When it comes to molecular cloning and genetic engineering, vectors play a crucial role. These vectors act as carriers, transporting foreign genetic material into host cells, allowing for replication and expression. In this article, we will explore the different types of vectors, such as plasmids, viral vectors, cosmids, and artificial chromosomes, and their various applications.
One type of vector commonly used is the expression vector, designed specifically for expressing target genes. Transcription vectors, on the other hand, are utilized for transcription purposes without translation. Additionally, shuttle vectors offer the advantage of being able to express in multiple host organisms. The processes of transforming vectors into bacteria, transfecting eukaryotic cells, and introducing viral vectors are known as transformation, transfection, and transduction, respectively.
Among the different vectors, artificial chromosomes stand out for their ability to carry larger gene sequences. Notable examples include bacterial artificial chromosomes (BACs), human artificial chromosomes (HACs), and yeast artificial chromosomes (YACs). These vectors offer unique capabilities and have contributed significantly to the field of genetic research and engineering.
In molecular cloning, vectors serve as DNA molecules that transport foreign genetic material into cells. Recombinant DNA refers to a vector containing foreign DNA that can replicate and express within the host organism. Two types of vectors commonly used in cloning are yeast artificial chromosomes (YACs) and bacterial artificial chromosomes (BACs). While both serve similar purposes, there are distinct differences between them.
YAC vectors are artificially constructed systems that utilize a specific region of yeast chromosomes to insert large segments of genetic material into yeast cells. On the other hand, BAC vectors utilize a specific region of E. coli chromosomes to insert large segments of DNA into E. coli cells. The key difference lies in the molecular components for replication contained within each vector type.
YAC vectors carry the necessary components for replication inside yeast, while BAC vectors contain molecular components for replication inside bacteria. Furthermore, YAC vectors can accommodate inserts ranging from 100 to 1000 kb in size, whereas BAC vectors can carry inserts ranging from 100 to 200 kb.
In summary, understanding the different types of vectors and their applications is essential for molecular cloning and genetic engineering. Whether it’s plasmids, viral vectors, cosmids, or artificial chromosomes like YACs and BACs, each vector offers unique advantages and serves as a valuable tool for manipulating genetic material.
Contents
What are YAC Vectors?
Yeast Artificial Chromosomes (YACs) are artificially constructed vectors that are used for the replication and insertion of foreign DNA sequences. These vectors are designed to mimic the structure and function of natural yeast chromosomes, allowing for the cloning and manipulation of large DNA fragments.
The construction of YAC vectors involves the incorporation of specific components taken from the yeast Saccharomyces cerevisiae. These components include the autonomously replicating system (ARS), which provides the origin of replication for autonomous replication within the yeast cell. The centromere (CEN) ensures proper segregation of the chromosome into daughter cells during reproduction. The telomeres (TEL) are the protective structures at the ends of the chromosome. These components are essential for the stability and replication of the YAC vector.
To make YAC vectors effective cloning vectors, they must also include selective marker genes and restriction sites. Selectable marker genes such as TRP1 and URA3 are included to identify and select the transformed yeast cells. The YAC vector may also include a bacterial selectable marker gene. Restriction sites like BamHI and EcoRI are incorporated to allow for the linearization and insertion of the DNA fragment of interest.
YAC vectors can carry large segments of foreign DNA, ranging from 1000 kb to 2000 kb, and transfer them into yeast cells. However, the transformation efficiency of YAC vectors is generally low, meaning the process of introducing the YAC vector into yeast cells is not very efficient.
The YAC vector system is valuable for various applications, particularly in the preparation of genomic libraries. By inserting large DNA fragments into YAC vectors, researchers can create libraries of genetic material for further analysis and manipulation. The YAC vectors provide a means to replicate and propagate these large DNA fragments within yeast cells.
In summary, YAC vectors are artificial chromosome-like structures that can carry and replicate large DNA fragments within yeast cells. They are constructed using elements from natural yeast chromosomes, including centromeres, telomeres, autonomously replicating sequences, and selectable marker genes. YAC vectors offer a useful tool for cloning and studying large DNA fragments and are widely used in genomic research.
