Contents
What is Cystine Tryptic Agar?
- Cystine Tryptic Agar (CTA), also known as cystine trypticase agar, is a semi-solid growth medium developed by Vera. It is primarily used for the identification and maintenance of microorganisms over extended periods when stored at appropriate temperatures.
- CTA is particularly useful for detecting bacterial motility and can contribute to fermentation reactions and differentiation of fastidious microorganisms such as Neisseria, pneumococci, streptococci, and non-spore-forming anaerobes.
- The medium can be used in two different formulations: CTA without carbohydrates, which is suitable for the maintenance of stock cultures, and CTA with carbohydrates, which enables the differentiation of fastidious organisms through fermentation reactions.
- CTA contains cystine and casein peptone, which provide essential nutrients for the growth of fastidious organisms. When a carbohydrate present in the medium is fermented by the microorganism, organic acids are produced, leading to acidification of the medium. This acidification causes a color change of the phenol red indicator from orange-red to yellow. A negative carbohydrate test (neutral orange color) occurs when peptone is deaminated in the absence of a carbohydrate source.
- In some cases, the addition of ascitic fluid to CTA media is recommended as an enrichment fluid to facilitate the cultivation of fastidious organisms such as Neisseria spp.
- Overall, Cystine Tryptic Agar is a valuable tool in microbiology laboratories for the identification and differentiation of various microorganisms, particularly those that are fastidious in nature. It provides a semi-solid growth medium that allows for the detection of motility and the assessment of fermentation reactions, aiding in the characterization of bacterial species.
Composition of Cystine Tryptic Agar
| Ingredients | Gm/L |
| Pancreatic Digest of Casein | 20.0gm |
| Sodium Chloride | 5.0gm |
| L-Cystine | 0.5gm |
| Sodium Sulfite | 0.5gm |
| Phenol Red | 17.0mg |
| Agar | 3.5gm |
Final pH 7.5 +/- 0.2 at 25ºC.
Principle of Cystine Tryptic Agar
The principle of Cystine Tryptic Agar (CTA) revolves around its composition and the detection of fermentation reactions and bacterial motility.
CTA contains cystine and peptone, which provide the necessary nutrients to support the growth of fastidious microorganisms. Additionally, the medium is supplemented with a specific carbohydrate at a 1% concentration to detect fermentation reactions.
The detection of fermentation reactions is based on the incorporation of the pH indicator dye, phenol red, into the medium. When a carbohydrate present in the medium is metabolized by the organism, organic acids are produced, resulting in acidification of the medium. This acid production leads to a decrease in pH, causing a visible color change in the medium from its initial red-pink color to yellow. The shift in color indicates positive fermentation.
However, it’s important to note that the peptone present in the medium is also degraded by the bacteria, producing alkaline end products. These alkaline substances can counteract the acidification caused by carbohydrate fermentation. The phenol red indicator changes from reddish-orange to yellow when the acid produced from carbohydrate fermentation surpasses the alkaline end products of peptone degradation. The color change typically occurs around pH 6.8, which is close to the original pH of the medium.
The addition of agar to the medium allows for the detection of bacterial motility. When the medium is inoculated along a stab line, motile organisms extend their growth beyond the stab line, resulting in turbidity or cloudiness throughout the medium. Non-motile organisms, on the other hand, only grow along the stab line and leave the surrounding medium clear.
By combining the detection of fermentation reactions and motility, Cystine Tryptic Agar enables differentiation and identification of microorganisms based on their metabolic capabilities and motility characteristics.
Intended Use
Cystine Tryptic Agar and CTA Medium (Cystine Trypticase(tm) Agar Medium) serve for the care of microorganisms and also for determination of motility in bacterial cells and, when combined with carbohydrate, to aid in the fermentation process of fastidious microorganisms, i.e., Neisseria, streptococci, pneumococci as well as nonsporeforming anaerobes.
Preparation of Cystine Tryptic Agar
To prepare Cystine Tryptic Agar, follow these steps:
- Suspend 28.51 grams of the Cystine Tryptic Agar powder in 1000 ml of distilled water. Ensure that the powder is evenly dispersed in the water.
- Heat the mixture to boiling while stirring continuously. This step is essential to dissolve the medium completely.
- Once the medium is fully dissolved, remove it from the heat source.
- Dispense the prepared Cystine Tryptic Agar into test tubes in 8-10 ml amounts. The volume can be adjusted based on your specific requirements.
- Sterilize the tubes containing the medium by autoclaving. Place the tubes in an autoclave chamber and subject them to a pressure of 15 lbs (pounds) per square inch at a temperature of 121°C (Celsius). Maintain these conditions for 15 minutes to ensure proper sterilization.
