What is Thyroid Gland?
- The thyroid gland is a pivotal endocrine organ situated in the anterior region of the neck, just below the Adam’s apple. This gland plays a crucial role in maintaining the body’s metabolic processes through the production of thyroid hormones, primarily thyroxine (T4) and triiodothyronine (T3), along with calcitonin. These hormones are essential for regulating metabolism, supporting growth and development, and maintaining serum calcium levels.
- Structurally, the thyroid is small yet highly vascularized, which is characteristic of endocrine glands. It is anatomically located between the 5th and 7th cervical vertebrae and has a unique configuration consisting of two lateral lobes connected by a narrow band of tissue known as the isthmus. This distinctive shape allows the gland to closely associate with adjacent anatomical structures, such as the parathyroid glands, which are responsible for regulating calcium homeostasis, and the recurrent laryngeal nerves, which innervate the vocal cords.
- The thyroid gland’s function is tightly regulated by the anterior pituitary gland, which releases thyroid-stimulating hormone (TSH). TSH, in turn, is regulated by the hypothalamus through the release of thyrotropin-releasing hormone (TRH). This regulatory mechanism ensures that thyroid hormone production meets the metabolic demands of the body. Notably, the gland is the largest pure endocrine gland in the human body, underscoring its significance.
- Thyroid hormones, T4 and T3, are synthesized from iodine, which the body must obtain through dietary sources. These hormones influence a multitude of physiological processes, including fat metabolism, thermogenesis, and growth and development, particularly in children. Their levels must be carefully balanced; an excess can lead to hyperthyroidism, characterized by accelerated metabolism, while insufficient production results in hypothyroidism, marked by a reduced metabolic rate.
- Additionally, the thyroid gland is involved in several pathological conditions, including inflammatory diseases such as thyroiditis and autoimmune disorders like Graves’ disease. These conditions can lead to significant dysfunction in hormone production. Furthermore, the thyroid gland is susceptible to various forms of cancer, including papillary, medullary, and follicular carcinoma, each necessitating different approaches to diagnosis and treatment.
- Calcitonin, another hormone secreted by the thyroid, plays a vital role in calcium metabolism. It helps to lower blood calcium levels by inhibiting osteoclast activity in the bones, thereby reducing the release of calcium into the bloodstream. This hormone, along with parathyroid hormone (PTH), helps to maintain calcium homeostasis, an essential aspect of various bodily functions.
Definition of Thyroid Gland
The thyroid gland is a butterfly-shaped endocrine organ located in the anterior neck that produces hormones such as thyroxine (T4), triiodothyronine (T3), and calcitonin, which regulate metabolism, growth, and calcium homeostasis. It plays a crucial role in maintaining the body’s metabolic rate and overall hormonal balance.
Anatomical Location of Thyroid Gland
The anatomical location of the thyroid gland is crucial for understanding its relationship with surrounding structures and its functional implications. This gland occupies a central position in the anterior neck, contributing significantly to various physiological processes.
- Location: The thyroid gland is situated in the anterior neck, spanning from the fifth cervical vertebra (C5) to the first thoracic vertebra (T1).
- Structural Configuration: The gland consists of two lateral lobes (left and right), which are joined by a central isthmus, giving it a characteristic butterfly shape. This unique morphology facilitates its positioning and function.
- Surrounding Structures: The lobes of the thyroid are intimately wrapped around the cricoid cartilage and the superior rings of the trachea. This close association allows the thyroid gland to interact with the airway and influences its function in regulating metabolism and other bodily processes.
- Visceral Compartment: The thyroid gland is located within the visceral compartment of the neck. This compartment also contains critical structures such as the trachea, esophagus, and pharynx. The visceral compartment is enveloped by pretracheal fascia, which provides structural support and delineates the space within the neck.
- Anatomical Relationships: The anatomical positioning of the thyroid gland has significant implications for surgical procedures. Given its proximity to important structures, including the recurrent laryngeal nerves and the parathyroid glands, surgical interventions involving the thyroid can be complex.
- Embryological Development: The thyroid gland develops embryologically from the pharynx during the third or fourth week of gestation. It gradually migrates downwards to its final position at the base of the neck.
- Clinical Relevance: Understanding the anatomical location is essential in diagnosing and treating thyroid disorders, as well as in planning surgical approaches to avoid damage to surrounding vital structures.
Structure of Thyroid Gland
The structure of the thyroid gland is integral to its function as a vital component of the endocrine system. This gland, which plays a crucial role in regulating metabolism and calcium homeostasis, exhibits specific anatomical characteristics that facilitate its hormonal activities.
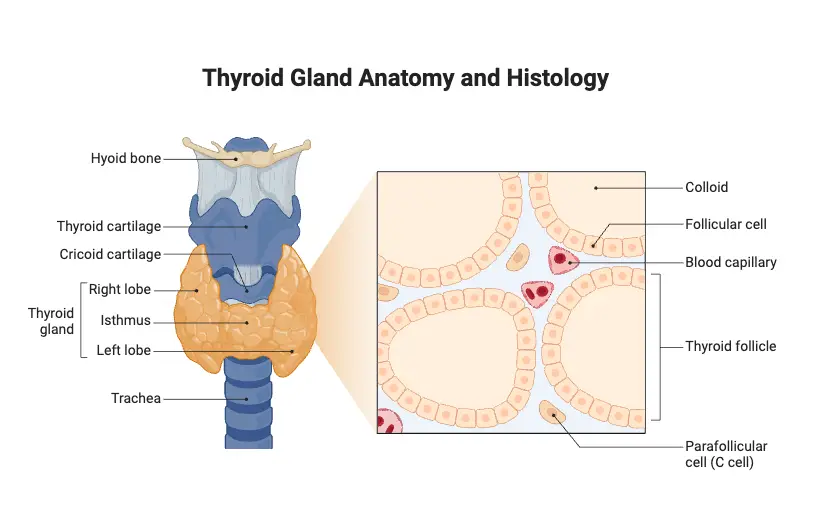
- Weight and Size: The thyroid gland typically weighs between 15 to 25 grams. Each of its two lobes is approximately 5 cm long and 3 cm wide, giving the gland a butterfly-like appearance.