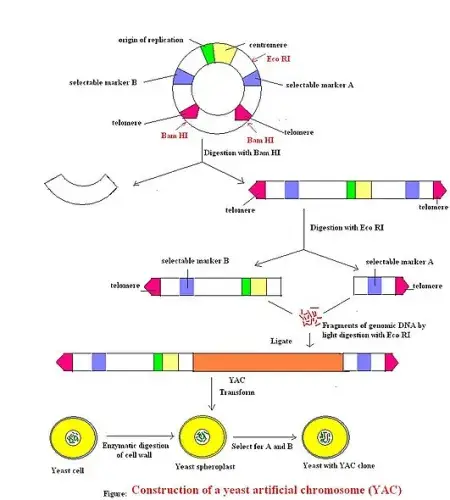
Construction of YAC Vectors
The construction of YAC vectors involves several steps to prepare the vector for the insertion of a DNA fragment of interest. Here is a general overview of the construction process:
- Linearization of a circular DNA plasmid: The first step is to linearize a circular DNA plasmid, typically through restriction digestion with an enzyme such as BamHI. This cleaves the plasmid at specific recognition sites, creating linear DNA fragments.
- Restriction digestion with EcoRI: The linearized DNA fragments are further digested using another restriction enzyme, such as EcoRI. This step helps create compatible ends on the vector and the DNA fragment that will be inserted.
- Ligation with the DNA fragment of interest: The DNA fragment of interest, typically ranging from 100 to 1000 kb in size, is obtained from another source. This DNA fragment is then ligated to the linearized YAC vector using DNA ligase. The ligase enzyme joins the compatible ends of the vector and the DNA fragment, creating a circular YAC vector with the inserted DNA fragment.
- Transformation into host yeast cells: The ligated YAC vector is then transformed into yeast cells, usually Saccharomyces cerevisiae. This process allows the YAC vector to enter the yeast cells, where it can replicate and propagate.
It’s important to note that YAC vectors can vary in their specific design and components. Examples of YAC vectors include YAC72 and YAC46, which have been widely used in research. These vectors have the capacity to accommodate DNA fragments ranging from 100 to 1000 kb in size.
Overall, the construction of YAC vectors involves the linearization of a circular plasmid, digestion with restriction enzymes, ligation with the DNA fragment of interest, and subsequent transformation into yeast cells. These steps enable the creation of YAC vectors capable of carrying and replicating large DNA fragments.
What are BAC Vectors?
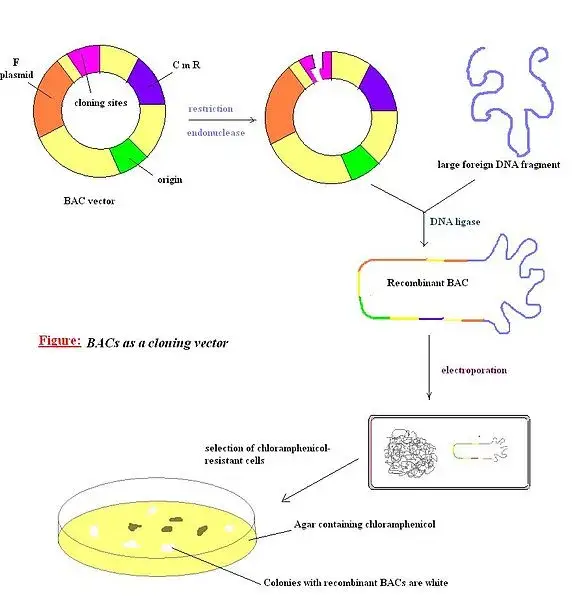
Bacterial Artificial Chromosomes (BACs) are DNA constructs commonly used for transformation and cloning in bacterial cells, particularly Escherichia coli (E. coli). These artificial chromosomes are based on functional fertility plasmids or F-plasmids, which serve as the backbone for the vector construction.
BAC vectors are circular and supercoiled DNA constructs that are designed for molecular cloning purposes. They can accommodate DNA fragments of relatively large sizes, typically up to 300 kb. Compared to YAC vectors, BAC vectors generally have smaller DNA insert sizes.
The structure of a BAC vector is based on the fertility plasmid/F-plasmid of E. coli. It includes specific regions and gene components to ensure efficient replication and transcription of the inserted DNA fragments. Some common components found in BAC vectors are:
- repE: This gene component mediates the assembly of the replication complex, allowing for the replication of the BAC vector within the bacterial host.