- After the sterilization process, cool the medium to approximately 50°C. This temperature allows for the addition of the appropriate carbohydrate without degrading its properties.
- Add the desired carbohydrate to the medium, following the recommended concentration for detection of fermentation reactions. Mix the medium thoroughly to ensure even distribution of the carbohydrate.
- Once the carbohydrate is added and mixed well, allow the medium in the test tubes to cool in an upright position. This ensures that the medium solidifies properly and maintains its intended consistency.
By following these steps, you can prepare Cystine Tryptic Agar, ready for use in the detection of fermentation reactions and other tests involving the identification and differentiation of microorganisms.
Plating Procedure of Cystine Tryptic Agar
The plating procedure for Cystine Tryptic Agar (CTA) involves the following steps:
- Prior to use, lock the caps of the CTA tubes to prevent leakage during the subsequent boiling step. Boil the tubes with the caps locked for a short period of time, then tighten the caps and allow them to cool before proceeding with the inoculation.
- Select suitable colonies for inoculation. Remove new colonies from a suitable medium, such as Chocolate Agar, but avoid selecting colonies from a selective primarily isolated plate.
- In the case of tests on fermentation with members of the Genus Neisseria, only the medium tube needs to be inoculated. For other organisms, such as facultative streptococci or strictly anaerobic organisms, you can inoculate by gently stabbing the middle of the medium using an inoculating needle. The needle should penetrate approximately halfway into the depth of the medium.
- Repeat the inoculation process for each tube that needs to be inoculated.
- Incubate the CTA tubes at a temperature of 35 ± 2°C. The caps can be left loose for aerobic incubation or tightened based on the specific requirements of the organisms being studied. For Neisseria, it is recommended to incubate the tubes with tightly fitting caps, particularly when they need to be kept inside a CO2 incubator. Regularly examine the tubes for up to 24 hours, looking for signs of growth (turbidity), evidence of motility, and acid production in carbohydrate-containing medium (indicated by a yellow color in the upper layer of the medium). Some strains may require an extended incubation period of up to 48-72 hours.
- CTA medium supports the growth of various organisms, including snobbly ones like Neisseria, Pasteurella, streptococci, Brucella, corynebacteria, and vibrios, without requiring additional serum, carbon dioxide, or other enrichments.
- To enhance the growth of certain organisms, particularly fast-fermenting anaerobes, it is beneficial to provide carbon dioxide during incubation. Anaerobic cultures prefer the presence of carbon dioxide, nitrogen, or hydrogen for optimal growth. Some strict anaerobes may not grow or exhibit limited growth in the absence of carbon dioxide.
Result Interpretation of Cystine Tryptic Agar
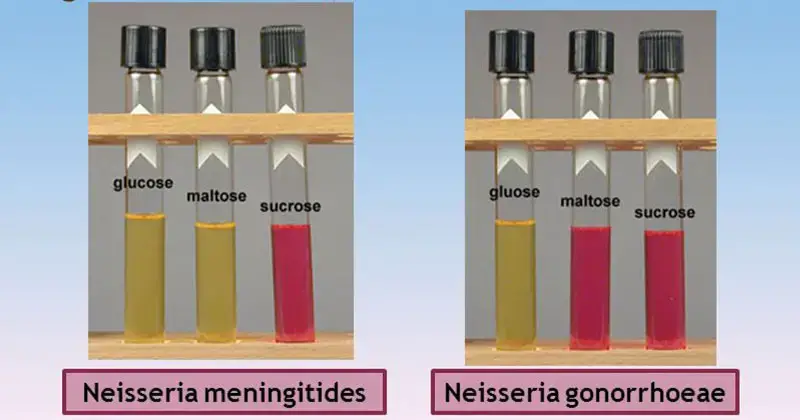
The interpretation of results obtained from Cystine Tryptic Agar (CTA) involves analyzing fermentation reactions and bacterial motility. Here’s how to interpret the results:
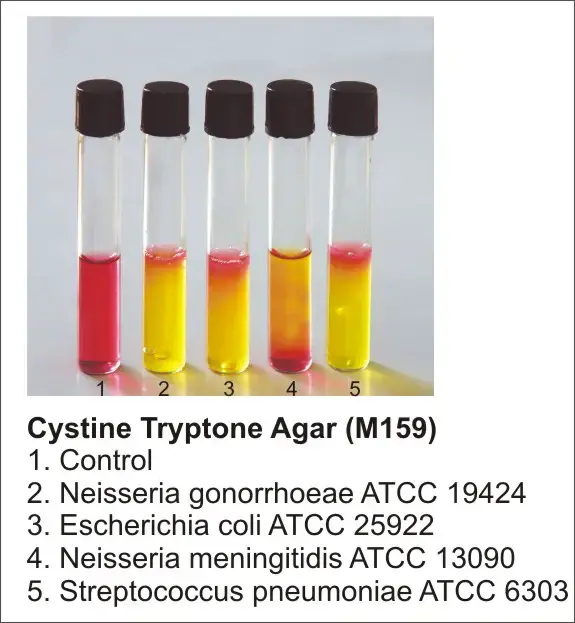
- Positive reaction: A positive result is indicated by the development of a yellow color change in the inoculated area (stab line) of the medium. This change in color indicates that the carbohydrate present in the medium has been metabolized and fermentation has occurred.