- Location: Positioned in the anterior neck, the thyroid gland lies in front of the trachea and below the larynx, spanning the region between the fifth cervical vertebra (C5) and the first thoracic vertebra (T1).
- Structural Configuration: The gland consists of two lateral lobes, referred to as the right (lobus dexter) and left (lobus sinister) lobes, which are interconnected by a central mass of tissue known as the isthmus. This anatomical arrangement enhances its functional integration within the neck.
- Capsular Composition: The thyroid gland is enveloped by a fibrous capsule that provides structural support. This capsule extends inward, forming septae that divide the gland into smaller lobules, thereby facilitating organization within the gland.
- Follicular Structure: The fundamental functional units of the thyroid gland are the thyroid follicles, which are hollow, spherical structures scattered throughout the gland. Each follicle is lined with cuboidal epithelial cells known as follicular cells, which are derived from endodermal tissue.
- Thyroid Hormone Production: Follicular cells synthesize and secrete thyroid hormones in the form of thyroglobulin, a glycoprotein stored in the follicular lumen as colloid. The colloid appears as an amber-colored, viscous liquid that serves as a precursor for the active hormones. Thyroxine (T4) is predominantly produced, while triiodothyronine (T3) is generated in smaller quantities. Notably, T4 can be converted into the more biologically active T3 in peripheral tissues.
- Parafollicular Cells: Interspersed among the follicular cells are parafollicular cells, also known as C cells. These cells originate from neural crest cells and are responsible for producing calcitonin, a hormone that plays a critical role in calcium homeostasis by promoting calcium deposition in bones, thus lowering blood calcium levels.
- Vascular Supply: The thyroid gland is highly vascularized, receiving its blood supply primarily from the superior and inferior thyroid arteries. This extensive vascular network ensures that the gland can efficiently deliver hormones into the bloodstream and facilitate metabolic processes.
- Pathological Relevance: The structural features of the thyroid gland have clinical significance, particularly in the context of thyroid diseases, such as hyperthyroidism and hypothyroidism, as well as in the evaluation of thyroid nodules and cancers.
Hormones of Thyroid Gland
The thyroid gland is an essential component of the endocrine system, producing hormones that significantly influence various physiological processes. The primary hormones secreted by the thyroid gland include thyroxine (T4), triiodothyronine (T3), and calcitonin. Each of these hormones plays a crucial role in regulating metabolism and calcium levels in the body.
- Thyroid Hormones: The thyroid hormones consist mainly of thyroxine (T4) and triiodothyronine (T3).
- Thyroxine (T4):
- Thyroxine contains four iodine atoms and is produced by the follicular cells of the thyroid gland.
- This hormone is crucial for increasing the basal metabolic rate (BMR) by enhancing oxygen consumption in most tissues, excluding the lungs, brain, testis, and retina.
- It is essential for the growth and development of skeletal tissues, particularly in children.
- Thyroxine regulates carbohydrate metabolism and promotes gluconeogenesis, which is the generation of glucose from non-carbohydrate sources.
- Additionally, it influences lipid metabolism and stimulates sodium-potassium ATPase activity, which plays a vital role in maintaining cellular ion balance.
- Thyroxine also contributes to the regulation of water and electrolyte balance within the body.
- The production of T4 is primarily regulated by thyroid-stimulating hormone (TSH) released from the pituitary gland.
- T4 is converted into T3 in peripheral tissues, with T3 being approximately five times more potent than T4 in stimulating metabolic processes.
- Deficiencies in thyroxine can lead to conditions such as goiter and hypothyroidism, whereas excess levels can result in thyrotoxicosis and hyperthyroidism.
- Triiodothyronine (T3):
- Triiodothyronine consists of three iodine atoms and is primarily formed from the conversion of T4.
- T3 is vital for regulating metabolic activity and has a more pronounced effect on target tissues compared to T4.
- Thyroxine (T4):
- Calcitonin:
- Calcitonin is a polypeptide hormone secreted by parafollicular cells (C cells) of the thyroid gland.
- Its secretion is primarily stimulated by elevated calcium levels in the bloodstream, rather than through feedback mechanisms involving the pituitary gland.
- The primary function of calcitonin is to lower blood calcium levels. It achieves this by acting antagonistically to parathyroid hormone (PTH).
- Calcitonin reduces osteoclastic activity, which decreases the release of calcium from bones into the bloodstream.
- Additionally, calcitonin promotes the transfer of calcium from the blood to the bone matrix, thereby aiding in bone formation and mineralization.
- This hormone has gained attention for its therapeutic potential, particularly in treating osteoporosis, where it serves a bone-sparing effect.
Some of the essential functions of the thyroid hormones
Thyroid hormones, primarily thyroxine (T4) and triiodothyronine (T3), play a pivotal role in the body’s physiological processes. These hormones, produced by the thyroid gland, are crucial for maintaining homeostasis and influencing various biological functions. Below is an exposition of the essential functions of thyroid hormones:
- Growth and Development: Thyroid hormones are fundamental to the growth, development, and differentiation of virtually all cells in the body. They are particularly significant during childhood and adolescence, promoting overall physical and mental maturation.
- Regulation of Basal Metabolic Rate (BMR): These hormones are instrumental in regulating BMR, which is the rate at which the body expends energy at rest. Elevated thyroid hormone levels typically increase BMR, thereby enhancing energy expenditure.
- Calcium Metabolism: Thyroid hormones facilitate calcium homeostasis in the body. They assist in the deposition of calcium and phosphate in the bones, thereby contributing to bone strength and density. Furthermore, they help lower serum calcium levels by promoting calcium storage in bones.
- Central Nervous System (CNS) Development: In pediatric populations, thyroid hormones are vital for the development and functioning of the CNS. They influence neurogenesis, synaptogenesis, and myelination, essential processes for cognitive function and neurological health.
- Somatic and Psychic Growth: Thyroid hormones stimulate both somatic (physical) and psychic (mental) growth, affecting mood, cognition, and overall mental health.