- parA and parB: These genes are responsible for the partitioning of genes during replication, ensuring proper distribution of genetic material to daughter cells.
- Selectable marker: BAC vectors typically contain a selectable marker, such as an antibiotic resistance gene or LacZ, which enables the identification and selection of transformed bacterial cells.
- T7 and SP6: These promoters are included in BAC vectors to facilitate the transcription of the inserted genes of interest.
- OriS: This element provides a unidirectional origin of replication for the BAC vector.
BAC vectors have been extensively used in various applications. During the Human Genome Project, BAC vectors played a crucial role in sequencing and mapping the human genome. They are also valuable tools in the study and modeling of genetic diseases such as Down syndrome and neurological disorders like Alzheimer’s disease. Additionally, BAC vectors are utilized for cloning the genomes of large DNA and RNA viruses.
It’s worth mentioning that there is a similar vector to BAC called PAC (P1-derived Artificial Chromosome), which is based on the P1 bacteriophage and used for cloning large DNA fragments.
In summary, BAC vectors are artificial chromosomes constructed from fertility plasmids, allowing for the transformation and cloning of large DNA fragments in bacterial cells. They have specific gene components for replication, partitioning, selectable markers, and transcription. BAC vectors have been instrumental in various genomic and genetic studies, offering stability, ease of construction, and the ability to clone large DNA fragments.
Difference between YAC and BAC Vectors
| Feature | YAC Vector | BAC Vector |
|---|---|---|
| Description | DNA constructs for cloning DNA in yeasts | DNA constructs for cloning DNA in bacteria |
| Gene Insert | 100-1000 kbp | 150-350 kbp |
| Chimerism | Often shows chimerism | No chimerism seen |
| Shape of the Construct | Linear | Circular |
| PTM | Utilizes yeast machinery with PTM mechanisms | Bacterial machinery lacks PTM mechanism |
| Number of Vectors | One vector per yeast cell | 1-2 vectors per bacterial cell |
| Stability | Less stable | More stable |
| Disadvantages | Gene deletion, recombination, inversion | None |
| Advantages | N/A | Quicker and more efficient generation, better chromosome coverage map |
| Applications | Gene mapping, chromosome walking, modeling genetic diseases, Human Genome Project | Large-scale genomic sequencing, cloning, functional genomics studies |
| Insert Length | Megabase-sized genomic inserts (1000 kb – 2000 kb) | Inserts of 200-300 kb or less |
| Construction | Difficult to purify intact, requires high concentration | Easy to purify intact, easily constructed |
| Gender | Cloning large fragments of genomic DNA into yeast | Cloning large genomic fragments into E. coli |
| Modifications | Yeast recombination can generate deletions and rearrangements in YACs | E. coli recombination prevents unwanted rearrangements in BACs |
| Maintenance | Laborious process, requires transfer into E. coli | Direct modification in E. coli, no need for DNA transfer |
| Definition | Constructed from yeast chromosome sequences | Based on functional fertility plasmid (F-plasmid) |
| Host | Transformed into yeast cells | Transformed into bacteria |
| Based on | Specific regions of the yeast chromosome | F-plasmid |
| Configuration | Linear | Circular |
| Copy Number | Single YAC vector per yeast cell | 1-2 BAC vectors per bacterial cell |
| Cloning Capacity | High cloning capacity (up to 1000 kb insert size) | Less cloning capacity (up to 200 kb insert size) |
Difference between YAC vs BAC Vectors
YAC Vector:
- Description: DNA constructs for cloning DNA in yeasts.
- Gene Insert: 100-1000 kbp.
- Chimerism: Often shows chimerism.
- Shape of the Construct: Linear.
- PTM: Utilizes yeast machinery with PTM mechanisms.
- Number of Vectors: One vector per yeast cell.
- Stability: Less stable.
- Disadvantages: Gene deletion, recombination, inversion.
- Advantages: N/A.
- Applications: Gene mapping, chromosome walking, modeling genetic diseases, Human Genome Project.
- Insert Length: Megabase-sized genomic inserts (1000 kb – 2000 kb).
- Construction: Difficult to purify intact, requires high concentration.
- Gender: Cloning large fragments of genomic DNA into yeast.
