface of the particle—resulting in a 3D image that is magnified. Therefore, the SEM does not have as much magnification ability as the TEM; however, it possesses much more detail relative to surface characteristics and resolutions. For example, images derived from the SEM are clearer and more useful for interpretive purposes—one of the distinguishing factors between the two. In addition, the SEM has other detectors as well: back-scattered electron detectors and X-ray detectors, which make for more flexibility and considerations.
- Scanning Transmission Electron Microscope (STEM)– Ultimately, SEM is one of the best devices to assess the morphology of a sample. Scanning Transmission Electron Microscope (STEM) As opposed to SEM and TEM, the STEM is a combination of scanning and transmission processes to create images. Ultimately, one could envision it as the idealization of both worlds, although it’s not commonly available for standard use in electron microscopy. The benefit is that it can create both types of images. STEM, as we discern from the title, is the amalgamation of the finest qualities of SEM and TEM. STEM functions in a scanning mode like SEM but also in a transmission mode like TEM; thus, it can take clear, quality resolution images from both the outside and inside. Generally, STEM is used when the desired information concerns both surface and internal structural features.
- Reflection Electron Microscope (REM)– Where TEM and SEM differ yet have similarities, the REM pictures aligned with a given sample as well from reflected electrons. The ability to diffract while simultaneously imaging and analyzing the sample presents one more chance to image from a different view—specifically, the surface features come more into play, and resolution tends to be better. For instance, REM does not penetrate as deeply to a sample as a TEM; it needs a more precise surface image. Therefore, it’s better for a more specific study of materials.
- Scanning Tunneling Microscope (STM)– Where other electron microscopes either enter the sample (the transmission variety) or register surface detail (the scanning variety), the STM operates on something called electron tunneling. Rather than seeing the sample, it senses the surface of the sample—but at the atomic or molecular level. Therefore, it renders an incredibly detailed, three-dimensional rendering of the surface—resolution quality above and beyond accuracy. It’s useful for experiments where surface activity and atomic interactions are desired.
6. Scanning Electron Microscope (SEM)
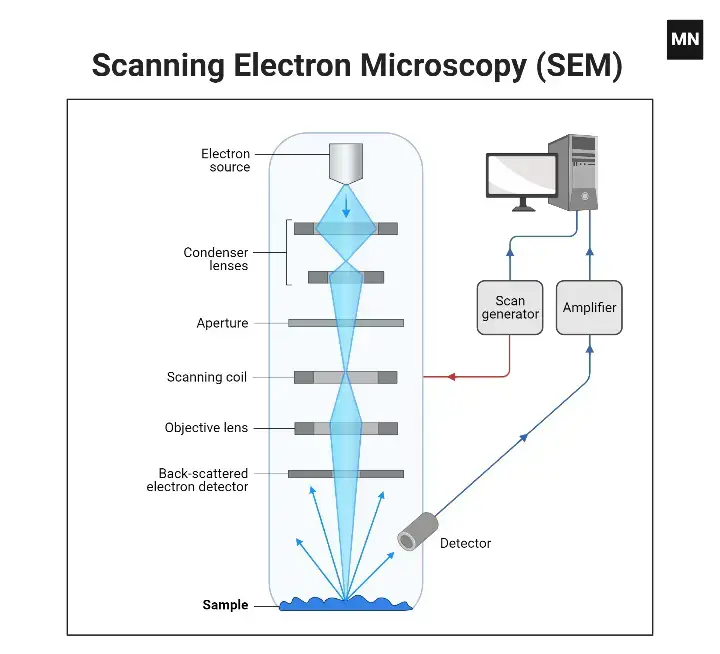
The SEM—one of the most remarkable types of microscopes—allows the trained user to view surfaces and sub-surfaces like never before, and by other means, doesn’t compare. The SEM produces quasi-three-dimensional images; for instance, it can assess the depth of surface features, measure surface roughness, and even the likelihood of determining surface composition far exceeds that of any optical microscope. But how does it operate? First, a high-energy electron beam is focused onto the specimen in a raster pattern. Upon collision with the specimen, electrons are backscattered, emitted, or diffracted. Yet, the SEM is unconcerned with the electrons that pass directly through the specimen like in a Transmission Electron Microscope (TEM). Instead, the SEM acquires information from electrons associated with the collisions occurring at the exact surface of interest. Thus, any such revelations—whether they are textural surface alterations or compositional interactions—merely enhance information gleaned from an ordinary SEM image.
However, the SEM—although it does not have as strong of magnification as the TEM counterpart—still produces high-resolution images. Thus, while its images may not be as small as atomic in scale, they’re still valuable because of the information about surface topology and the clearer view of surfaces. Surface topology is made possible by the detectors that gather this information for the SEM. In a way, they’re gathering information from thousands of different electrons, which assist in rendering not only a clear image but also a more detailed, contextualized one.
Back-Scattered Electron Detectors: These detect the backscattered electrons that escape from a sample surface. The number of backscattered electrons measured corresponds to the atomic number of a sample and thus gives compositional information.
Secondary Electron Detectors: These detect secondary electrons that escape the sample surface as a result of incident beam bombardment. This provides surface/topographical information.
X-ray Detectors: These detect information from emitted X-rays. If a sample gives off X-rays, specialized detectors can be used to assess elemental analysis of the sample for additional chemical information. The end result is a surface entity in 3D.
Where some microscopes might penetrate beyond the surface and explore the depths of what’s underneath, SEM is a surface-seeking vision and, many times, assumptions are made from that surface level. Yet, SEM is a critical function across many fields of study from material investigation to biological uses. It takes us closer to an understanding of something we would not normally be able to observe. SEM is used broadly in material sciences, biological uses, and semiconductor defect testing. A researcher might use SEM to determine if a material is defective, to examine the surface morphology of cells, or to determine the elemental composition of a material.
SEM is used when one wants to see what kind of surface morphology exists or when very high-powered resolution is required. Yet it’s not to say that the SEM doesn’t have shortcomings of its own—its relatively incapacity to achieve higher magnification than the TEM for one—but the depth of field and surface details make it an instrument too important to ignore. The SEM alters the playing field of what can be examined with both materials and biological specimens at such low levels, providing relatively high resolution as well as some of the most crucial compositional data.
Application of Scanning Electron Microscope (SEM)
- Used to study the surface area of microorganisms in detail.
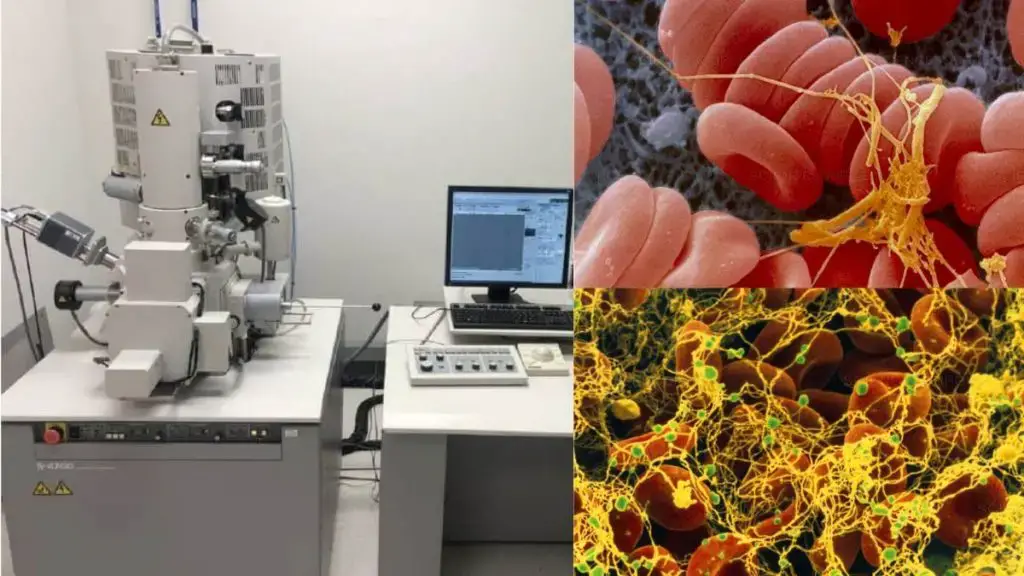
7. Transmission Electron Microscope (TEM)
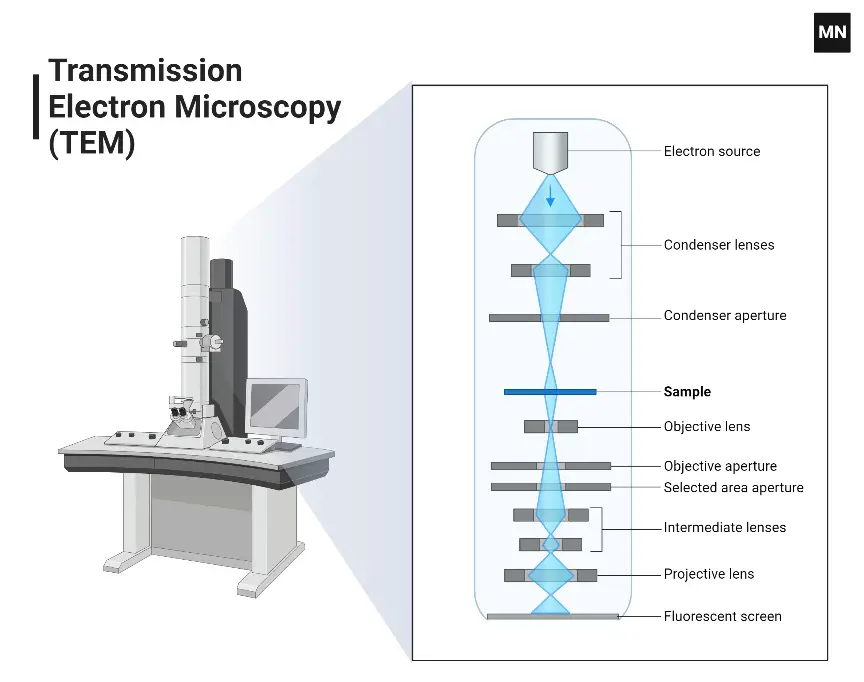
The Transmission Electron Microscope (TEM) stands as a specialized category within the broader family of electron microscopes. Its operational mechanism and imaging capabilities are distinct, offering researchers unparalleled insights into the nanoscopic realm. The following elucidates the fundamental principles and attributes of the TEM:
- Principle of Operation:
- The TEM operates by utilizing transmitted electrons to generate a magnified representation of a specimen. This transmission is facilitated by the interaction of the electron beam with the specimen’s constituents.