- Cardiovascular Function: These hormones have a direct impact on cardiovascular physiology, increasing heart rate and the strength of heart contractions, thus enhancing cardiac output.
- Metabolism of Macromolecules: Thyroid hormones are crucial for the metabolism of carbohydrates, fats, and proteins. They enhance the catabolism of these macromolecules, facilitating energy production and utilization in the body.
- Vitamin Metabolism: The hormones also influence the metabolism of vitamins, ensuring that essential nutrients are processed efficiently to meet the body’s demands.
- Thermoregulation: By controlling metabolic processes, thyroid hormones play a significant role in regulating body temperature. They enhance heat production, which is particularly vital during colder conditions.
- Lipid Metabolism: Thyroid hormones aid in the degradation of cholesterol and triglycerides, thereby influencing lipid profiles and overall metabolic health.
- Electrolyte Balance: They contribute to maintaining electrolyte balance, which is crucial for normal cellular function and overall homeostasis.
- Erythropoiesis: Thyroid hormones support red blood cell (RBC) formation by promoting erythropoietin production and enhancing iron metabolism.
- Mitochondrial Activity: These hormones enhance mitochondrial metabolism, increasing cellular respiration and energy production. Consequently, they boost the oxygen consumption of cells and tissues.
- Mood and Behavior: There is a significant link between thyroid hormone levels and mood regulation. Abnormal levels can lead to mood disorders, such as depression or anxiety.
- Gut Motility: Thyroid hormones stimulate gastrointestinal motility, facilitating the digestive process and nutrient absorption.
- Beta-Adrenergic Sensitivity: They enhance the sensitivity of beta-adrenergic receptors to catecholamines, which can improve the body’s responsiveness to stress and hormonal signals.
Embryology and histology of Thyroid Gland
The thyroid gland is a critical endocrine organ that undergoes complex developmental processes during embryogenesis and exhibits distinctive histological features. Understanding the embryology and histology of the thyroid gland is essential for grasping its function and potential pathologies.
- Embryology:
- The thyroid gland originates from the endodermal layer during the third gestational week of development. Specifically, it arises from the fusion of the fourth pharyngeal pouch and the base of the tongue along the median line.
- By the tenth week of gestation, the developing fetus begins to utilize iodine, which is essential for synthesizing thyroid hormones. At this stage, levels of thyroxine (T4) and thyroid-stimulating hormone (TSH) can be detected in fetal blood.
- The synthesis of thyroid hormones, particularly T4, increases during the second trimester, reflecting the development of the fetal thyroid gland. Concurrently, the hypothalamus also develops, which plays a crucial role in the synthesis of thyrotropin-releasing hormone (TRH), subsequently stimulating TSH production.
- It is important to note that while TRH can cross the placenta from mother to fetus, TSH does not. Thus, the fetal thyroid gland’s function is primarily influenced by maternal hormone levels.
- Triiodothyronine (T3) levels begin to rise towards the end of the second trimester, with its synthesis increasing after birth.
- The development of the thyroid gland is governed by key transcription factors: thyroid transcription factor 1 (TTF-1 or NKX2A), thyroid transcription factor 2 (TTF-2 or FKHL15), and paired homeobox-8 (PAX-8). These factors are essential for follicular cell proliferation and the expression of thyroid-specific proteins, including the TSH receptor and thyroglobulin.
- Mutations in these transcription factors can lead to congenital hypothyroidism due to thyroid agenesis or reduced hormone secretion.
- Histology:
- The thyroid gland’s fundamental structural unit is the thyroid follicle, which ranges from 100 to 300 micrometers in diameter. Each follicle comprises a single layer of follicular cells surrounding a central lumen filled with colloid.
- Follicular cells synthesize thyroglobulin, a precursor for thyroid hormones, and secrete it into the colloidal lumen. This colloid serves as a storage form of thyroid hormones.
- The apical surface of the follicular cells faces the colloid, facilitating the uptake of iodide and the subsequent synthesis of T3 and T4. The basal aspect of these cells is richly vascularized, allowing for the efficient release of hormones into the bloodstream.
- Additionally, the thyroid gland contains parafollicular cells, also known as C-cells, which secrete calcitonin. This hormone plays a role in regulating calcium homeostasis in the body by reducing blood calcium levels through its effects on bone metabolism.
Thyroid hormone synthesis
Thyroid hormone synthesis is a complex biochemical process that occurs within the thyroid gland, essential for regulating metabolism and growth in the human body. The primary hormones produced are thyroxine (T4) and triiodothyronine (T3), with T4 being the predominant form synthesized while T3 is more biologically active. The synthesis of these hormones can be broken down into four distinct stages:
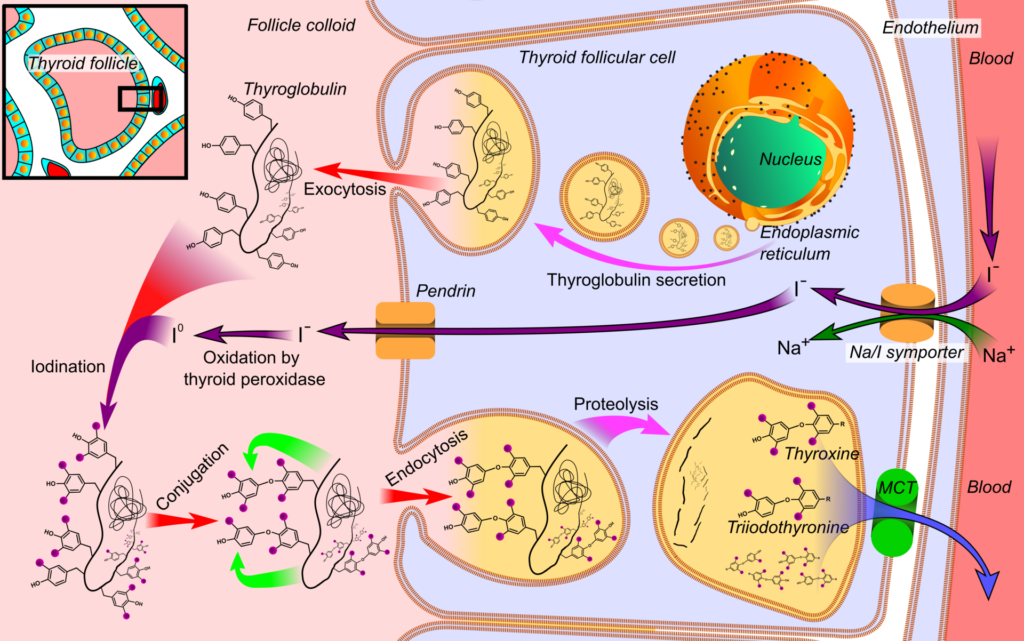
- Iodine Uptake:
- The initial stage involves the active transport of iodine into thyroid follicular cells via the sodium-iodide symporter (Na+/I- symporter).