- Negative reaction: A negative result is observed when the medium retains a red-pink or deep red color. This indicates that the carbohydrate has not been utilized by the organism, and instead, peptone degradation has occurred. The growth in this case may exhibit a deep red to orange color, indicating the breakdown of peptone rather than carbohydrate fermentation.
- Positive test: A positive motility test is indicated by turbidity or cloudy growth that extends from the line of inoculation (stab line). This indicates that the organism is motile and can spread beyond the initial point of inoculation.
- Negative test: A negative motility test is observed when growth is limited only along the stab line, leaving the surroundings clear. This suggests that the organism is non-motile and does not exhibit significant movement away from the point of inoculation.
By analyzing the fermentation reactions and motility characteristics observed on CTA, it is possible to make interpretations about the metabolic capabilities and motility of the tested microorganisms. These results contribute to the identification and differentiation of different bacterial species.
Storage of Cystine Tryptic Agar
This product should be stored between 2 and 8 degrees Celsius. A proper storage environment prolongs the shelf life and quality of the product. Overheating and freezing can cause extreme degradation of the media. The media must not be used past the expiration dates. The expiration dates are for not-opened tubes that are properly maintained.
Quality Control
The quality control of Cystine Tryptic Agar (CTA) is an essential step to ensure the reliability and consistency of the medium. The following information outlines the quality control measures for CTA:
- Manufacturing Compliance: Each lot number of CTA Agar is manufactured, packaged, and processed in accordance with current Good Manufacturing Practice (GMP) regulations. This ensures that the medium is produced under controlled conditions and meets the required standards.
- Quality Control Organisms: Control organisms are used to assess the performance and acceptability of each lot of CTA Agar. These control organisms are specifically selected and tested to verify the medium’s ability to support their growth and exhibit the expected reactions.
- Testing Procedures: Testing of the control organisms should be performed in accordance with established laboratory quality control procedures. These procedures may include inoculating the medium with control strains, incubating under specified conditions, and assessing the growth, fermentation reactions, and motility as appropriate.
- Interpretation of Results: The quality control results obtained from the control organisms should meet the expected criteria established for the specific lot of CTA Agar. If any aberrant results are observed, indicating a potential issue with the medium, the sample results should not be reported until the issue is resolved.
Uses of Cystine Tryptic Agar
Cystine Tryptic Agar (CTA) has several important uses in microbiology. Some of its main applications include:
- Identification and Maintenance: CTA is commonly used for the identification and maintenance of the gonococcus (Neisseria gonorrhoeae) and other bacteria. It provides a suitable medium for the growth and characterization of these organisms, aiding in their identification and study.
- Carbohydrate Fermentation: CTA is utilized for the determination of carbohydrate fermentation by fastidious microorganisms. By adding specific carbohydrates to the medium, the ability of the microorganisms to ferment these carbohydrates can be assessed. This allows for the differentiation and identification of these organisms based on their metabolic capabilities.
- Bacterial Motility Detection: CTA is also used for the detection of bacterial motility. The semi-solid nature of the medium allows motile organisms to spread away from the line of inoculation, resulting in turbidity or cloudy growth. This aids in the assessment of bacterial motility, which can be an important characteristic for the identification and classification of microorganisms.
- Maintenance of Fastidious Organisms: Additionally, the base of CTA can serve as a holding medium for the maintenance of fastidious microorganisms. The medium provides the necessary nutrients and conditions to support the survival and growth of these organisms over an extended period, allowing for their storage and preservation.
Limitations of Cystine Tryptic Agar
Cystine Tryptic Agar (CTA) has certain limitations that should be considered when using the medium. These limitations include:
- Complete Identification: While CTA can aid in the identification of microorganisms, it is recommended to perform additional biochemical, immunological, molecular, or mass spectrometry testing on colonies from pure culture for a more comprehensive identification.
- Limited Acid Production: Neisseria organisms, such as gonococci, may produce only small amounts of acid as they primarily utilize carbohydrates oxidatively. This limited acid production can affect the interpretation of fermentation reactions on CTA.
- pH Indicator and Abnormal Reactions: Prolonged incubation on CTA may result in changes in the pH indicator or abnormal lactose/sucrose reactions with Neisseria pathogens. This can affect the reliability of the results and make interpretation challenging.
