What is Integral Protein?
- Integral proteins, formally recognized as integral membrane proteins, are a distinct class of proteins characterized by their specific functional domains that anchor them securely within the cellular membrane. These proteins embed themselves within the membrane through regions enriched with particular amino acids that exhibit affinity towards the central portion of the plasma membrane. Such regions, predominantly hydrophobic in nature, interact with the hydrophobic internal layer of the plasma membrane.
- A quintessential feature of many integral proteins is their ability to traverse the plasma membrane multiple times. However, this is not a universal trait; certain integral proteins possess only a singular domain that penetrates the hydrophobic core of the membrane.
- The stability and positioning of these proteins within the membrane are governed by the synergistic effects of non-polar interactions and the repulsive forces exerted when attempting to penetrate a water-rich environment. Beyond their structural role, integral proteins can participate in a myriad of cellular reactions, underscoring their functional versatility.
- In the broader context of membrane proteins, integral proteins can be juxtaposed with peripheral proteins. While both associate with the plasma membrane, their modes of interaction differ markedly.
- Peripheral proteins primarily adhere to the phospholipid head groups and can be dislodged with relative ease, indicating a more transient association with the membrane. In stark contrast, the unique chemical environment surrounding integral proteins ensures their permanent residency within the plasma membrane.
- Furthermore, it is imperative to distinguish between integral, or intrinsic, membrane proteins (IMPs) and transmembrane proteins. While all transmembrane proteins fall under the category of IMPs, the converse is not true.
- IMPs constitute a substantial proportion of the protein repertoire encoded within an organism’s genome. When these proteins span the membrane, they are enveloped by annular lipids, which are specifically those lipids in immediate proximity to a membrane protein.
- The intimate association of these proteins with the membrane is so robust that their extraction necessitates the use of specialized agents like detergents, nonpolar solvents, or occasionally, denaturing agents.
Definition of Integral Protein
An integral protein is a type of protein that is permanently anchored within the cellular membrane, often spanning its entire width, and plays crucial roles in various cellular functions due to its specific functional domains.
Integral Protein Types
Integral proteins, a pivotal component of cellular membranes, can be broadly categorized into two primary types based on their interaction and orientation within the lipid bilayer. These classifications are:
- Integral Polytopic Proteins (Transmembrane Proteins)
- Transmembrane Protein (TM): This is the predominant form of integral polytopic proteins. These proteins traverse the entirety of the biological membrane.
- Single-pass Membrane Proteins: As the name suggests, these proteins cross the membrane a singular time. They can be further sub-classified into:
- Type I: Oriented such that their carboxyl-terminus faces the cytosol.
- Type II: Positioned with their amino-terminus directed towards the cytosol.
- Multi-pass Membrane Proteins: These proteins navigate through the membrane multiple times, creating a labyrinthine structure.
- Type III: Characterized by multiple transmembrane domains within a single polypeptide chain.
- Type IV: Comprises multiple polypeptide units that collectively form a conduit or channel through the membrane.
- Type V: These proteins are tethered to the lipid bilayer via covalently bonded lipids.
- Type VI: A hybrid category, these proteins possess both transmembrane domains and lipid anchors.
- Single-pass Membrane Proteins: As the name suggests, these proteins cross the membrane a singular time. They can be further sub-classified into:
- Transmembrane Protein (TM): This is the predominant form of integral polytopic proteins. These proteins traverse the entirety of the biological membrane.
- Integral Monotopic ProteinsIntegral monotopic proteins exhibit a unique interaction with the cellular membrane. Unlike their polytopic counterparts, they associate with only one face of the membrane and do not traverse the entire lipid bilayer. Their association is unilateral, providing a distinct mode of interaction compared to transmembrane proteins.