- Specimen Thickness:
- A quintessential aspect of TEM imaging is the requirement for extremely thin specimens. These specimens typically do not exceed a thickness of 100 nm. To put this into perspective, they are approximately 200 times thinner than those used in conventional compound microscopes.
- Electron Interaction and Imaging:
- The electron beam, focused onto the specimen by a condenser lens, interacts with the specimen’s components. Post-interaction, electrons are emitted from the specimen. These emitted electrons are subsequently channeled through a series of electromagnetic lenses, including both objective and ocular lenses.
- Upon reaching the fluorescent screen, these electrons stimulate the screen to produce an enlarged image. This image generation is a consequence of the electron-screen interaction, where the impinging electrons induce fluorescence, thereby visualizing the specimen’s details.
- Image Characteristics:
- The TEM is renowned for producing two-dimensional images. These images, while being monochromatic, are characterized by their exceptional resolution. The inherent high resolution of TEM images is a testament to the instrument’s capability to discern minute details, often at the molecular or atomic scale.
- In terms of magnification, the TEM offers a broad range, with capabilities extending from a modest 2X to a staggering 50,000X. This wide magnification spectrum ensures that researchers can tailor the imaging scale to their specific requirements.
- Prevalence in Scientific Research:
- Among electron microscopes, the TEM is notably the most ubiquitous. Its widespread adoption in scientific research can be attributed to its ability to provide detailed insights into structures that are beyond the resolution limits of optical microscopes.
Application of Transmission Electron Microscope (TEM)
- It used to study the internal structure of a specimen.

8. Confocal Microscopy
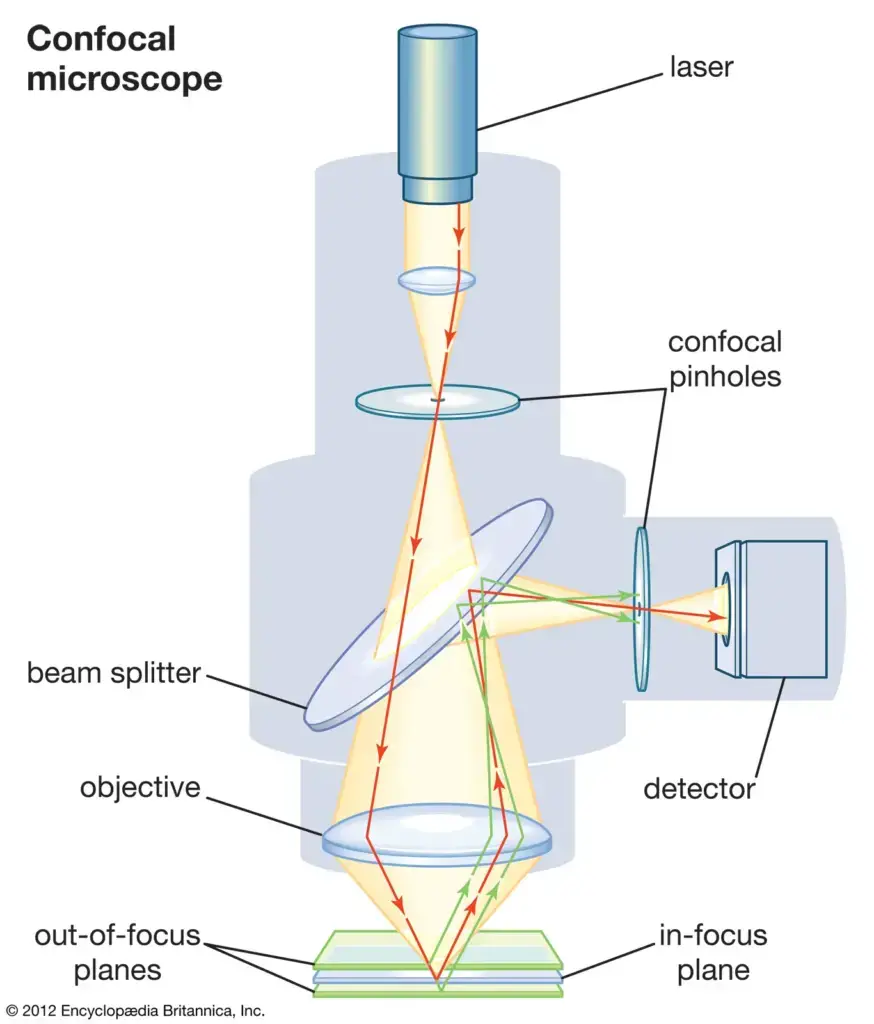
Confocal Microscopy, often referred to as Confocal Laser Scanning Microscopy (CLSM) or Laser Confocal Scanning Microscopy (LCSM), represents a pivotal advancement in the realm of optical microscopy.
- Principle of Operation:
- Central to the functionality of confocal microscopy is the strategic use of a spatial pinhole. Positioned at the confocal plane, this pinhole plays a crucial role in eliminating out-of-focus light, thereby enhancing both the optical resolution and contrast of the resultant micrograph. By selectively allowing only in-focus light to reach the detector, the confocal microscope ensures that the image is derived from a precise, thin plane within the specimen.
- Three-Dimensional Imaging:
- One of the standout features of confocal microscopy is its ability to perform optical sectioning. By sequentially capturing a series of two-dimensional images at varying depths within a specimen, the microscope facilitates the reconstruction of a comprehensive three-dimensional representation of the sample’s internal structures. This capability is invaluable for understanding the spatial relationships and intricate architectures within biological specimens, among others.
- Advantages:
- The inherent design and operational principles of confocal microscopy offer several advantages over traditional optical microscopy. Notably, the enhanced resolution and contrast, coupled with the ability to generate three-dimensional images, make it a preferred choice for detailed studies of cellular and subcellular structures.
Application
Confocal Microscopy is used extensively in the scientific and industrial communities and typical applications are in life sciences, semiconductor inspection, and materials science.
Other Types of Electron Microscopes
Electron microscopy, with its ability to provide unparalleled resolution, has evolved into various specialized forms, each tailored to specific applications and research needs. Beyond the commonly known Transmission Electron Microscope (TEM) and Scanning Electron Microscope (SEM), there exist other types of electron microscopes, each with unique functionalities:
- Reflection Electron Microscope (REM):
- The Reflection Electron Microscope operates by utilizing the beams of electrons that are reflected or scattered off the specimen’s surface to generate an image.
- This microscope integrates techniques from diffraction, imaging, and spectroscopy, offering a multifaceted approach to specimen analysis.
- Scanning Transmission Electron Microscope (STEM):
- The STEM is a hybrid instrument that amalgamates the technologies of both SEM and TEM.
- By combining the principles of these two microscopes, STEM can offer versatile imaging capabilities, capturing both surface and internal structural details of specimens.
- Scanning Tunneling Microscope (STM):
- Distinct from other electron microscopes, the STM operates based on the quantum mechanical phenomenon known as electron tunneling.
- Instead of penetrating the specimen, the STM scans the atomic and molecular structures present on the specimen’s surface. The resulting images are three-dimensional representations of these surface atoms and molecules.
- The STM’s ability to visualize atomic-scale details on surfaces has revolutionized the field of surface science, providing insights into atomic arrangements and molecular architectures.