- This transport mechanism is primarily stimulated by thyroid-stimulating hormone (TSH). The uptake of iodine is subject to concentration-dependent autocontrol, where increased intracellular iodine levels inhibit further transport.
- Various anions, such as perchlorate, pertechnetate, and thiocyanate, can disrupt this iodine transport, highlighting a critical step in the synthesis process.
- Iodine Oxidation:
- The second stage is characterized by the oxidation of iodide to iodine, a reaction facilitated by the enzyme thyroperoxidase, which requires hydrogen peroxide (H2O2) as a cofactor.
- This reaction occurs in the follicular lumen, where the oxidation process is critical for preparing iodine for subsequent binding to tyrosine residues in thyroglobulin. Certain medications, such as propylthiouracil and methimazole, can inhibit this enzymatic step, thereby affecting hormone synthesis.
- Iodination of Tyrosine:
- The third stage involves the iodination of tyrosine residues present in thyroglobulin, a process known as organification.
- During this stage, iodine binds to tyrosine, resulting in the formation of two important intermediates: monoiodotyrosine (MIT) and diiodotyrosine (DIT). These are considered inactive forms of thyroid hormones.
- Approximately 70% of the protein content in the thyroid gland is comprised of thyroglobulin, a glycoprotein with a molecular weight of around 660 kDa, which includes numerous tyrosine residues.
- Coupling Reactions:
- The final stage of synthesis involves the coupling of MIT and DIT to produce the active thyroid hormones:
- MIT + DIT → T3
- DIT + DIT → T4
- Additionally, T3 can also be generated from the metabolism of T4 in peripheral tissues, emphasizing the interconnectedness of these processes.
- The final stage of synthesis involves the coupling of MIT and DIT to produce the active thyroid hormones:
The synthesized thyroglobulin, containing significant quantities of MIT, DIT, T3, and T4 residues, is transported to the apical membrane of the follicular cells and secreted into the follicular lumen via exocytosis. This storage form is crucial for the subsequent release of T3 and T4 into circulation when required.
Thyroid hormone secretion
Thyroid hormone secretion is a meticulously regulated process that involves the release of T3 (triiodothyronine) and T4 (thyroxine) from the thyroid gland into the bloodstream. These hormones are critical for maintaining metabolic homeostasis and influencing various physiological functions. The process can be delineated into several key stages:
- Storage of Thyroid Hormones:
- Thyroid hormones are synthesized and stored in the colloid of follicular cells, bound to a glycoprotein known as thyroglobulin. This storage mechanism allows for a readily available supply of hormones when needed.
- Stimulus for Secretion:
- The secretion of thyroid hormones is primarily triggered by thyroid-stimulating hormone (TSH) from the anterior pituitary gland. Increased TSH levels lead to several cellular adaptations, including an enhancement in the number of apical microvilli on follicular cells.
- Pinocytosis of Colloid:
- Following TSH stimulation, the follicular cells engage in pinocytosis, a process in which colloid droplets containing thyroglobulin and thyroid hormones are engulfed. Microtubules assist in transporting these droplets back to the apical region of the follicular cell.
- Formation of Fagolysosomes:
- Hydrolysis of Thyroglobulin:
- Within the fagolysosomes, lysosomal proteases are activated to hydrolyze thyroglobulin. This enzymatic breakdown releases tyrosine residues, which are crucial for the formation of active thyroid hormones, T3 and T4.
- Hormone Release:
- The active forms of thyroid hormones (T3 and T4) then exit the follicular cells through facilitated diffusion into the bloodstream. This process ensures that hormones are swiftly delivered to target tissues where they exert their metabolic effects.
- Recycling of Iodotyrosines:
- Not all iodinated compounds generated during the hydrolysis process are released into circulation. Monoiodotyrosine (MIT) and diiodotyrosine (DIT) are not secreted and are instead recycled through deiodination.
- In this recycling process, T3 can also be generated from T4 through the action of the enzyme dehalogenase, which facilitates the removal of iodine atoms from these compounds.
- Iodine Reutilization:
- Approximately 50% of the iodine contained within the thyroglobulin structure can be reclaimed and reused due to these deiodination reactions. This process is particularly vital for maintaining iodine levels in the body and preventing deficiencies.
- Clinical Relevance:
- In cases where the deiodinase enzyme is deficient, individuals may experience iodine deficiency, leading to hypothyroid goiter. Such patients may require iodine replacement therapy to restore normal thyroid function.
Thyroid hormone transport
Thyroid hormone transport is a crucial physiological process that regulates the distribution and availability of T3 (triiodothyronine) and T4 (thyroxine) hormones in the bloodstream. Upon their release from the thyroid gland, these hormones bind to specific carrier proteins synthesized primarily in the liver, which play a significant role in maintaining hormonal balance. The transport mechanism can be outlined as follows:
- Binding to Carrier Proteins:
- Once thyroid hormones enter circulation, they predominantly bind to plasma proteins, which renders them inactive. This binding mechanism serves a dual purpose: it reduces the excretion of hormones through urine and acts as a reservoir to modulate hormone availability in the bloodstream.
- Main Carrier Proteins:
- The primary carrier proteins involved in thyroid hormone transport include:
- Thyroxine-Binding Globulin (TBG):
- TBG is the most significant transport protein, with a molecular weight of 54 kDa.
- Although its plasma concentration is relatively low, it binds approximately 75% of circulating T4.
