Contents
Overview of Kinyoun stain
Kinyoun method also known as Kinyoun stain (cold method), was first developed by Joseph J. Kinyoun. It is a differential staining method sue to differentiate between acid-fast and non-acid-fast bacteria. This staining method is used for the detection of acid-fast microorganisms such as Mycobacterium and Nocardia and the apicomplexan genus Cryptosporidium.
Their waxy mycolic acid-containing cell wall allows them to be stained with Acid-Fast better than a Gram-Stain. This special ability help them to prevent the decolorization by acid-alcohol, that’s why they are called acid-fast.
It completed in different steps such as the application of a primary stain (basic fuchsin), a decolorizer (acid-alcohol), and a counterstain (methylene blue). The Kinyoun method does not require any physical mordant (heating) unlike the Ziehl-Neelsen stain.
In the Ziehl-Neelsen stain method, we used heat as a physical mordant and phenol as a chemical mordant. While in case of Kinyoun method, instead of heat we uses high concentration of carbol fuschin.
Purpose of Kinyoun stain
The purpose of Kinyoun’s Acid Fast stain is to detection of acid-fast Mycobacterium spp. and other parasites i.e Cryptosporidium and Isopora spp.
Kinyoun stain Principle
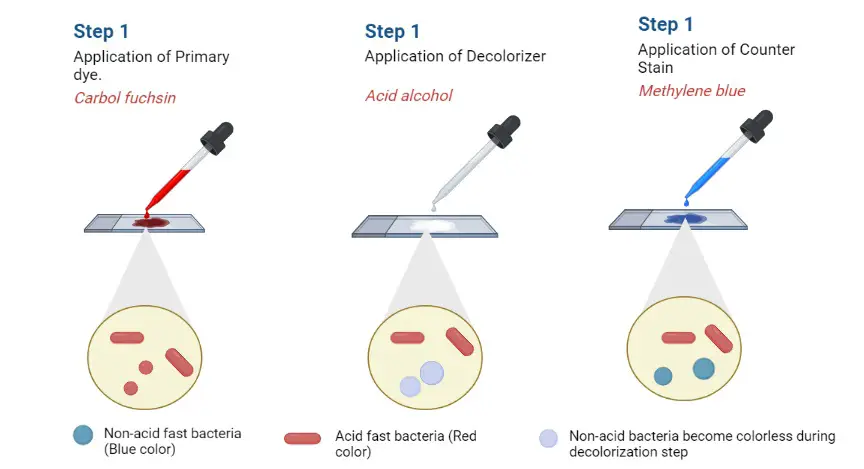
Kinyoun staining procedure also known as cold carbolfuchsin because no heat is applied during the staining process. Teh primary dye used in this method is the aniline dye, basic fuchsin, it stains all the cells present red. Mycobacteria has a unique ability to resist decolorization by
Acid-alcohol, that’s why they are known as acid-fast. They will keep their red coloration throughout the staining process.
When the decolorizer is added, a hydrochloric acid-ethanol mixture, it will decolorize the non-acid-fast material present within the sample. The final step of this staining method is the application of counterstain such as methylene blue or brilliant green.
If methylene blue is used, it will stain blue the other cells and background of the material, and if we use brilliant green, the background and other cells will be stained in green color.
Nonmycobacterial organisms with various degrees of acid-fastness include Rhodococcus species, Nocardia species, Legionella micdadei, and the cysts of Cryptosporidium, Isospora, Cyclospora and microsporidia.
Kinyoun Stain Reagents
- Kinyoun Carbol Fuchsin Stain: For 100ml mix 3.33g Basic Fuchsin, 6.67g Phenol, 16.7ml Ethanol, and 83.3 ml of Deionized Water.
- Carbol Fuchsin Decolorizer: For 100ml mix 3ml Hydrochloric Acid with 97 ml of Ethanol.
- Counterstain Methylene Blue: Mix 0.3g Methylene Blue with 100ml of Deionized Water.
- Counterstain Brilliant Green: Mix 1g Brilliant Green with 100ml of Deionized Water.
Kinyoun staining Procedure
- First, prepare the smear on slide.
- Flood the slide with primary stain, Kinyoun Carbol Fuchsin Stain, and wait for 2 minutes.
- Rinse the slide with water.
- Flood the slide with a decolorizer, until no more color drains from the slide (approx 3 to 5 seconds).
- Again wash the slide with water.
- Flood the slide with counterstain, Methylene Blue or Brilliant Green. Wait for 30 seconds.
- Rinse the slide with water.
- Air-dry the slide.
- Now, slide is ready to observe under microscope.
Result of Kinyoun staining
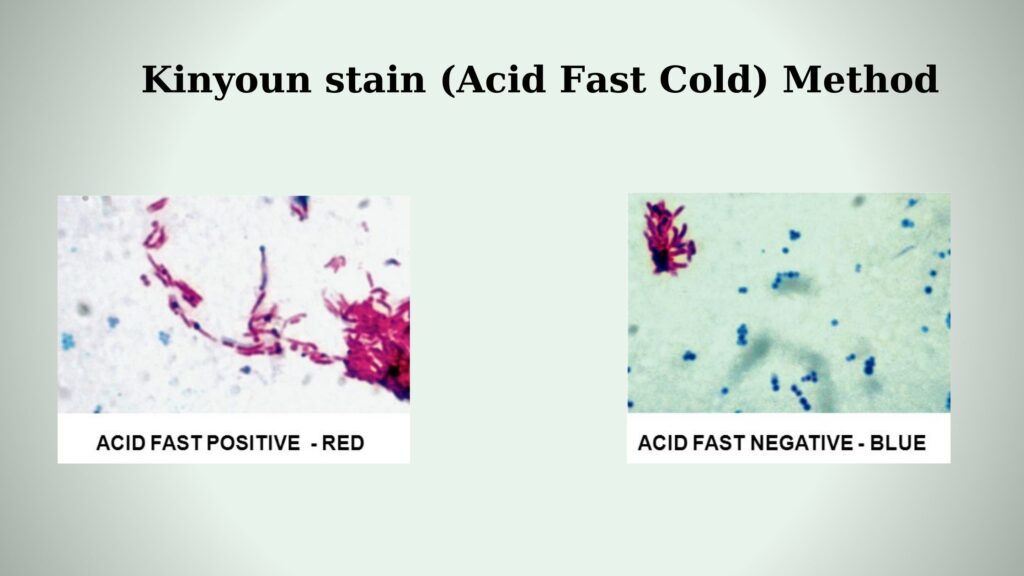
Acid-fast mycobacteria: They will appear as dark pink to red bacilli against a blue (methylene blue) or green (brilliant green) background under microscope. Mycobacteria are cylindrical in shape, 1 to 10-µm long rods, which may appear in curved or bent shapes.
Non-acid fast bacteria: These organisms and background material will stain blue or green depending on the counterstain used.
Limitation
- Rapidly growing mycobacteria may fail to stain because their ability to retain acid-fast dyes is varied.
- Be aware of adequate safety precautions and procedures required when handling specimens that are submitted for mycobacterial evaluation.
- Mycobacterial staining should always be used as an adjunct to culture methods since culture techniques are much more sensitive than all acid-fast staining procedures.
- The Counterstain (methylene blue or brilliant green) and Decolorizer should be stored at room temperature and protected from light. Under these conditions, they have a shelf life of 52 weeks from the date of manufacture.