9. Polarizing microscopes

A polarizing microscope is an enhancement over the basic optical microscope that someone would normally possess; yet, it differs from the light that it uses. A polarizing microscope uses polarized light instead. This means images are clearer, contrast is higher, and one can determine if a material is inherently birefringent. Polarized light only vibrates in one direction. Thus, when the opportunity to constrain such vibration is offered, a clearer picture occurs. Yet, the true power of the polarizing microscope lies in disclosing characteristics not able to be seen while hiding under the guise of natural light. It’s an unfortunately titled “petrographic microscope” due to how necessary it is for research within geology and mineralogy. The ability to study the optics of minerals through analyzing incredibly thin slices of rock allows it to diagnose materials in the first place.
Furthermore, in conjunction with birefringence (the splitting of light after penetrating certain substances), it’s even more justified to use. It’s within those minute details and light differentiations that the answers occur to assess what something actually is. It all begins with the illuminator lamp of the microscope, creating regular light which is then converted into polarized light. A polarizer takes light and causes it to vibrate on one plane and one plane only. Thus, the light that is emitted is plane-polarized.
Next, the plane-polarized light is shined through the anisotropic substance. Anisotropic materials are unique because they possess a differing index of refraction in differing aspects of light. Thus, when the light hits the material, it is split—this is called birefringence. It is converted into two waves—the ordinary wave and the extraordinary wave—which propagate in orthogonal directions (perpendicular to each other). This is how the patterns form. Then, these two waves move through the specimen. They take different routes based upon how they interact with the internal structure of the material.
They are then reunited—recombined—by the analyzer so the ocular can see both waves at one time and make the image one. And what is that image one? An overlay—sometimes quite beautiful—of the specimen relative to a polarized light field. A polarizing microscope is not a super high-powered microscope with a better magnification—it is something that allows people to see what bright field microscopes cannot. Such precision makes this device indispensable for geology and other scientific fields—materials science, forensic applications, and even biology—as it’s been shown that light and matter are close enough to reveal what any given material actually is.

Polarizing Microscope Parts
- Polarizer: The polarizer is located between the illuminator and the slide on the specimen stage. It is one of the more significant parts. Its function is simple, yet required. It exists to change the light emitted from the light source—non-polarized light—as it enters the plane of view to plane-polarized light so that subsequent interaction with the specimen enhances the visual properties of the sample, especially those related to inner composition.
- Analyzer: An additional important component to the orientation is the analyzer situated in the light path above the objectives. The specimen creates analyzed light. The purpose of the analyzer is to bring back together the two waves—ordinary and extraordinary—that have been split apart. The bringing back together is what is seen, and for casting rendering, this is required of the polarized light.
- Accessory Plates: These are the plates positioned anterior to the analyzer. For example, compensators and retardation plates affect the light incident on the specimen. They overlay certain visual effects over the technician’s perception of the optical path difference between two such waves. This is essential for focus and contrast. Thus, these plates operate as an essential component in ascertaining the relative qualities of the specimen.
- Specialized Stage: Most microscopes come with a non-adjustable stage, and other “fixed” stages are fixed in place permanently. However, the polarizing microscope has a circular stage that rotates 360°. This is important because it allows the user to rotate the specimen to the exact angle required. Such positioning is essential for determining the impact of polarized light at every angle.
Uses of Polarizing Microscope
- Mineralogy and Petrography
- Mineral identification through optical properties
- Rock composition analysis
- Crystal structure determination
- Materials Science
- Polymer orientation studies
- Liquid crystal analysis
- Stress distribution visualization
- Biology
- Pharmaceutical Industry
- Crystal form identification
- Drug formulation analysis
- Quality control of crystalline materials
- Forensics
- Fiber analysis
- Paint chip examination
- Crystal residue identification
Limitations of Polarizing Microscope
The polarizing microscope, while offering unique advantages in certain scientific domains, is not without its limitations. These constraints, inherent to its design and operational principles, are outlined below:
- Necessity for Anisotropic Specimens: One of the primary limitations of the polarizing microscope is its dependency on anisotropic specimens. Anisotropic materials possess different properties in different directions, which is essential for the microscope to produce the desired birefringence effect. Consequently, isotropic materials, which exhibit uniform properties in all directions, are not suitable for examination under a polarizing microscope. This requirement restricts the range of specimens that can be effectively studied using this technique.
- Limited Scope of Application: The specialized nature of the polarizing microscope means that its applications are somewhat niche. While it excels in specific fields like geology and certain biological studies, it may not be the instrument of choice for a broad spectrum of scientific investigations. Its utility is primarily confined to those areas where the study of birefringence or the properties of anisotropic materials is paramount.
2. Scanning Probe Microscope
A scanning probe microscope (SPM) is a type of microscope that uses a very sharp probe to scan the surface of a sample and create high-resolution images of its topography. SPMs are capable of producing images with a resolution of just a few nanometers, making them useful for studying the properties of small structures and surfaces.
There are several different types of SPMs, including atomic force microscopes (AFMs), scanning tunneling microscopes (STMs), and scanning near-field optical microscopes (SNOMs). These instruments work by using a probe to interact with the sample in various ways, such as by measuring the force between the probe and the sample, by tunneling electrons through the gap between the probe and the sample, or by measuring the near-field optical properties of the sample.
SPMs are widely used in a variety of fields, including materials science, biology, and nanotechnology, to study the surface properties of samples and to analyze the structure and composition of small structures. They are also used to fabricate and manipulate small structures, such as nanowires and nanotubes, and to study the properties of individual atoms and molecules.
Principle of Scanning Probe Microscope
The probe tip of the scanning probe microscope is affixed to the end of a cantilever. The tip is so sharp that it can glide precisely and precisely across the sample’s surface, scanning each and every atom. The tip is positioned close to the surface of the sample, causing the cantilever to deflect under the influence of forces. This distance of deflection is measured by the laser. After scanning, the final image is obtained on a computer.
Types of Scanning Probe Microscope
There are several different types of scanning probe microscopes (SPMs), each of which uses a different method to scan the surface of a sample and measure its properties. Some common types of SPMs include:
- Atomic force microscopes (AFMs): AFMs use a sharp, cantilevered probe to measure the forces between the probe and the sample as the probe is scanned across the surface. The probe is typically made of a very hard and stiff material, such as silicon, and is attached to a piezoelectric element that moves the probe in very small increments. AFMs can be used to measure the topography of a sample, as well as its electrical, mechanical, and magnetic properties.
- Scanning tunneling microscopes (STMs): STMs use a very sharp probe to measure the tunneling current between the probe and the sample as the probe is scanned across the surface. The probe is typically made of a metallic material, such as tungsten, and is mounted on a piezoelectric element that moves the probe in very small increments. STMs can be used to measure the topography of a sample and to study the electronic properties of surfaces.
- Scanning near-field optical microscopes (SNOMs): SNOMs use a probe with a very sharp tip, typically made of a metallic material such as gold, to measure the near-field optical properties of a sample. The probe is typically mounted on a piezoelectric element that moves the probe in very small increments. SNOMs can be used to study the optical properties of samples at the nanoscale, such as the absorption and scattering of light.
- Scanning capacitance microscopes (SCMs): SCMs use a probe with a sharp tip to measure the capacitance between the probe and the sample as the probe is scanned across the surface. The probe is typically made of a metallic material and is mounted on a piezoelectric element that moves the probe in very small increments. SCMs can be used to measure the topography of a sample and to study its electrical properties.
- Scanning Kelvin probe microscopes (SKPMs): SKPMs use a probe with a sharp tip to measure the electrostatic potential of a sample as the probe is scanned across the surface. The probe is typically made of a metallic material and is mounted on a piezoelectric element that moves the probe in very small increments. SKPMs can be used to measure the topography of a sample and to study its electrical properties.
Application of Scanning Probe Microscope
- It is used to examine various sample qualities, such as electrical properties.
- This microscope is used to analyse the magnetic property of the sample.
- With the use of this microscope, information on the sample can be transferred.
3. Dissecting Microscope (Stereo Microscope)
The Dissecting Microscope, commonly referred to as the Stereo Microscope, stands as a distinct category within the realm of light microscopy. Unlike traditional microscopes that transmit light through specimens, the dissecting microscope operates on the principle of reflected light microscopy.
- The core functionality of the dissecting microscope is based on the reflection of light off the surface of the specimen. This reflected light is then captured by the microscope’s optics to produce a magnified image.
- The unique design and optical arrangement of the dissecting microscope allow for the visualization of three-dimensional specimens. This is in contrast to many other light microscopes, which are designed primarily for the examination of thin, two-dimensional specimens mounted on slides.
- As the name suggests, the dissecting microscope is an invaluable tool in the realm of biological dissections. It provides researchers and students with a clear, magnified view of their specimen, facilitating precision in their work.
- Beyond dissections, the stereo microscope’s ability to visualize three-dimensional objects makes it a versatile instrument, suitable for a range of applications from examining mineral samples in geology to inspecting electronic components in technology.
Stereo Microscope Diagram
Principle of Stereo Microscope
The Dissecting Microscope, also known as the Stereo Microscope, operates on a distinct optical principle that sets it apart from traditional compound microscopes. At its core, the stereo microscope is designed to provide a three-dimensional perspective of the specimen under examination.