- Modifications: Yeast recombination can generate deletions and rearrangements in YACs.
- Maintenance: Laborious process, requires transfer into E. coli.
- Definition: Constructed from yeast chromosome sequences.
- Host: Transformed into yeast cells.
- Based on: Specific regions of the yeast chromosome.
- Configuration: Linear.
- Copy Number: Single YAC vector per yeast cell.
- Cloning Capacity: High cloning capacity (up to 1000 kb insert size).
BAC Vector:
- Description: DNA constructs for cloning DNA in bacteria.
- Gene Insert: 150-350 kbp.
- Chimerism: No chimerism seen.
- Shape of the Construct: Circular.
- PTM: Bacterial machinery lacks PTM mechanism.
- Number of Vectors: 1-2 vectors per bacterial cell.
- Stability: More stable.
- Disadvantages: None.
- Advantages: Quicker and more efficient generation, better chromosome coverage map.
- Applications: Large-scale genomic sequencing, cloning, functional genomics studies.
- Insert Length: Inserts of 200-300 kb or less.
- Construction: Easy to purify intact, easily constructed.
- Gender: Cloning large genomic fragments into E. coli.
- Modifications: E. coli recombination prevents unwanted rearrangements in BACs.
- Maintenance: Direct modification in E. coli, no need for DNA transfer.
- Definition: Based on functional fertility plasmid (F-plasmid).
- Host: Transformed into bacteria.
- Based on: F-plasmid.
- Configuration: Circular.
- Copy Number: 1-2 BAC vectors per bacterial cell.
- Cloning Capacity: Less cloning capacity (up to 200 kb insert size).
FAQ
Which is better, YAC or BAC?
Determining whether YAC or BAC vectors are better depends on the specific research goals and requirements. Each vector has its advantages and disadvantages. YAC vectors have a higher cloning capacity and can accommodate larger gene inserts, making them suitable for cloning large fragments of genomic DNA. However, they tend to be less stable and can experience chimerism and rearrangements. On the other hand, BAC vectors are more stable, have a lower cloning capacity, and are easier to manipulate in bacterial systems. BAC vectors are commonly used for large-scale genomic sequencing and functional genomics studies. Ultimately, the choice between YAC and BAC vectors depends on the specific experimental needs and the characteristics of the target DNA.
Why are shuttle vectors called so?
Shuttle vectors are called so because they can “shuttle” or transfer between different host organisms, typically between bacteria and yeast or bacteria and mammalian cells. These vectors contain specific elements required for replication and maintenance in both host organisms, allowing researchers to clone and manipulate DNA in one host and then transfer it to another host for further analysis or expression.
What is MCS in cloning?
MCS stands for Multiple Cloning Site. It refers to a region within a vector that contains multiple unique restriction enzyme recognition sites. These sites enable the insertion of DNA fragments into the vector at specific locations, allowing researchers to clone and manipulate genes of interest.
Is BAC a shuttle vector?
BAC vectors are not typically considered shuttle vectors in the conventional sense. While they can be transferred between different bacterial strains, they are primarily used for cloning and studying DNA in bacteria. Shuttle vectors are more commonly associated with vectors that can shuttle between different species or organisms, such as between bacteria and yeast or bacteria and mammalian cells.
What are the characteristics of a shuttle vector?
Characteristics of a shuttle vector typically include:
Ability to replicate and be maintained in multiple host organisms.
Presence of origin(s) of replication for each host organism.
Compatibility with the replication machinery of each host organism.
Selection markers for each host organism.
Cloning sites or MCS for easy insertion of DNA fragments.
Elements for efficient gene expression or functional studies in each host organism.
Proper regulatory elements for gene expression control in each host organism.
Stability and compatibility with the genetic background of both host organisms.
Ease of manipulation and purification for reliable and efficient cloning experiments.
These characteristics enable researchers to clone, manipulate, and express genes of interest in different host organisms using a single shuttle vector.
Related Posts
- Difference Between Homologous Chromosomes and Sister Chromatids
- Difference between Monocarpic and Polycarpic Plants
- Differences Between Poisonous and Non-poisonous Snakes
- Differences Between Sensitivity, Specificity, False positive, False negative
- Anabolism vs Catabolism – Differences Between Anabolism and Catabolism