- TBG facilitates the diffusion of T4 into extracellular fluids, and while its levels can increase total T3 and T4, it does not influence the levels of free T3 and T4.
- Thyroxine-Binding Prealbumin (Transthyretin, TTR):
- TTR has a molecular weight of 55 kDa and exhibits lower binding affinity compared to TBG.
- It is present in plasma at about 1/100th the concentration of TBG and binds thyroid hormones at a reduced rate.
- Serum Albumin:
- With a molecular weight of 65 kDa, serum albumin is the most abundant plasma protein, but it binds thyroid hormones less effectively.
- Thyroxine-Binding Globulin (TBG):
- The primary carrier proteins involved in thyroid hormone transport include:
- Differential Binding and Activity:
- T3 binds to carrier proteins in significantly lower amounts compared to T4. Consequently, T3 remains more active in the intracellular environment.
- The affinity of carrier proteins for T4 means that when T4 enters cells, it typically binds to cytoplasmic proteins that facilitate its conversion into the more active T3.
- Half-Life of Thyroid Hormones:
- The half-life of T4 in circulation is approximately six days, allowing for a steady release of T4 to maintain physiological functions.
- In contrast, T3 has a much shorter half-life of less than one day, which aligns with its higher activity level in cellular processes.
- Physiological Implications:
- The reversible binding of thyroid hormones to carrier proteins ensures that the free, biologically active form of these hormones is maintained at levels necessary for metabolic regulation. This dynamic equilibrium allows for rapid hormonal responses to physiological demands while preventing excess hormone levels that could lead to pathological conditions.
Diseases and Disorders of Thyroid Gland
Diseases and disorders of the thyroid gland encompass a wide range of conditions that significantly impact metabolic processes and overall health. The thyroid gland, responsible for producing essential hormones, can develop various abnormalities, each with distinct etiologies and manifestations. Below is an overview of the key diseases and disorders associated with the thyroid gland:
- Goiter: Goiter refers to an abnormal enlargement of the thyroid gland. It can be categorized into several types:
- Uni-nodular Goiter: Characterized by a single enlarged nodule in the thyroid gland.
- Multinodular Goiter: Involves multiple nodules and is often associated with varying hormone levels.
- Diffuse Goiter: Represents uniform enlargement of the thyroid without discrete nodules.
- Colloid Nodular Goiter: The most common non-neoplastic lesion, where thyroid follicles are filled with colloid and lined by squamous follicular cells.
- Hyperthyroidism (Thyrotoxicosis): This condition is marked by an excessive secretion of thyroid hormones, leading to a hypermetabolic state. Increased levels of T3 and T4 result in symptoms such as:
- Palpitations and tachycardia
- Nervousness and anxiety
- Increased sweating and weight loss
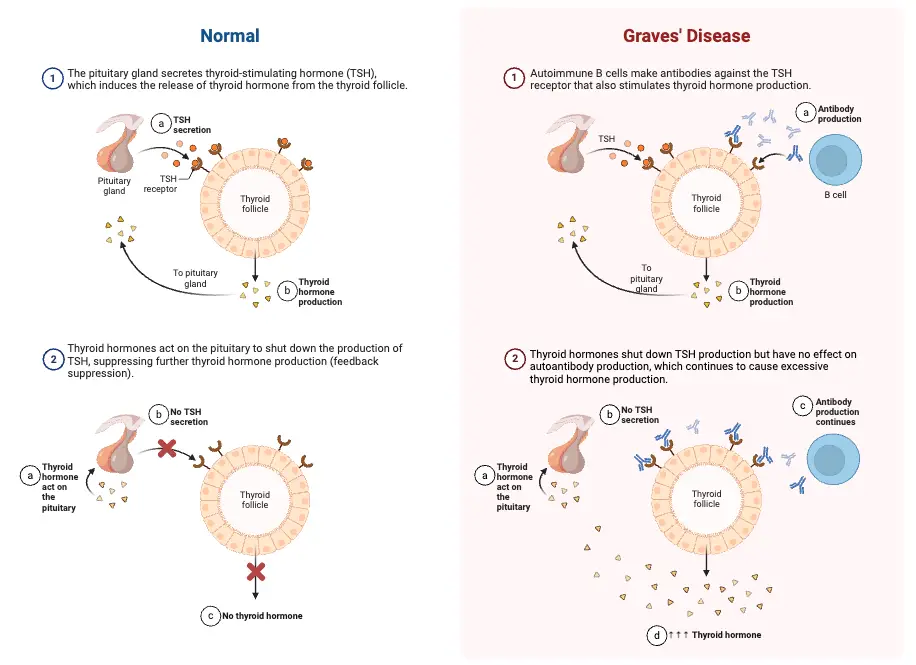
- Graves’ Disease: An autoimmune disorder primarily affecting women aged 30 to 50, Graves’ disease is characterized by the hypersecretion of thyroid hormones. The immune system produces antibodies that mimic thyroid-stimulating hormone (TSH), resulting in:
- Prolonged palpitations
- Exophthalmos (protrusion of the eyeballs)
- Dermopathy (myxedema)
- Increased metabolic activity, which may lead to weight loss and heat intolerance
- Hypothyroidism: This condition arises from diminished production of thyroid hormones due to various factors, including autoimmune disorders, iodine deficiency, or thyroiditis. It manifests differently in individuals:
- Cretinism: A severe form of hypothyroidism in infants characterized by physical and mental retardation, stunted growth, and developmental delays. This can result from maternal iodine deficiency or genetic factors affecting thyroid function.
- Myxedema: Occurring in adults, this condition presents with symptoms such as:
- Puffiness of the skin
- Low body temperature and bradycardia
- Reduced basal metabolic rate (BMR)
- Mental sluggishness and fatigue
- Thyroid Cancer: Thyroid carcinomas can originate from either follicular epithelium or parafollicular C-cells, often presenting as painless nodules. Types of thyroid cancer include:
- Papillary Carcinoma: The most common form, typically slow-growing and highly treatable.
- Follicular Carcinoma: More aggressive than papillary carcinoma, can metastasize to distant organs.
- Anaplastic Carcinoma: A rare and aggressive form with a poor prognosis.
- Medullary Carcinoma: Arising from C-cells, often associated with multiple endocrine neoplasia (MEN) syndromes.
- Thyroid Tumors: These can be benign or malignant. Benign adenomas are relatively common, while malignancies are more frequent in older adults. Some benign tumors may secrete excess thyroid hormones, leading to hyperthyroidism symptoms. Regular monitoring, including imaging studies, is essential for determining the tumor’s nature and guiding treatment.
- Exophthalmic Goiter: Closely linked with Graves’ disease, exophthalmic goiter involves protrusion of the eyeballs (exophthalmos) and is accompanied by symptoms of hyperthyroidism. This condition highlights the systemic effects of excessive thyroid hormones, particularly on ocular health and overall physiology.
Effects of thyroid hormones
Thyroid hormones, primarily thyroxine (T4) and triiodothyronine (T3), exert a broad range of effects on cellular functions, growth, metabolism, and various physiological systems. Understanding these effects is crucial for students and educators alike, as thyroid hormones play a pivotal role in maintaining homeostasis and influencing overall health.
- Cellular Effects:
- At the cellular level, T3 facilitates the synthesis of proteins, including enzymes, through transcription and translation processes. These proteins are produced after T3 interacts with specific receptors in the nucleus.
- An increase in protein synthesis is accompanied by a rise in catabolism, significantly boosting the basal metabolic rate, which can escalate by 60-100% in instances of excessive thyroid hormone secretion.
- Thyroid hormones enhance mitochondrial activity, increasing mRNA synthesis and protein production, particularly respiratory chain proteins. This stimulation leads to enhanced ATP synthesis and oxygen consumption, indicating a direct correlation between thyroid hormones and cellular energy metabolism.
- The production of transport enzymes, such as the Na⁺-K⁺-ATPase pump, increases due to the heightened protein synthesis. This pump regulates sodium and potassium ion concentrations across cell membranes, which further accelerates metabolic processes.
- The Ca²⁺-ATPase enzyme also plays a crucial role in maintaining calcium ion homeostasis, particularly in muscle contractions and neurotransmitter release.
- Effects on Growth:
- Thyroid hormones are essential for normal growth and development, influencing both specific and general growth processes. In children, hypothyroidism often results in stunted growth due to premature closure of the epiphyses, while hyperthyroid children may exhibit increased height.
- These hormones are also critical for brain development during both pre- and post-natal periods. A deficiency in maternal thyroid hormone can lead to significant developmental delays and neurological impairments in the fetus.
- Normal serum levels of T4 (5-12 µg/dL) and T3 (80-200 ng/dL) are crucial for ensuring appropriate growth; early detection and treatment of thyroid hormone deficiencies in newborns can lead to normal development.
- Metabolic Effects:
- Thyroid hormones regulate carbohydrate metabolism by promoting both anabolic and catabolic pathways. They enhance the synthesis of enzymes involved in carbohydrate metabolism, thereby increasing glucose uptake by cells and stimulating both glycolysis and gluconeogenesis.
- In lipid metabolism, thyroid hormones also exhibit both anabolic and catabolic properties. They stimulate lipolysis in adipose tissue, resulting in elevated free fatty acid concentrations in the bloodstream. Notably, while cholesterol and triglyceride levels are expected to rise, they often remain low due to the increased receptor synthesis for LDL and cholesterol in the liver, facilitating their clearance from circulation.
- Additionally, thyroid hormones enhance protein metabolism by promoting amino acid transport and increasing protein synthesis. This anabolic effect supports normal growth and development, particularly in fetal stages, by synthesizing insulin-like growth factors.
- Effects on Physiological Systems:
- In the cardiovascular system, thyroid hormones primarily exert their effects through the modulation of catecholamines. They increase the number of β-adrenergic receptors, which results in elevated heart rate, stroke volume, and cardiac output. In hyperthyroidism, this can lead to warm, moist skin and symptoms of restlessness due to heightened sympathetic activity.
- In contrast, hypothyroidism leads to a reduction in β-adrenergic receptor expression, resulting in decreased heart rate and cold, dry skin. Studies have shown that cardiac parameters significantly fluctuate between hyperthyroid and hypothyroid patients.
- Respiratory effects are also evident, as increased metabolic activity leads to heightened oxygen consumption and carbon dioxide production, prompting hyperventilation.
- Gastrointestinal activity is stimulated by thyroid hormones, resulting in increased appetite and digestive processes. Conversely, excessive thyroid hormone can lead to diarrhea, while hypothyroidism is often associated with constipation.
- In terms of skeletal effects, thyroid hormones influence bone metabolism by stimulating the activity of both osteoblasts and osteoclasts. While they promote bone growth in normal physiology, excessive levels can lead to increased bone resorption, thereby heightening the risk of osteoporosis, particularly in postmenopausal women.
- Moreover, thyroid hormones impact the central nervous system, influencing muscle tone and neuromuscular coordination. Symptoms such as muscle tremors and fatigue are common in hyperthyroid individuals, while hypothyroidism may lead to lethargy and increased sleepiness.
- Finally, thyroid hormones facilitate erythropoiesis by stimulating erythroid stem cells in response to oxygen deprivation. They increase erythropoietin production in the kidneys, further promoting red blood cell synthesis, which is often diminished in hypothyroid patients.
6 Steps of Thyroid Hormone Synthesis
Thyroid hormone synthesis is a complex biochemical process essential for the production of the hormones thyroxine (T4) and triiodothyronine (T3). These hormones play critical roles in regulating metabolism, growth, and development. The synthesis process occurs within the thyroid follicular cells and can be delineated into six key steps, which can be remembered using the mnemonic “ATE ICE.”
- Active Transport of Iodide:
- The process begins with the active transport of iodide into the follicular cells. This is facilitated by the sodium-iodide symporter (NIS), which utilizes a sodium gradient established by the sodium-potassium ATPase.
- The transport of iodide is crucial because it provides the necessary substrate for thyroid hormone synthesis.
- Thyroglobulin Formation:
- Within the ribosomes of the follicular cells, thyroglobulin (Tg) is synthesized. This large protein is rich in the amino acid tyrosine and serves as the precursor for thyroid hormones.
- After its formation, thyroglobulin is packaged into secretory vesicles for transport to the follicle lumen.
- Exocytosis of Thyroglobulin:
- The secretory vesicles undergo exocytosis, releasing thyroglobulin into the lumen of the thyroid follicle, where it is stored as colloid.
- This colloid serves as a scaffold for subsequent hormone synthesis, enabling the iodination and coupling processes that follow.
- Iodination of Thyroglobulin:
- In the follicle lumen, iodide becomes reactive through the action of the enzyme thyroid peroxidase.
- Iodide atoms attach to the tyrosine residues within the thyroglobulin molecule, resulting in the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT).
- Coupling Reactions:
- The next critical step involves the coupling of MIT and DIT, which generates the active hormone triiodothyronine (T3).
- Additionally, coupling two DIT molecules results in the synthesis of thyroxine (T4), the primary form of thyroid hormone released into circulation.
- Endocytosis and Proteolysis:
- The final step is the endocytosis of iodinated thyroglobulin back into the follicular cells.
- Within lysosomes, thyroglobulin undergoes proteolysis, whereby iodinated tyrosine residues are cleaved from the protein scaffold. This process liberates the free T3 and T4 hormones for release into the bloodstream.
Both T3 and T4 are lipophilic hormones predominantly transported in the blood bound to plasma proteins, such as thyronine-binding globulin (TBG) and albumin. While T3 is biologically more potent than T4, it has a shorter half-life due to a lower affinity for these binding proteins, with less than 1% of T3 and T4 remaining in an unbound free form.
In peripheral tissues, T4 can be converted into T3 through deiodination, enhancing its metabolic effects. Moreover, both T3 and T4 can be inactivated by the removal of iodine, primarily in the liver and kidneys. Due to its longer half-life, T4 is often preferred for therapeutic purposes in treating hypothyroidism, as it allows for more stable plasma concentrations compared to T3.
Hypothalamus-hypophysis-thyroid axe
The hypothalamus-hypophysis-thyroid axis (HPT axis) regulates thyroid hormone synthesis and secretion through a feedback loop involving the hypothalamus, the pituitary gland (hypophysis), and the thyroid gland. This system ensures the proper levels of thyroid hormones necessary for metabolic and physiological functions.
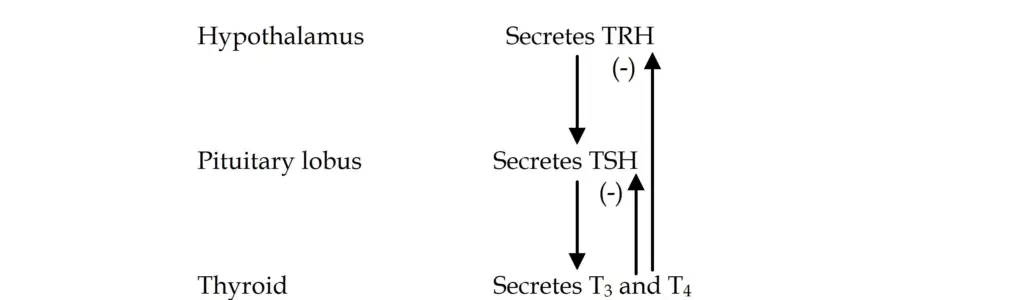
- Synthesis of Thyrotropin-Releasing Hormone (TRH):
- The process begins in the hypothalamus, where thyrotropin-releasing hormone (TRH) is synthesized in the periventricular nucleus. TRH is a tripeptide hormone and plays a pivotal role in stimulating the release of thyroid-stimulating hormone (TSH) from the anterior pituitary.
- TRH is transported from the hypothalamus to the anterior pituitary via the hypophyseal portal circulation. Upon reaching the thyrotrope cells of the anterior pituitary, TRH binds to specific receptors on the surface of these cells.
- Stimulation of TSH Secretion:
- The interaction of TRH with its receptors activates Gq proteins, which then trigger phospholipase C. This enzyme cleaves membrane phospholipids into diacylglycerol (DAG) and inositol triphosphate (IP3), both of which serve as secondary messengers.
- IP3 stimulates the release of calcium ions from the endoplasmic reticulum, while DAG activates protein kinase C. Together, these signals facilitate the secretion of TSH from the anterior pituitary into the bloodstream.
- TSH Structure and Activation:
- TSH is a glycoprotein hormone composed of two subunits: alpha (α) and beta (β). While the α subunit is shared with other hormones like luteinizing hormone (LH) and follicle-stimulating hormone (FSH), the β subunit is unique to TSH and determines its receptor specificity.
- Once released into circulation, TSH binds to TSH receptors on the thyroid gland’s follicular cells, where it activates a Gs protein that stimulates adenylate cyclase. This, in turn, increases cyclic AMP (cAMP) levels, which play a key role in promoting thyroid hormone synthesis.
- Thyroid Hormone Production and Feedback Mechanism:
- TSH stimulates the thyroid gland to synthesize and secrete thyroxine (T4) and triiodothyronine (T3), essential hormones for regulating metabolism. The production of T3 and T4 depends on the availability of iodide and the activity of thyroid peroxidase.
- However, if circulating levels of T3 and T4 become too high, they exert negative feedback on both the hypothalamus and the pituitary gland. This feedback loop inhibits the secretion of TRH from the hypothalamus and TSH from the pituitary, thereby reducing further thyroid hormone production.
- Circadian Rhythm and TSH Regulation:
- TSH secretion is pulsatile and follows a circadian rhythm, with its highest levels occurring at night and peaking around midnight. Throughout the day, TSH levels gradually decline, reflecting the body’s changing metabolic needs.
- Besides TRH, TSH secretion is influenced by other factors. Estrogen can enhance TSH secretion, while somatostatin, dopamine, and glucocorticoids suppress it. Additionally, T3 and T4 themselves inhibit TSH release through feedback regulation.
- Effects of TSH:
- The actions of TSH on the thyroid gland can be divided into three time-dependent effects:
- Immediate Effects: TSH rapidly stimulates the synthesis of thyroid hormones within minutes.
