Unsaturated fish oils are especially vulnerable to oxidation, resulting in peroxides when stored in cold or freezing conditions for storage. Peroxides are precursors to breakdown products that can cause rancid taste in fat. Peroxide levels are an indicator of oxidation in the initial stages of lipid degradation. The index is less reliable in the latter phase of the process, as peroxide degradation rises.
Peroxide quantity (POV) refers to the content of reactive oxygen expressed in milliequivalents (meq) of free iodine per kilogramme fat. It is measured by titrating the Iodine dissolved from potassium iodide using sodium thiosulphate as a solution. Oils that have a POV of less than 10 meq/kg are considered to be fresh. The taste of rancid is evident when the POV falls between 20 to 40 meq/kg. When interpreting these figures however it is important to be aware of the specific fat or oil that is involved.
Contents
Purpose and Scope
This method is used to determine the amount the peroxide value for animals fats and oils such as oil and fats from vegetables and for flavor and fragrance ingredients. Peroxide is an indication of the quantity of oxygen as peroxide particularly hydroperoxides, in a particular substance. The value of the peroxide is an indication of the degree of oxidation present.
Principle of Peroxide Value Test
The sample is treated in solution using a mix of acetic acid and an organic solvent, and then the solution of potassium iodide. The iodine that is liberated is then titrated using the standard sodium thiosulfate solution. Peroxide values are measured in milliequivalents of peroxide/kg , or in millimololes of peroxide/L.
Reaction
Requirement
Instruments
- Evaporating flasks with stoppers (250 ml capacity)
- Rotary evaporator with vacuum pump
- Pipettes (1 ml, 5 ml, 10 ml, 20 ml)
- Measuring cylinders (25 ml, 100 ml)
- Stop watches
- Microburette (2 ml)
- Burette (50 ml)
- Erlenmeyer flasks (100 ml, 200 ml) with stoppers
- Balance with at least 0.1 g sensitivity
Reagents
a) 0.01N Na2S2O3 solution
Dissolve 25 grams of Na2S2O3.5H20 in freshly boiled distillate water, and then make 1000 milliliters. Let it sit for a couple of days. Add 10ml of iso-amylalcohol to stabilize the mixture. If required dilute 10 times using freshly boiling distillate water. Store in the dark brown bottle. Standardization of the N2S2O3 Solution
- Make 20 ml from 0.01N K2Cr2O7 solution into 250 ml flask with a stopper.
- Make 10 ml with 10 percent Kl solution, and five ml of 25% H2SO4.
- The flask should be immediately shut off and then stand for 5 minutes in darkness.
- Add 100ml of distillation water, then shake.
- Titrate the solution with 0.01N Na2S2O3 solution, until the yellow color almost disappears.
- Add 1 millilitre of 1.5 percent starch solution for indicator. Continue the titration process until the dark blue color disappears.
- Perform a blank test using 20ml of distilled water instead of the K2Cr2O7 solution.
- Calculation:
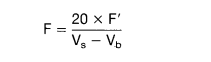
where
- F = factor of 0.01N Na2S3O3 solution
- F’ = factor of 0.01 N K2Cr2O7 solution
- Vs = titration volume of sample (ml)
- Vb = titration volume of blank (ml)
b) Chloroform-acetic acid mixture (2:3).
- Mix CH3COOH and CHCl3, 2:3 in volume.
- Flush with clean, dry nitrogen gas.
c) Saturated Kl solution
- Dissolve 100 grams Kl in 70 ml of freshly boiling distillate water.
- Keep the solution in the form of the precipitated crystals in an opaque bottle.
d) 1.5% starch solution
- Take a weight of 1.5 grams of soluble starch into a beaker.
- Include 100 ml of distilled water. Boil and heat until 30 sec.
e) 0.01 N K2Cr2O7 standard solution
- Take a weight of 4.9035 grams of K2Cr2O7 that was dried at a temperature of 100-110 degrees Celsius for 3-4 hours.
- It will dissolve into distilled water and create up to 1000ml.
- If required dilute 10 times with the distilled water.
Factor F’ = 4.9035/W ; where W is the actual weight of K2Cr2O7 used.
f) 10% (w/v) Kl solution
- Dissolve 10 grams of Kl within distilled water and create up to 100 ml
g) 25% H2SO4 solution
- Mix 25 grams (13.5 milliliters) of H2SO4 concentrated and 75ml of distilled water
Procedure of Peroxide Value Test
- Take about 0.3 grams of fat or one ml extract that contains about 0.3 grams of fat in the 250-ml flask, with a stopper.
- Get rid of solvent by using a rotary evaporator at a lower pressure of forty degrees C (water-bath temperatures).
- Add 10ml of the CHCl3-CH3COOH mixture to dissolve fats with shaking.
- Add 1 ml of saturated Kl.
- Then stop the bottle and stand in darkness for 5 minutes.
- And then add 20 ml of distilled water, and shake
- Titrate the liberated iodine using 0.01 Na2S2O3 solution, until it turns light yellow colour. Add 1 millilitres of 1.5 percent starch solution to serve as an indicator, and then titrate until it is colourless.
- Test the blanks similarly, but without the fats.
Calculation
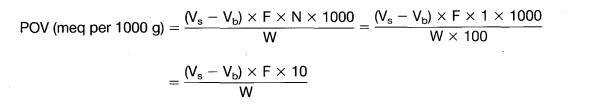
where
- Vs = titration volume of sample (ml);
- Vb = titration volume of blank (ml);
- F = factor of 0.01N Na2S2O3 solution;
- W = weight of fat in volume of extract used (g);
- N = normality of Na2S2O3 solution (in this case N/100)
POV (millimoles per 1000 g) = 0.5 x (Vs – Vb) x N x 1000/W
References
- https://aquadocs.org/bitstream/handle/1834/41021/C-07.pdf?sequence=1&isAllowed=y
- https://ifrafragrance.org/docs/default-source/guidelines/20190910-revised-ifra-analytical-method-on-peroxide-value.pdf?sfvrsn=c4a931e2_0
- https://www.ysi.com/File%20Library/Documents/Titration%20Applications/XA00078-Determination-of-Peroxide-Application-Note.pdf