- Aerobic Incubation Requirement: CTA requires aerobic incubation, as incubation in CO2 may lead to erroneous results. Proper incubation conditions should be maintained to ensure accurate and reliable results.
- Heavy Inoculum Requirement: CTA requires a sufficient inoculum size for reliable results. A lack of adequate inoculum may lead to erroneous or inconclusive results, as there may not be enough microorganisms present to produce detectable reactions.
- Improper Inoculation Technique: Inoculating the medium improperly or not reaching the bottom of the tube can result in weak acid reactions. This can make it difficult to interpret the test results accurately.
- Alkaline By-Products and Reversion Reaction: Peptone utilization in CTA results in the production of alkaline by-products. Prolonged incubation can lead to a reversion reaction, where the alkaline by-products mask the acid by-products formed from carbohydrate utilization. This can affect the interpretation of fermentation reactions.
- Requirement for Additional Compounds: Some strains of gonococci may require additional compounds not provided by the CTA medium formulations. As a result, these strains may not grow or exhibit proper reactions on CTA, necessitating the use of alternative media.
FAQ
What is the purpose of Cystine Tryptic Agar?
Cystine Tryptic Agar is a versatile medium used for the identification, maintenance, and differentiation of microorganisms. It aids in assessing carbohydrate fermentation, detecting bacterial motility, and supporting the growth of fastidious organisms.
How is CTA different from other agar media?
CTA contains cystine and casein peptone, which provide essential nutrients for the growth of fastidious microorganisms. Additionally, it allows for the assessment of fermentation reactions and detection of motility.
What types of microorganisms can be identified using CTA?
CTA is commonly used for the identification of fastidious microorganisms such as Neisseria, pneumococci, streptococci, and non-spore-forming anaerobes.
Can CTA be used for determining carbohydrate fermentation patterns?
Yes, CTA can be supplemented with specific carbohydrates to determine the fermentation patterns of microorganisms. The change in color of the medium indicates positive or negative fermentation reactions.
How is motility assessed using CTA?
Motility is assessed by observing the growth pattern of microorganisms in CTA. Positive motility is indicated by turbidity or cloudy growth extending from the line of inoculation, while negative motility shows growth only along the stab line.
Does CTA require specific incubation conditions?
Yes, incubation of CTA should be done at a temperature of 35 ± 2°C. The incubation conditions (aerobic or anaerobic) may vary depending on the organisms being studied.
What are the limitations of CTA?
Some limitations of CTA include the need for biochemical or molecular testing for complete identification, limited acid production by certain organisms, and the requirement for a heavy inoculum for reliable results. Other limitations include the potential for abnormal reactions with prolonged incubation and the need for additional compounds for specific strains of microorganisms.
Can CTA be used for long-term storage of microorganisms?
CTA can serve as a holding medium for the maintenance of fastidious microorganisms, allowing for extended storage periods when kept under appropriate conditions.
Are there any specific recommendations for using CTA with Neisseria species?
For Neisseria species, tightly fitting caps are recommended during incubation, particularly in the case of tubes that need to be kept inside a CO2 incubator. Loose caps are advised when incubating in an incubator without CO2.
Is CTA suitable for all types of microorganisms?
While CTA is useful for a wide range of microorganisms, it may not be suitable for all strains or species. Some microorganisms may require additional compounds or specific media formulations for proper growth and identification.
References
- https://tools.thermofisher.com/content/sfs/manuals/IFU452981.pdf
- http://biotrading.com/assets/productinformatie/hardy/ifu/c5510-5514.pdf
- https://legacy.bd.com/europe/regulatory/Assets/IFU/Difco_BBL/252310.pdf
- https://www.fishersci.com/shop/products/remel-cta-w-1-sucrose-cystine-trypticase-agar/R060660
- https://us.vwr.com/store/product/11795683/cta-cystine-tryptic-agar-hardy-diagnostics
- https://hardydiagnostics.com/c5512
- https://hardydiagnostics.com/c5511
- https://www.mercedesscientific.com/buy/product/cystine-trypticase-agar/CLENCa0fb173b5095ab48c08b63bcc425ac48
- https://www.amerigoscientific.com/cta-cystine-tryptic-agar-with-lactose-3ml-13x100mm-tube-optically-clear-glass-tube-item-58998.html
Related Posts
- Robertson’s Cooked Meat Medium (RCM Medium) – Composition, Preparation, Uses
- Nutrient Agar – Principle, Composition, Preparation, Results, Uses
- Viral Transport Media (VTM) – Definition, Principle, Preparation, Application
- Decarboxylase Broth Protocol
- Culture Media Examples, Components and Primary Purpose