- Intermediate Effects: Over the course of hours, TSH promotes the growth of thyroid follicular cells and enhances their ability to produce hormones.
- Chronic Effects: Long-term exposure to elevated TSH levels leads to thyroid hypertrophy and hyperplasia, which can result in an enlarged thyroid (goiter).
- Importantly, TSH does not regulate the peripheral conversion of T4 to T3, a process primarily occurring in the liver and kidneys.
- The actions of TSH on the thyroid gland can be divided into three time-dependent effects:
- Hormonal Interactions:
- In addition to regulating thyroid function, TRH also influences the secretion of other pituitary hormones, including growth hormone (GH), follicle-stimulating hormone (FSH), and prolactin (PRL).
- Noradrenaline stimulates TRH secretion, while somatostatin and serotonin inhibit it. These interactions highlight the intricate hormonal balance maintained by the HPT axis.
Vasculature of Thyroid Gland
The vasculature of the thyroid gland is essential for its function, as it ensures an adequate blood supply for hormone secretion and metabolic processes. The thyroid is highly vascularized, which facilitates the rapid delivery of hormones into the bloodstream. This section outlines the arterial supply and venous drainage of the thyroid gland, highlighting their anatomical significance.
- Arterial Supply: The thyroid gland receives its blood supply primarily from two main arteries:
- Superior Thyroid Artery:
- This artery originates as the first branch of the external carotid artery.
- It courses downward and forward, supplying the upper part of the thyroid gland.
- Its proximity to the external branch of the superior laryngeal nerve, which innervates the larynx, is clinically relevant, particularly during surgical procedures.
- Inferior Thyroid Artery:
- Arising from the thyrocervical trunk (a branch of the subclavian artery), this artery supplies the lower portion of the thyroid gland.
- It runs closely alongside the recurrent laryngeal nerve, which also innervates the larynx, making its anatomical relationship significant during thyroid surgeries.
- Thyroid Ima Artery:
- Present in approximately 10% of the population, this additional artery emerges from the brachiocephalic trunk.
- It supplies the anterior surface and isthmus of the thyroid gland, providing an alternative route for blood supply, which may be crucial in specific clinical scenarios.
- Superior Thyroid Artery:
- Venous Drainage: The venous drainage of the thyroid gland is accomplished through the following veins:
- Superior Thyroid Vein:
- This vein drains the upper part of the thyroid gland and empties into the internal jugular vein.
- Middle Thyroid Vein:
- Also draining into the internal jugular vein, it collects blood from the lateral aspects of the gland.
- Inferior Thyroid Vein:
- This vein drains the lower portions of the thyroid and empties into the brachiocephalic vein.
- A venous plexus surrounds the thyroid gland, allowing for efficient drainage of blood and maintaining venous return to the systemic circulation.
- Superior Thyroid Vein:
Functions of Thyroid Gland
The thyroid gland serves multiple critical functions that are essential for maintaining metabolic homeostasis and supporting growth and development in the human body. Its primary role revolves around the production of hormones that significantly influence various physiological processes.
- Production of Thyroid Hormones:
- The thyroid gland synthesizes two primary hormones, thyroxine (T4) and triiodothyronine (T3), both of which are crucial for metabolic activities.
- These hormones contain iodine, which is vital for their synthesis, thereby highlighting the gland’s role in iodine metabolism.
- Regulation of Basal Metabolic Rate (BMR):
- Thyroid hormones play a fundamental role in regulating the basal metabolic rate, which is the rate at which the body expends energy at rest.
- They influence various metabolic pathways, including glucose, fat, and protein metabolism, thereby promoting energy production and utilization.
- Impact on Growth and Development:
- Thyroid hormones are essential for normal growth and development, particularly in tissues such as the brain and kidneys.
- Adequate levels of these hormones are necessary for the development of neural tissues and skeletal growth in children.
- Regulation of Calcium Levels:
- Calcitonin, produced by the parafollicular cells of the thyroid gland, is involved in regulating calcium ion concentrations in the bloodstream.
- This hormone acts to lower blood calcium levels by inhibiting osteoclastic activity in bones, which reduces the release of calcium into circulation.
- Influence on Iodine Metabolism:
- As the primary organ responsible for iodine metabolism, the thyroid gland ensures that adequate iodine levels are maintained in the body, which is critical for the synthesis of thyroid hormones.
- Support for Cellular Metabolism:
- Thyroid hormones stimulate metabolic processes in nearly all tissues, enhancing oxygen consumption and promoting heat production.
- This metabolic stimulation is essential for overall energy balance and thermoregulation.
- Homeostasis and Feedback Mechanisms:
- The secretion of thyroid hormones is regulated through feedback mechanisms involving the pituitary gland, particularly via thyroid-stimulating hormone (TSH).
- This regulation ensures that hormone levels remain within an optimal range to meet the body’s physiological demands.
- Bursuk, E. (2012). Introduction to Thyroid: Anatomy and Functions. InTech. doi: 10.5772/37942
- Allen E, Fingeret A. Anatomy, Head and Neck, Thyroid. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470452/
- Khan YS, Farhana A. Histology, Thyroid Gland. [Updated 2022 Dec 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551659/
- https://www.onlinebiologynotes.com/what-is-thyroid-gland/
- https://www.gastroepato.it/en_tiroide.htm
- https://teachmephysiology.com/endocrine-system/thyroid-parathyroid-gland/thyroid-gland/
- https://reference.medscape.com/article/835535-overview?form=fpf
- https://teachmeanatomy.info/neck/viscera/thyroid-gland/
- https://en.wikipedia.org/wiki/Thyroid
- https://oer.unimed.edu.ng/LECTURE%20NOTES/1/2/Dr-Akpalaba-Immaculata-ANATOMY-OF-THE-THYROID-GLAND.pdf
- https://www.medicalnewstoday.com/articles/thyroid-gland-function
- https://www.thoughtco.com/thyroid-gland-anatomy-373251
- https://www.geeksforgeeks.org/thyroid-gland-anatomy-function-and-clinical-aspects/