Optical Principle:
- The foundational principle of the stereo microscope lies in its dual optical pathways. Unlike the singular pathway in conventional microscopes, the stereo microscope employs two separate light paths, each corresponding to one of the ocular lenses. This bifurcated light path is what facilitates the generation of a stereoscopic or three-dimensional image.
- Each of the two ocular lenses focuses on a slightly different angle of the specimen, capturing two distinct images. When viewed together, these images converge to produce a depth perception, allowing the observer to discern the three-dimensional structure of the specimen.
Illumination System:
- The stereo microscope is equipped with both top and bottom illumination systems. The top light, often referred to as incident light, illuminates the surface of the specimen, making it particularly useful for dissecting or examining opaque objects.
- Conversely, the bottom light, or transmitted light, shines through the specimen, enhancing the visibility of transparent or semi-transparent samples.
Objective Lenses and Stage Design:
- The objective lenses of the stereo microscope are housed within a cylindrical cone, distinguishing it from the exposed objectives of compound microscopes. This design not only protects the lenses but also facilitates the microscope’s unique optical configuration.
- Accommodating the needs of various specimens, the stage of the stereo microscope is typically larger than that of its compound counterpart. A notable feature is the inclusion of a groove or restraining mechanism, ensuring the stability of the specimen during examination.
Applications of Stereo Microscope
The Dissecting Microscope, commonly referred to as the Stereo Microscope, is a versatile instrument with a wide range of applications across various scientific and technical domains. Its unique design, which offers a three-dimensional view of specimens, makes it particularly suited for tasks requiring intricate detail and depth perception. Here are some of the primary uses of the Dissecting or Stereo Microscope:
- Biological Procedures:
- Dissection and Microsurgery: The stereo microscope is an indispensable tool in biological laboratories, especially during dissection procedures. Its ability to provide a detailed, three-dimensional view allows for precise manipulation and examination of small biological specimens, tissues, and organs. Additionally, it is employed in microsurgical procedures where precision and depth perception are paramount.
- Archaeological and Geological Examination:
- The microscope is frequently used in the fields of archaeology and geology. Archaeologists utilize it to closely examine and analyze artifacts, ensuring that every detail, no matter how minute, is observed. Similarly, geologists employ the stereo microscope to study rock samples, minerals, and other geological specimens, gaining insights into their composition, structure, and history.
- Electronics and Precision Engineering:
- Nano Electric Appliance Manufacturing and Repair: The intricate nature of modern electronics, from microchips to circuit boards, necessitates tools that offer precision and clarity. The stereo microscope is invaluable in the manufacturing, inspection, and repair of such components. Its ability to magnify and provide a three-dimensional view ensures that even the smallest of components can be accurately placed, soldered, or repaired.
- Watchmaking: The world of horology, or watchmaking, requires a keen eye and a steady hand. The stereo microscope aids watchmakers in assembling the tiny components of a watch mechanism, ensuring its accurate and reliable function.
- Mobile Phone Repair: With the increasing complexity of mobile phones, repairing them requires a tool that can provide a clear view of their intricate internal components. The stereo microscope is often used to inspect and repair the minute parts of mobile phones, from circuitry to connectors.
Limitations of Dissecting Microscope or Stereo Microscope
The Dissecting Microscope, also known as the Stereo Microscope, is a valuable tool in various scientific and technical domains due to its ability to provide a three-dimensional view of specimens. However, like all scientific instruments, it comes with its own set of limitations. Here are some of the inherent constraints associated with the Dissecting or Stereo Microscope:
- Limited Applicability:
- While the stereo microscope is indispensable for specific tasks requiring depth perception and detailed observation of three-dimensional objects, its utility is restricted to these particular applications. It may not be the preferred choice for tasks that require high magnification or the examination of cellular and sub-cellular structures.
- Magnification Constraints:
- One of the primary limitations of the stereo microscope is its relatively low magnification capabilities. Unlike compound microscopes or electron microscopes, which can magnify specimens to a much higher degree, the stereo microscope’s magnification range is comparatively limited. This constraint makes it unsuitable for observing ultra-fine details or structures at the cellular or molecular level.
- Economic Considerations:
- The stereo microscope, especially high-quality models with advanced features, can be a significant investment. The cost associated with acquiring, maintaining, and upgrading these microscopes might be prohibitive for some institutions, researchers, or hobbyists, particularly when their usage might be infrequent or specialized.
4. Inverted Microscopes

The Inverted Microscope, as the name suggests, is a unique variation of the conventional light microscope, characterized by an inverted design where the positions of key components are reversed. This specialized microscope is tailored to cater to specific observational needs, offering distinct advantages in certain research and clinical applications.
- Design and Configuration: The primary distinction of the Inverted Microscope lies in its structural configuration. Unlike the traditional upright microscope, where the light source and condenser are positioned below the specimen and the objective lenses are above, the Inverted Microscope flips this arrangement. The objective lenses and turret are situated beneath the stage, while the illuminator and condenser are positioned above it. This inversion means that observers must direct their gaze upwards to visualize the specimen.
- Operational Principle: Fundamentally, the Inverted Microscope operates on the same principles as its upright counterpart. It harnesses the properties of light to magnify and elucidate the intricate details of specimens. However, the inverted design facilitates specific observational scenarios, particularly when examining specimens in larger containers or when observing living cells in culture dishes.
- Instrumentation: While the core components of the Inverted Microscope mirror those of standard microscopes, their arrangement is what sets it apart. The light source, typically located at the base in traditional microscopes, is positioned at the top in the inverted design. Conversely, the turret and objective lenses, which are usually above the stage in upright microscopes, are located below the stage in the Inverted Microscope. This design allows for more direct access to the specimen, especially when using larger or unconventional containers.
- Digital Enhancements: With the advent of technology, many modern Inverted Microscopes are equipped with digital cameras. These cameras enable researchers and clinicians to capture high-resolution images or videos of their specimens, facilitating detailed analysis, documentation, and sharing of findings.
Uses of Inverted Microscope
The Inverted Microscope, characterized by its unique design with objective lenses positioned below the stage, has found a myriad of applications in various scientific domains. Its specialized configuration facilitates the observation of specimens that might be challenging to examine under conventional upright microscopes. Here, we delve into some of the primary uses of the Inverted Microscope in scientific research.
- Metallurgical Analysis: In the realm of metallurgy, the Inverted Microscope is an indispensable tool. It is employed to scrutinize the microstructure of metals and minerals. By illuminating these specimens from above and observing them from below, researchers can gain insights into the grain structure, phase distribution, and other microstructural features of metallic samples. This information is pivotal in understanding the properties and performance of metals, aiding in the development of new alloys and improving existing ones.
- Cytological Studies: The field of cytology, which focuses on the study of cells, also benefits from the capabilities of the Inverted Microscope. Researchers utilize this instrument to closely monitor the process of cell division, allowing them to observe the intricate stages of mitosis and meiosis in real-time. The microscope’s design is particularly advantageous when studying cells in culture dishes, as it provides unobstructed access to the specimen.
- Microbiological Investigations: In microbiology, the Inverted Microscope proves to be a valuable asset, especially in the identification and study of specific microorganisms. For instance, it is employed in the detection of Mycobacterium tuberculosis, the causative agent of tuberculosis. Additionally, it facilitates the observation of the growth and behavior of certain pathogens, such as Phytophthora spp., in culture. By providing a clear view of these microorganisms, the Inverted Microscope aids in understanding their morphology, life cycle, and interactions with their environment.
Limitations of Inverted Microscope
The Inverted Microscope, while a valuable tool in various scientific applications, is not without its limitations. These constraints, inherent to its design and functionality, can sometimes pose challenges to researchers and impede certain microscopic investigations. Here, we elucidate some of the primary limitations associated with the Inverted Microscope.
- Limited Availability and High Cost: One of the primary constraints of the Inverted Microscope is its limited availability in many research settings. Unlike conventional upright microscopes, which are more ubiquitous in laboratories, the Inverted Microscope is less commonly found. Moreover, the specialized design and features of the Inverted Microscope often come with a higher price tag, making it a significant investment for many institutions. This cost factor can be a deterrent for some research facilities, especially those operating with constrained budgets.
- Specimen Thickness Impediments: The design of the Inverted Microscope, which necessitates the placement of specimens above the objective lenses, introduces challenges when working with slides or petri dishes of varying thicknesses. The thickness of the slide or dish can influence the quality of imaging. Specifically, if the slide or dish is too thick, it can introduce aberrations or distortions in the captured images. This limitation necessitates careful consideration of the specimen holder’s dimensions to ensure optimal imaging results.
5. Metallurgical Microscopes

The realm of microscopy has been enriched by the advent of the Metallurgical Microscope, a specialized instrument tailored for the meticulous examination of metals and their intricate structures. This microscope stands distinct from its counterparts, primarily due to its reliance on reflected light as opposed to transmitted light, a feature that enables the detailed observation of opaque specimens.
- Fundamental Design and Instrumentation: At its core, the Metallurgical Microscope shares similarities with the conventional optical microscope in terms of its fundamental design and components. However, the distinguishing feature lies in its illumination system. Instead of allowing light to pass through the specimen, as is the case with transmitted light microscopy, the Metallurgical Microscope employs a method where light is reflected off the surface of the specimen. This approach is crucial, given the opaque nature of the materials it is designed to study.
- Applications in Metallography: The primary domain of the Metallurgical Microscope is metallography, the science of analyzing the physical structure and components of metals and alloys. Through this microscope, scientists and researchers can delve into the microstructures of metals, gaining insights into grain boundaries, inclusions, phases, and other metallurgical phenomena. Such detailed examinations are pivotal in understanding the properties, behavior, and performance of metals under various conditions.
- Scope of Study: While the primary focus of the Metallurgical Microscope is metals and alloys, its utility extends beyond this. The microscope is adept at examining other opaque materials such as ceramics, certain minerals, and rocks. This versatility makes it an invaluable tool in diverse fields, from materials science to geology.
In summation, the Metallurgical Microscope is a testament to the advancements in microscopy, offering a specialized lens through which the intricate world of metals and other opaque materials can be explored with precision and clarity.

6. The Digital Microscope

In the ever-evolving realm of microscopy, the Digital Microscope stands as a testament to the seamless integration of traditional optical techniques with modern digital technology. This innovative instrument has revolutionized the way microscopic observations are conducted, offering enhanced visualization, analysis, and sharing capabilities.
- Core Design and Features: Distinct from traditional microscopes, the Digital Microscope eschews the conventional ocular lens. Instead, it incorporates a digital camera, which captures and relays images directly to a screen for digital display. This design is underpinned by a sophisticated computerized system that amalgamates the microscope, camera, display monitor, and specialized software. The absence of eyepieces is compensated by the camera, which serves as the primary medium for capturing microscopic images.
- Image Processing and Analysis: One of the standout features of the Digital Microscope is its advanced image processing capabilities. The integrated software facilitates a myriad of functions, from basic magnification and focusing to intricate analyses such as size measurement and color correction. Furthermore, the software enables users to capture both still images and videos, offering flexibility in documentation and analysis. The ability to manipulate images, adjust color contrast, control brightness, and even produce graphic recordings underscores the versatility of this instrument.
- Three-Dimensional Visualization: Beyond two-dimensional imaging, certain advanced Digital Microscopes are equipped to project three-dimensional images, providing a more comprehensive view of specimens and enhancing depth perception.
- Applications Across Domains: The Digital Microscope’s prowess extends across a multitude of fields. Its precision and advanced imaging capabilities make it an invaluable tool in microbiology, pathology, and cytology. Surgeons leverage its capabilities during intricate procedures, while researchers in nanotechnology benefit from its detailed imaging. Furthermore, its role in forensics and various industrial sectors underscores its versatility. The instrument’s ability to process and display images digitally positions it as a preferred choice over traditional compound microscopes in many scenarios.
In conclusion, the Digital Microscope represents a paradigm shift in microscopy, bridging the gap between optical techniques and digital technology. Its multifaceted capabilities, ranging from advanced image processing to diverse applications, underscore its significance in contemporary scientific and industrial endeavors.
7. USB Microscope
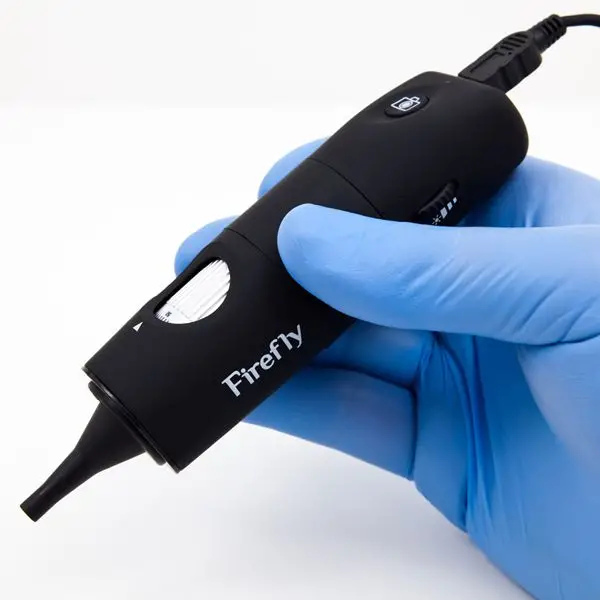
The realm of microscopy has witnessed numerous advancements, and the USB Microscope stands as a testament to the integration of digital technology with traditional microscopic techniques. This instrument, characterized by its simplicity and portability, offers a unique approach to microscopic observation, leveraging the capabilities of modern computers.
- Design and Instrumentation: The USB Microscope is fundamentally a low-power digital microscope, distinguished by its ability to interface directly with a computer via a USB port. At its core, it employs a digital camera equipped with a high-power macro lens, capable of achieving magnifications up to 200X. The design is streamlined, comprising primarily of a light source, typically an LED, the aforementioned digital camera, and the integral USB port for connectivity.
- Operational Mechanism: The operational principle of the USB Microscope is straightforward. The LED illuminates the specimen, and the light reflected from the specimen is captured by the digital camera. This image is then transmitted digitally to the connected computer, where it is displayed on the screen. The digital nature of the microscope allows for the captured images or videos to be saved, edited, or processed using specialized computer software.
- Applications and Utility: The USB Microscope finds its niche in a variety of applications. It is adept at examining objects such as insects, coins, gemstones, jewelry, intricate scripts, and crystalline structures. Beyond these, it also finds utility in medical examinations, particularly in endoscopic and ENT procedures, offering a detailed view of internal structures.
- Advantages and Limitations: One of the standout features of the USB Microscope is its affordability and portability. Its compact design and direct computer interface make it a convenient tool for both professionals and hobbyists. However, it’s worth noting that while it offers several advantages, it does come with its set of limitations, primarily its relatively low magnification when compared to more advanced microscopes.
In summation, the USB Microscope represents a fusion of traditional microscopy with digital technology, offering a unique and accessible means of microscopic exploration. While it may not replace high-end microscopes in specialized applications, its versatility and ease of use make it a valuable tool for a wide range of users.
8. The Pocket Microscope

The realm of microscopy, traditionally dominated by large, stationary instruments, has seen the emergence of compact, portable devices designed for on-the-go observations. The Pocket Microscope stands as a testament to this evolution, offering a blend of convenience and functionality.
- Design and Components: The Pocket Microscope is characterized by its diminutive size, designed specifically for easy portability. Despite its compact nature, it houses essential components integral to its operation. At its core, it features an eyepiece for direct observation. Illumination is provided by an LED, powered by an onboard battery. To aid in the reflection and direction of light onto the specimen, a mirror is incorporated into the design. Furthermore, a stage is present to securely hold and position the sample for observation. Some advanced variants of the Pocket Microscope also come equipped with a digital camera, allowing users to capture and store images of their observations.
- Applications and Utility: Given its design and magnification capabilities, the Pocket Microscope is best suited for general observational purposes. It excels in examining objects that are millimeter-scale in size. This makes it particularly useful for inspecting jewelry, gemstones, intricate components in watchmaking, electronic circuits, insects, and other similar objects.
- Limitations: While the Pocket Microscope offers the advantage of portability and ease of use, it does come with inherent limitations. Its magnification capability, typically capped at around 100X, restricts its ability to observe truly microscopic entities, such as microorganisms. As such, it cannot replace the functionality of more advanced, high-magnification microscopes in specialized applications.
In conclusion, the Pocket Microscope represents a significant stride in making microscopy accessible and convenient for a broader audience. While it may not cater to the needs of advanced scientific research, its versatility and portability make it an invaluable tool for a myriad of general observational purposes.
9. The Acoustic Microscope
Acoustic microscopy stands as a distinctive branch within the realm of microscopy, diverging from traditional methods by harnessing the power of high-frequency ultrasound waves, rather than light or electrons, to generate detailed images of specimens. This innovative approach offers a unique perspective, particularly in visualizing the internal structures of objects.
Fundamental Principle
Central to the operation of an acoustic microscope is the principle of ultrasonic wave propagation and reflection. A transducer, acting as the heart of the system, plays a dual role: it transforms electrical signals into ultrasonic waves and vice versa. When these sound waves, typically ranging from 5 MHz to 400 MHz, are directed towards a specimen, they interact with it, leading to both reflection and transmission of waves. The nature of these interactions, whether it’s the amplitude, phase, or time of return of the reflected sound, is meticulously processed to generate an image.
Imaging Modalities
Two primary imaging techniques are employed in acoustic microscopy:
- Pulse-Echo Mode: Utilizing a singular transducer, this method focuses on analyzing the reflected sound waves or echoes.
- Transmission Mode: This technique employs a pair of transducers, with one capturing transmitted sound waves and the other detecting reflected waves. In both methodologies, meticulous scanning of the specimen, pixel by pixel, results in the formation of a two-dimensional image.