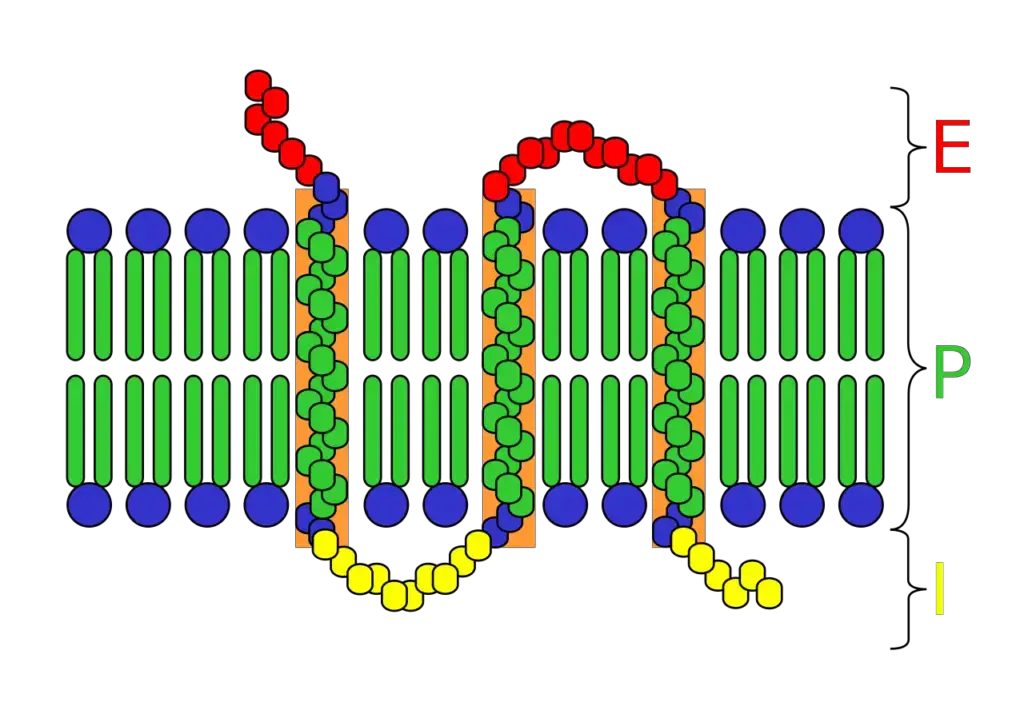
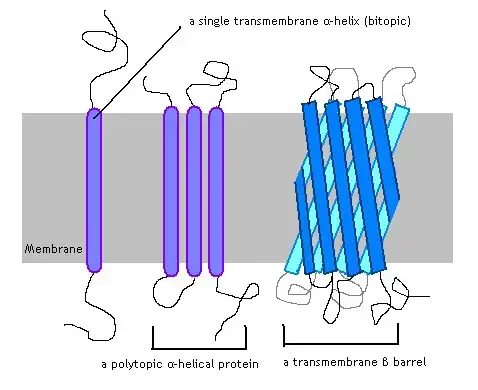
Integral Protein Structure
Integral proteins, vital components of cellular membranes, exhibit distinct structural configurations that facilitate their interaction with and within the lipid bilayer. The elucidation of their three-dimensional structures has been achieved through advanced techniques like X-ray crystallography and nuclear magnetic resonance spectroscopy. Despite the inherent challenges in studying these proteins, primarily due to extraction and crystallization complexities, significant insights have been garnered.
Three primary structural motifs are observed in integral proteins:
- Alpha (α) Helix:
- This configuration is characterized by a coiled chain of amino acids, reminiscent of a spiral staircase. The inherent non-polar nature of the alpha helix, primarily due to the sequence of amino acids, ensures its stable positioning within the hydrophobic region of the lipid bilayer.
- Some proteins, termed transmembrane proteins, employ multiple alpha helices to span the entirety of the membrane. This arrangement can lead to the formation of protein channels, facilitating the passage of specific molecules through the plasma membrane.
- Depending on their interaction with the lipid bilayer, integral proteins can be further classified. Integral monotopic proteins interact with only one face of the membrane, while transmembrane proteins traverse the entire lipid bilayer. The latter can be single-pass or multi-pass, based on the number of times they cross the membrane. Their categorization extends to Type I-VI, based on their orientation and interaction with the membrane.
- Beta Barrel:
- This structure is formed when multiple beta sheets, which are flattened chains of amino acids, extend through the membrane to create a pore. The exterior of these sheets is enriched with hydrophobic residues, ensuring the protein’s stable integration within the plasma membrane.
- Amphipathic Lipid Anchor:
- Distinct from the proteinaceous structures, the lipid anchor is a hydrophobic moiety covalently attached to certain proteins. This modification, which is not genetically encoded but rather introduced post-translationally, allows the protein to embed within the plasma membrane. The lipid anchor associates with the hydrophobic tails of the phospholipids, ensuring the protein’s stable positioning within the membrane.
Integral Protein Functions
Integral membrane proteins (IMPs) are pivotal entities embedded within cellular membranes, playing diverse and multifaceted roles essential for cellular function and intercellular communication. Their functions can be broadly categorized as follows:
- Transporters and Channels:
- IMPs facilitate the selective transport of molecules across the cell membrane. They can act as carriers, binding to specific molecules and transporting them across the membrane, or form channels that allow the passive diffusion of specific ions or molecules.
- Receptors:
- Certain IMPs function as receptors, detecting extracellular signals such as hormones. Upon ligand binding, these receptors undergo conformational changes, initiating intracellular signaling cascades that modulate cellular responses.
- Enzymes:
- Some IMPs possess enzymatic activity, catalyzing specific biochemical reactions at the membrane interface. Their membrane-bound nature ensures the localized action and regulation of these reactions.
- Structural Anchoring:
- IMPs provide structural integrity to the membrane, anchoring it to the cytoskeleton or extracellular matrix. This anchorage is crucial for maintaining cellular shape, stability, and facilitating cell movement.
- Signal Transduction:
- IMPs are instrumental in transducing extracellular signals into intracellular responses. They can relay information from the external environment to the cell’s interior, modulating cellular activities in response to external stimuli.
- Cell Adhesion:
- Certain IMPs mediate cell-cell or cell-matrix interactions, ensuring tissue integrity and facilitating processes like tissue repair, immune responses, and embryonic development.
- Energy Transduction:
- Complexes like ATP synthase, an integral protein residing on the inner mitochondrial membrane, exemplify the role of IMPs in energy transduction. Utilizing ion gradients, ATP synthase synthesizes ATP, the primary energy currency of the cell.
- Neurotransmitter Production:
- Some integral proteins associated with neurotransmitter synthesis are strategically positioned at neuron termini. This ensures the efficient release of neurotransmitters, facilitating neural signal transmission.
Examples of integral membrane proteins
Integral membrane proteins (IMPs) are pivotal components of cellular membranes, playing diverse roles in various cellular processes. Here are some notable examples of integral membrane proteins:
- Insulin Receptor:
- This receptor is crucial for glucose metabolism. Upon binding with insulin, it initiates a cascade of intracellular events, facilitating glucose uptake by cells.
- Cell Adhesion Molecules (CAMs):
- These proteins mediate cell-cell and cell-matrix interactions. Examples include:
- Integrins: Mediate cell attachment to the extracellular matrix and play roles in cell signaling.
- Cadherins: Facilitate calcium-dependent cell-cell adhesion.
- NCAMs (Neural Cell Adhesion Molecules): Prominent in neural cells, they aid in cell-cell interactions.
- Selectins: Involved in leukocyte-endothelial cell interactions.
- These proteins mediate cell-cell and cell-matrix interactions. Examples include:
- Receptor Proteins:
- These proteins detect extracellular signals, transducing them into intracellular responses. They play a pivotal role in cellular communication and response mechanisms.
- Glycophorin:
- Predominantly found in red blood cells, glycophorin is involved in maintaining cell shape and facilitating cell-cell interactions.
- Rhodopsin:
- A light-sensitive receptor protein, rhodopsin plays a central role in the phototransduction pathway in the retina, enabling vision.
- Band 3:
- This protein is essential for the exchange of chloride and bicarbonate ions across the plasma membrane, aiding in maintaining acid-base balance.
- CD36:
- A multifunctional protein, CD36 is involved in lipid uptake, inflammation, and angiogenesis.
- