Contents
Definition of Tollens’ Test
It is a type of biochemical test which is used to distinguish reducing sugars from non-reducing sugars. This biochemical test is also known as the silver mirror test based on the end product of this test. This test was also used to differentiate between aldehydes and ketones through routine qualitative organic analysis.
Tollens’ Reagent
- In tollens test, a chemical reagent is used for the detection of an aldehyde functional group, an aromatic aldehyde functional group, or an alpha hydroxy ketone functional group, this chemical reagent is known as Tollen Reagent.
- This reagent was first discovered by German chemist Bernhard Tollens, that’s why it’s termed as Tollens Reagent.
- The Tollens reagent is a mixture of silver nitrate (AgNO3) and Ammonia (NH3).
Preparation of Tollens’ Reagent
This reagent has a short shelf life, that’s why it is not commercially sold. Therefore, the reagent is directly prepared in the laboratory by following this method;
- Add a few drops of dilute NaOH into an aqueous solution of silver nitrate. The aqueous solution of silver nitrate contains silver aqua complexes wherein water acts as a ligand.
- The hydroxide ions now turn these silver aquo complexes into silver oxides.
- This silver oxide (given by Ag2O) precipitates as a brown solid from this solution.
- The brown precipitate of silver oxide generated in step 1 is now dissolved with aqueous ammonia.
- The solution formed from this addition of aqueous ammonia contains the [Ag(NH3)2]+ complex. This complex is the primary component of Tollens Reagent.
Principle of Tollens’ Test
Tollen’s reagent is the mixture of silver nitrate (AgNO3) and liquid ammonia (NH3), which results in the formation of a complex. The aqueous solution of silver nitrate helps in the formation of a silver aqua complex while the water functions as a ligand.
Now, the hydroxide ions convert the aqua complexes into silver oxides (Ag2O) which appear as a brown precipitate. This brown precipitate dissolved by aqueous ammonia resulting in the formation of the [Ag(NH3)2]+ complex. This complex is considered as the primary component of the Tollen’s reagent and is a strong oxidizing agent.
Now, the aldehyde groups present in the sugars are oxidized by this complex and form a carboxylic acid. At the same time, the silver ions present in the reagent are reduced to metallic silver, as a result of this reduction leads to the formation of a silver mirror on the bottom and sides of the test tube. However, an α-hydroxy ketone gives a positive Tollen’s test as the Tollen’s reagent oxidizes the α-hydroxy ketone into an aldehyde.
Reactions of Tollens’ Test
2AgNO3 + 2NaOH → Ag2O (brown ppt) + 2NaNO3 + H2O
Ag2O (brown ppt) + 4NH3 + 2NaNO3 + H2O → 2[Ag(NH3)2]NO3 + 2NaOH
Glucose + 2[Ag(NH3)2]NO3 + H2O → 2 Ag(silver mirror) + 4 NH3 + Gluconic acid + 2 H+
Requirements for Tollens’ Test
- Reagent: Tollen’s reagent, Test sample
- Materials required: Test tubes, Test tube stand, Pipette.
- Equipment: Water bath.
Procedure of Tollens’ Test
- Add 1 ml of the test sample in one test tube and 1 ml of distilled water in another as blank.
- Add 2 ml of Tollen’s reagent to both the test tubes.
- Keep both the test tubes in a water bath for 1 min.
- Observe the formation of color and note it down.
Result of Tollens’ Test
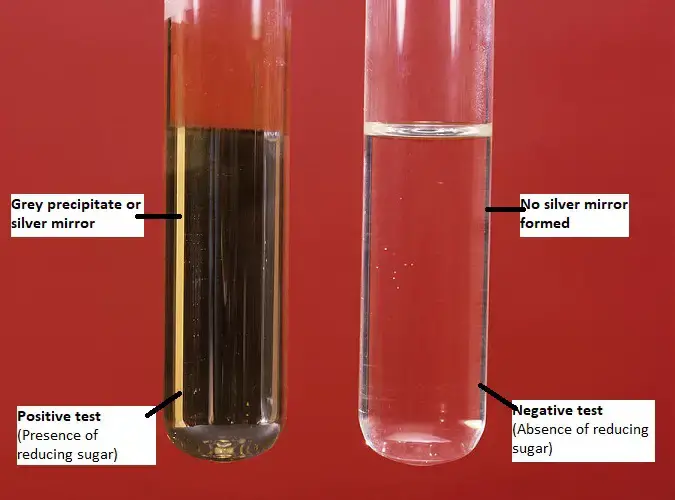
- Positive result: If a dark grey precipitate or silver mirror forms on the bottom and sides of the test tube. It indicates the given sample contains reducing sugars/ aldoses.
- Negative result: If no precipitate is formed. It indicates the given sample doesn’t contain any reducing sugars/ aldoses/ α-hydroxy ketoses.
Tollens’ test Application
- In chemical laboratories, this test is used for qualitative organic analysis, which helps to distinguishes aldehydes from ketones.
- It also helps in the differentiation of reducing sugars from non-reducing sugars.
Limitations of Tollens’ test
- Some carbohydrates lack the aldehyde group, they might give a false positive result on Tollen’s test because of the isomerization of such sugars under alkaline conditions.
- The reagent must be prepared immediately prior to its use as an explosive substrate can be formed if it is allowed to dry.
- Cleaned the glasswares with concentrated KOH (Potassium Hydroxide) because if they are not perfectly clean, a dark precipitate instead of grey may appear in the solution.