Instrumentation
Beyond the pivotal transducer, which acts both as a loudspeaker and microphone, the acoustic microscope relies on computer software to interpret the transformed electrical signals into coherent images. This system’s prowess lies in its ability to delve into the internal intricacies of specimens, capturing even submicron-level features without compromising the integrity of the sample.
Applications
The acoustic microscope’s capabilities have found resonance across various sectors:
- Electronics: Instrumental in quality assurance and production, especially in imaging circuit boards and identifying microchip defects.
- Industrial: Employed across the chemical, pharmaceutical, and ceramic industries for product quality assessments.
- Biomedical: Offers insights into cellular structures, motility, and elasticity, proving invaluable for cytological and histological examinations.
Limitations
Despite its innovative approach and diverse applications, acoustic microscopy is not without its challenges:
- Sound propagation necessitates a medium.
- Sound manipulation poses difficulties.
- The image processing time can be protracted.
- The associated costs can be prohibitive.
In summation, acoustic microscopy, with its unique sound-based imaging technique, offers a fresh perspective in the microscopy domain. While it presents certain challenges, its potential applications across industries underscore its significance in modern microscopy.
10. X-Ray Microscopes
The X-Ray Microscope, distinct from traditional optical microscopes, employs X-rays to illuminate specimens, yielding magnified images. This technique’s inherent advantage lies in X-rays’ ability to penetrate most materials, eliminating the need for specialized sample preparation or staining. Typically, X-rays ranging from 100 to 1,000 eV, equivalent to a wavelength of approximately 1 nm, are utilized. However, contemporary X-Ray microscopes have evolved to use wavelengths between 0.1 to 10 nm.
Underlying Principle
The foundational principle of X-Ray microscopy is rooted in the interaction between matter and X-rays. When molecules encounter X-rays, ionization ensues. This interaction excites the electrons of the atom to a higher energy state. As these electrons revert to their ground state, they release energy in the form of X-rays, which possess a specific energy and wavelength characteristic of the element in question.
Upon illuminating the specimen with high-energy X-rays, various interactions occur: some X-rays scatter, others penetrate the sample, and a fraction is absorbed. The ionized molecules subsequently emit X-rays, which are captured either photographically or through a detection system, culminating in the formation of an image.
Instrumentation
- X-ray Tube: This component, reminiscent of cathode ray tubes, is responsible for X-ray generation. It houses a vacuum system with a metallic anode and a tungsten filament cathode. When subjected to high voltage, the cathode emits electron beams that, upon striking the anode, release X-ray photons.
- Collimator: This device focuses the generated X-rays into a parallel beam, a process termed collimation. It comprises two sets of metallic plates, allowing only a narrow beam to pass, resulting in a collimated X-ray beam.
- Monochromator: This optical apparatus polarizes an unpolarized X-ray beam. It can either be a filter or a crystal, such as quartz or NaCl.
- Detection System: This encompasses various detectors:
- Imaging Detectors: Utilize photographic plates or X-ray films.
- Scintillator Detectors: Comprise a scintillator and a photomultiplier tube. Upon X-ray interaction, scintillators emit visible light, which is then converted into electric pulses.
- Semiconductor Detectors: These employ silicon and lithium plates. X-ray interaction produces an electron and a hole, which are detected and processed to form images.
- Other detectors, such as the Geiger-Muller tube and Proportional counter, are occasionally employed.
Applications
X-Ray microscopes have found utility across various domains:
- Crystal and polymer identification and characterization.
- Quality control in metallurgy, petroleum, ceramics, and glass manufacturing sectors.
- Geological studies encompassing rock, mineral, and soil composition analysis.
Limitations
Despite its potential, X-Ray microscopy is not devoid of challenges:
- Imaging can be intricate.
- The process is costly, time-intensive, and necessitates intricate instrumentation.
11. Near-infrared microscopes
- Near-infrared microscopes (NIR microscopes) are a type of microscope that uses near-infrared light to produce images of samples. Near-infrared light has a wavelength that is slightly longer than visible light and is therefore not visible to the human eye. NIR microscopes are often used to study the structure and function of tissues and cells, as well as the distribution and interaction of molecules within these systems.
- NIR microscopes work by illuminating the sample with near-infrared light and detecting the light that is scattered or absorbed by the sample. The resulting image can provide information about the distribution and concentration of various molecules within the sample. NIR microscopes are particularly useful for studying biological samples, as many biological molecules absorb near-infrared light and can therefore be visualized using this technique.
- NIR microscopes are commonly used in fields such as biology, materials science, and medicine to study the structure and function of tissues and cells, as well as the distribution and interaction of molecules within these systems. They are also used to study the properties of materials and to analyze the chemical composition of samples.
12. Raman microscopes
- Raman microscopes are a type of microscope that uses lasers to excite vibrations in the bonds of molecules, which can then be detected and used to identify the molecules present in a sample. Raman microscopes work by shining a laser beam onto the sample and detecting the light that is scattered by the sample. The intensity and wavelength of the scattered light are unique for each type of molecule, and can be used to identify the specific molecules present in the sample.
- Raman microscopes are commonly used in fields such as materials science, biology, and chemistry to study the chemical composition of samples and to analyze the properties of materials. They are particularly useful for studying samples that are difficult to analyze using other techniques, such as samples that are too small or too complex to be studied with other types of microscopes.
- Raman microscopes are also useful for studying samples that are too weak or too unstable to be studied using other techniques, such as samples that are sensitive to heat or other forms of damage. They are also used to study the structure and function of tissues and cells, and to analyze the distribution and interaction of molecules within these systems.
Differences between Simple Microscope and Compound Microscope
Simple microscopes and compound microscopes are both types of optical microscopes that use lenses and light to magnify and analyze samples. However, there are several key differences between these two types of microscopes:
- Magnification: Simple microscopes have a lower magnification compared to compound microscopes. Simple microscopes typically have a magnification range of 2x to 10x, while compound microscopes can have a magnification range of up to 1000x or more.
- Objective lenses: Simple microscopes have a single objective lens, while compound microscopes have multiple objective lenses that can be used to achieve different levels of magnification.
- Illumination: Simple microscopes typically use natural light or an external light source, such as a lamp, to illuminate the sample. Compound microscopes often have an internal light source, such as a bulb or a LED, that is used to illuminate the sample.
- Eyepieces: Simple microscopes have a single eyepiece, while compound microscopes have two eyepieces that are used to provide a three-dimensional view of the sample.
- Image quality: Simple microscopes often produce lower quality images compared to compound microscopes due to their lower magnification and less sophisticated optical system. Compound microscopes are capable of producing higher resolution images with greater detail.
- Applications: Simple microscopes are often used for basic observations and are suitable for examining larger, more transparent samples. Compound microscopes are more powerful and are commonly used for more advanced studies and for examining smaller, more opaque samples.
Differences between Phase-contrast Microscope and Fluorescent Microscope
Phase-contrast microscopes and fluorescent microscopes are two different types of microscopes that are used to observe and study samples at a very small scale.
Phase-contrast microscopes use a special type of objective lens that is designed to enhance the contrast of samples that are transparent or have low refractive indices. This makes it easier to see and study the details of these types of samples. Phase-contrast microscopes do not require the use of dyes or stains, as the contrast is created through the interaction of light with the sample itself.
Fluorescent microscopes, on the other hand, use special dyes or stains that are applied to the sample and emit light when exposed to a specific wavelength of light. This allows researchers to visualize specific structures or molecules within the sample. Fluorescent microscopes require the use of a special light source, called a fluorescence lamp, to excite the fluorophores in the sample and emit light.
There are a few key differences between phase-contrast microscopes and fluorescent microscopes:
- Image contrast: Phase-contrast microscopes enhance the contrast of transparent or low-index samples through the interaction of light with the sample, while fluorescent microscopes use dyes or stains to create contrast.
- Sample preparation: Phase-contrast microscopes do not require the use of dyes or stains, while fluorescent microscopes require the application of special dyes or stains to the sample.
- Light source: Phase-contrast microscopes use standard light sources, while fluorescent microscopes require a special light source, called a fluorescence lamp, to excite the fluorophores in the sample.
- Image resolution: Fluorescent microscopes typically have higher resolution than phase-contrast microscopes, as the use of dyes or stains can help to enhance the contrast and clarity of the sample.
Overall, phase-contrast microscopes and fluorescent microscopes are both useful tools for studying samples at a very small scale, but they are used in different ways and for different purposes.
Differences between Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM) and Confocal Microscopy
Scanning electron microscopes (SEM), transmission electron microscopes (TEM), and confocal microscopes are all types of microscopes that are used to observe and study samples at a very small scale. However, they use different techniques and have different capabilities.
- Scanning electron microscopes (SEM): SEMs use a focused beam of electrons to scan the surface of a sample and create an image of its surface features. They are particularly useful for studying the surface features of samples, such as the topography and composition of materials. SEMs can achieve high resolution and can be used to study samples at a variety of scales, from micrometers to nanometers.
- Transmission electron microscopes (TEM): TEMs use a beam of electrons that is transmitted through a sample to create an image of the internal structure of the sample. They are particularly useful for studying the microstructure and internal features of samples, such as the arrangement of atoms and molecules. TEMs can achieve very high resolution and are often used to study samples at the nanoscale.
- Confocal microscopes: Confocal microscopes use a laser to scan a sample and create an image by focusing the laser onto a small, specific area of the sample. They are particularly useful for studying samples with a high degree of depth or thickness, as they can create detailed images of the sample’s structure at different depths. Confocal microscopes are often used to study biological samples, such as cells and tissues.
There are a few key differences between these types of microscopes:
- Image contrast: SEMs create images based on the interaction of electrons with the sample surface, while TEMs create images based on the interaction of electrons with the internal structure of the sample. Confocal microscopes create images based on the reflection of a laser off the sample.
- Sample preparation: SEMs and TEMs typically require samples to be prepared in a specific way, such as by coating them with a thin layer of metal or by embedding them in a resin. Confocal microscopes typically do not require any special sample preparation.
- Resolution: TEMs can achieve the highest resolution of the three types of microscopes, followed by SEMs and confocal microscopes.
- Scale: SEMs and TEMs can be used to study samples at a variety of scales, from micrometers to nanometers, while confocal microscopes are typically used to study samples at the micrometer scale.
Differences Between Bright-field Microscope and Dark-Field Microscope
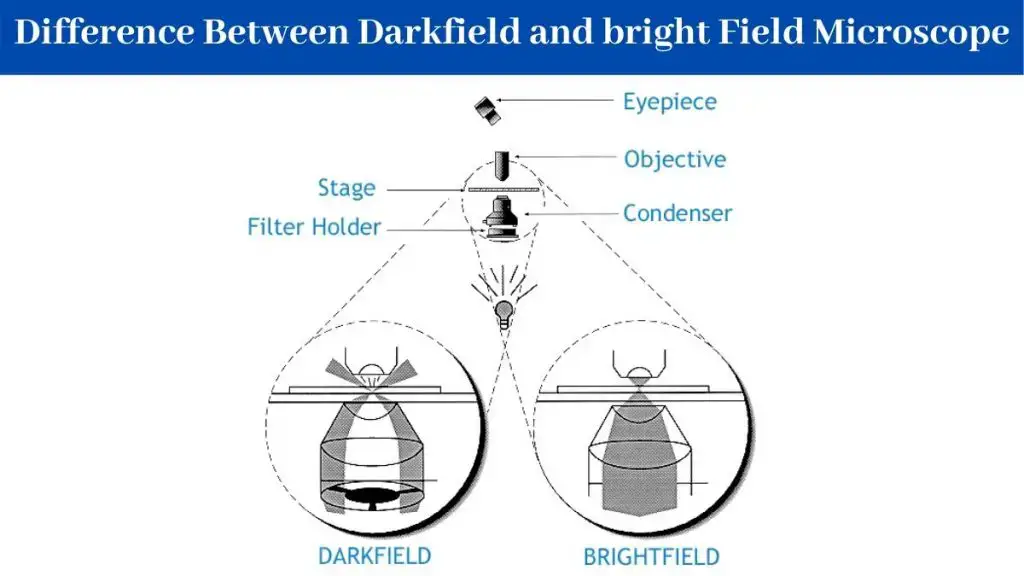
There are several key differences between bright-field microscopy and dark-field microscopy:
- Illumination: In bright-field microscopy, the sample is illuminated from below, and the light passes through the transparent or semi-transparent sample and is focused by the objective lens onto the eyepiece. In dark-field microscopy, the light source is located below the stage and the sample is illuminated from the side at a shallow angle. The light that is scattered by the sample is focused by the objective lens onto the eyepiece.
- Image contrast: In bright-field microscopy, the sample appears bright against a dark background. In dark-field microscopy, the sample appears bright against a dark background.
- Sample types: Bright-field microscopy is best suited for studying opaque or semi-transparent samples, while dark-field microscopy is best suited for studying transparent or semi-transparent samples.
- Details and structures: Bright-field microscopy is not as effective at observing fine details and structures as dark-field microscopy.
- Color: Bright-field microscopy can produce a true-color image of the sample, while dark-field microscopy cannot.
- Field of view: Bright-field microscopy has a wider field of view compared to dark-field microscopy.
- Operator skill: Dark-field microscopy requires a highly skilled operator to properly align the microscope and adjust the light source.
Differences between Scanning Probe Microscope and Stereo Microscope
Scanning probe microscopes (SPMs) and stereo microscopes are two different types of microscopes that are used to observe and study samples at a small scale. They have different capabilities and are used for different purposes.
- Scanning probe microscopes (SPMs): SPMs use a very small probe, often just a few nanometers in size, to scan the surface of a sample and create an image of its surface features. SPMs are particularly useful for studying the surface features of samples at the nanoscale, such as the topography and composition of materials. There are several different types of SPMs, including atomic force microscopes (AFMs), scanning tunneling microscopes (STMs), and scanning near-field optical microscopes (SNOMs).
- Stereo microscopes: Stereo microscopes, also known as dissecting microscopes or low-power microscopes, use two objective lenses to create a three-dimensional image of the sample. They are particularly useful for studying samples that have a high degree of depth or thickness, such as biological samples or small mechanical parts. Stereo microscopes are often used for dissection, inspection, and assembly tasks.
There are a few key differences between these types of microscopes:
- Image contrast: SPMs create images based on the interaction of the probe with the sample surface, while stereo microscopes create images based on the refraction of light through the sample.
- Sample preparation: SPMs typically do not require any special sample preparation, while stereo microscopes may require samples to be mounted on a slide or in a special holder.
- Resolution: SPMs can achieve very high resolution, often at the atomic scale, while stereo microscopes typically have lower resolution.
- Scale: SPMs are typically used to study samples at the nanoscale, while stereo microscopes are typically used to study samples at the micrometer scale.
Overall, SPMs and stereo microscopes are both useful tools for studying samples at a small scale, but they are used for different purposes and have different capabilities.
Differences between Electron Microscope and Compound Microscope
Electron microscopes and compound microscopes are two different types of microscopes that are used to observe and study samples at a small scale. They have different capabilities and are used for different purposes.
- Electron microscopes: Electron microscopes use a beam of electrons to create an image of the sample. There are two main types of electron microscopes: transmission electron microscopes (TEMs) and scanning electron microscopes (SEMs). TEMs create an image of the internal structure of the sample by transmitting a beam of electrons through the sample, while SEMs create an image of the surface features of the sample by scanning the surface with a beam of electrons. Electron microscopes can achieve very high resolution and are often used to study samples at the nanoscale.
- Compound microscopes: Compound microscopes use light to create an image of the sample. They have two main components: an objective lens that is located close to the sample and a eyepiece lens that is located near the observer’s eye. Compound microscopes are often used to study biological samples, such as cells and tissues, as well as small mechanical parts. They can achieve high resolution, but are typically limited to studying samples at the micrometer scale.
There are a few key differences between these types of microscopes:
- Image contrast: Electron microscopes create images based on the interaction of electrons with the sample, while compound microscopes create images based on the refraction of light through the sample.
- Sample preparation: Electron microscopes often require samples to be prepared in a specific way, such as by coating them with a thin layer of metal or by embedding them in a resin. Compound microscopes typically do not require any special sample preparation.
- Resolution: Electron microscopes can achieve very high resolution, often at the atomic scale, while compound microscopes typically have lower resolution.
- Scale: Electron microscopes are typically used to study samples at the nanoscale, while compound microscopes are typically used to study samples at the micrometer scale.
Overall, electron microscopes and compound microscopes are both useful tools for studying samples at a small scale, but they are used for different purposes and have different capabilities.
Types of Microscopes with their applications ppt
Types of Microscopes with their applications Video
FAQ
Q1. What are two types of electron microscopes?
There are two main types of electron microscopes: transmission electron microscopes (TEMs) and scanning electron microscopes (SEMs).
- Transmission electron microscopes (TEMs): TEMs use a beam of electrons to create an image of a thin sample by transmitting the electrons through the sample and recording the resulting image on a fluorescent screen or a digital detector. TEMs are capable of producing high-resolution images of samples with a resolution of just a few nanometers, making them useful for studying the internal structure of materials and small biological specimens.
- Scanning electron microscopes (SEMs): SEMs use a beam of electrons to create an image of the surface of a sample by scanning the surface with the beam and recording the resulting image on a fluorescent screen or a digital detector. SEMs are capable of producing high-resolution images of the surface features of a sample, with a resolution of just a few nanometers. They are commonly used to study the surface features of materials and biological specimens.
Q2. What are the types of microscopes?
There are several different types of microscopes, each of which uses a different method to magnify and analyze samples. Some common types of microscopes include:
- Optical microscopes: These use lenses and light to magnify and analyze samples, and include microscopes such as compound microscopes, stereo microscopes, and polarizing microscopes.
- Electron microscopes: These use a beam of electrons to create an image of a sample and include transmission electron microscopes (TEMs) and scanning electron microscopes (SEMs).
- Scanning probe microscopes (SPMs): These use a very sharp probe to scan the surface of a sample and create high-resolution images of its topography. Examples include atomic force microscopes (AFMs), scanning tunneling microscopes (STMs), and scanning near-field optical microscopes (SNOMs).
- Confocal microscopes: These use lasers and specialized optics to produce high-resolution images of samples by selectively illuminating small areas of the sample at a time.
- Fluorescence microscopes: These use fluorescent dyes to highlight specific structures or molecules in a sample and are commonly used in biological research.
- X-ray microscopes: These use X-rays to produce images of the internal structure of samples and are often used to study materials and biological specimens.
- Near-infrared microscopes: These use near-infrared light to produce images of samples and are often used to study the structure and function of tissues and cells.
- Raman microscopes: These use lasers to excite vibrations in the bonds of molecules, which can then be detected and used to identify the molecules present in a sample.
Q3. which of the following types of microscopes reveals the surface features of small molecules?
Scanning electron microscopes (SEMs) are capable of revealing the surface features of small molecules. SEMs use a beam of electrons to create an image of the surface of a sample by scanning the surface with the beam and recording the resulting image on a fluorescent screen or a digital detector. SEMs are capable of producing high-resolution images of the surface features of a sample, with a resolution of just a few nanometers. They are commonly used to study the surface features of materials and biological specimens, including small molecules.
Q4. Which of the following types of microscopes can magnify more than 2000x?
Transmission electron microscopes (TEMs) are capable of magnifying samples by more than 2000x. TEMs use a beam of electrons to create an image of a thin sample by transmitting the electrons through the sample and recording the resulting image on a fluorescent screen or a digital detector. TEMs are capable of producing high-resolution images of samples with a resolution of just a few nanometers, and are capable of magnifying samples by more than 2000x. They are commonly used to study the internal structure of materials and small biological specimens.
Q5. Explain why the several different types of microscopes are all necessary?
There are several different types of microscopes because each type of microscope is designed to analyze samples in a specific way and is best suited to certain types of applications. Different types of microscopes use different techniques to magnify and analyze samples, and each type has its own unique capabilities and limitations.
For example, optical microscopes, such as compound microscopes and stereo microscopes, use lenses and light to magnify and analyze samples, and are best suited for studying samples that are transparent or moderately opaque. These types of microscopes are commonly used in fields such as biology, materials science, and engineering to study the surface features of samples and to perform tasks such as dissection, assembly, and inspection.
On the other hand, electron microscopes, such as transmission electron microscopes (TEMs) and scanning electron microscopes (SEMs), use a beam of electrons to create an image of a sample and are best suited for studying samples that are too small or too dense to be studied with an optical microscope. These types of microscopes are commonly used in fields such as materials science, biology, and nanotechnology to study the internal structure of materials and small biological specimens.
Scanning probe microscopes (SPMs), such as atomic force microscopes (AFMs) and scanning tunneling microscopes (STMs), use a very sharp probe to scan the surface of a sample and create high-resolution images of its topography. SPMs are best suited for studying the surface properties of samples at the nanoscale, such as the electrical, mechanical, and magnetic properties of materials.
In summary, the different types of microscopes are all necessary because they allow researchers to study samples in different ways and to analyze a wide range of properties, including the surface and internal structure of materials, the electrical and optical properties of samples, and the topography of surfaces at the nanoscale.
Q6. what types of microscopes are used to study viruses?
There are several different types of microscopes that can be used to study viruses, depending on the properties of the virus and the specific type of analysis that is being performed. Some common types of microscopes that are used to study viruses include:
- Optical microscopes: These use lenses and light to magnify and analyze samples, and can be used to study the size and shape of viruses, as well as their surface features. Examples of optical microscopes that are commonly used to study viruses include compound microscopes, stereo microscopes, and polarizing microscopes.
- Electron microscopes: These use a beam of electrons to create an image of a sample and can be used to study the internal structure of viruses and other small biological specimens. Examples of electron microscopes that are commonly used to study viruses include transmission electron microscopes (TEMs) and scanning electron microscopes (SEMs).
- Scanning probe microscopes (SPMs): These use a very sharp probe to scan the surface of a sample and create high-resolution images of its topography. Examples of SPMs that are commonly used to study viruses include atomic force microscopes (AFMs) and scanning tunneling microscopes (STMs).
- Confocal microscopes: These use lasers and specialized optics to produce high-resolution images of samples by selectively illuminating small areas of the sample at a time. Confocal microscopes can be used to study the structure and distribution of viruses in tissues and cells.
- Fluorescence microscopes: These use fluorescent dyes to highlight specific structures or molecules in a sample and can be used to study the distribution and interaction of viruses with host cells.
- X-ray microscopes: These use X-rays to produce images of the internal structure of samples and can be used to study the internal structure of viruses and other small biological specimens.
- Near-infrared microscopes: These use near-infrared light to produce images of samples and can be used to study the distribution and interaction of viruses with host cells.
- Raman microscopes: These use lasers to excite vibrations in the bonds of molecules, which can then be detected and used to identify the molecules present in a sample. Raman microscopes can be used to study the chemical composition of viruses and other small biological specimens.
Quiz
Which type of microscope uses a beam of electrons to produce an image?
a) Light microscope
b) Transmission Electron Microscope (TEM)
c) Polarizing microscope
d) Acoustic microscope
Which microscope is primarily used for observing live cells in culture?
a) Inverted Microscope
b) Scanning Electron Microscope (SEM)
c) Polarizing microscope
d) X-Ray Microscope
Which microscope uses polarized light to enhance image quality and contrast?
a) Acoustic microscope
b) Digital microscope
c) Polarizing microscope
d) USB microscope
Which microscope is designed for portability and can easily fit in a pocket?
a) Transmission Electron Microscope (TEM)
b) Digital microscope
c) USB microscope
d) Pocket microscope
Which microscope uses sound waves for imaging?
a) Acoustic microscope
b) X-Ray Microscope
c) Polarizing microscope
d) Digital microscope
Which microscope is used to study the internal structures of metals and alloys?
a) Metallurgical Microscope
b) Inverted Microscope
c) Scanning Electron Microscope (SEM)
d) Transmission Electron Microscope (TEM)
Which microscope lacks an ocular lens and displays images digitally?
a) Polarizing microscope
b) Digital microscope
c) Acoustic microscope
d) X-Ray Microscope
Which microscope is used for observing objects at the atomic and molecular level on specimen surfaces?
a) Scanning Tunneling Microscope (STM)
b) Scanning Electron Microscope (SEM)
c) Transmission Electron Microscope (TEM)
d) Acoustic microscope
Which microscope uses X-rays to illuminate samples?
a) Digital microscope
b) Acoustic microscope
c) X-Ray Microscope
d) Polarizing microscope
Which microscope is connected to a computer via a USB port and is used for observing larger objects like insects, coins, and jewelry?
a) Digital microscope
b) USB microscope
c) Polarizing microscope
d) Acoustic microscope
FAQ
What is a microscope?
A microscope is a scientific instrument used to magnify and visualize objects or specimens that are too small to be seen with the naked eye.
What are the main types of microscopes?
The main types of microscopes include optical microscopes (such as compound and stereo microscopes), electron microscopes (such as TEM and SEM), and other specialized microscopes like polarizing microscopes and atomic force microscopes.
How does an optical microscope work?
Optical microscopes use visible light to illuminate specimens. They contain lenses that magnify and focus the light to produce an enlarged image of the specimen.
What is the difference between a compound microscope and a stereo microscope?
Compound microscopes are used for high magnification and are suitable for viewing thin specimens on slides. Stereo microscopes provide lower magnification but offer a 3D view of larger, solid objects.
How do electron microscopes differ from optical microscopes?
Electron microscopes use a beam of electrons instead of visible light to magnify specimens. They offer much higher resolution and can visualize objects at the nanoscale.
What is the purpose of a polarizing microscope?
A polarizing microscope uses polarized light to enhance image quality and contrast, making it particularly useful for studying minerals, geological samples, and materials with birefringent properties.
What are some common applications of electron microscopes?
Electron microscopes are used in various fields, including biology, materials science, and nanotechnology, to study the structure and composition of materials at the microscopic and nanoscopic levels.
Can you explain the working principle of a scanning electron microscope (SEM)?
SEM uses a focused beam of electrons to scan the surface of a specimen. By detecting emitted and backscattered electrons, it creates a 3D image with high resolution.
What are the limitations of optical microscopes compared to electron microscopes?
Optical microscopes have limited resolution and cannot visualize objects smaller than the wavelength of visible light. Electron microscopes can achieve much higher resolution.
Are there portable microscopes available for field use?
Yes, there are portable microscopes like pocket microscopes and USB microscopes designed for fieldwork and on-the-go observations. They are often used for tasks like inspecting jewelry, insects, or small objects.
Reference
- http://www.biologyreference.com/La-Ma/Light-Microscopy.html
- https://en.wikipedia.org/wiki/Scanning_electron_microscope
- https://opticsmag.com/types-of-microscopes/
- https://www.microscopeworld.com/p-3658-types-of-microscopes.aspx
- https://microscopeinternational.com/types-of-microscopes/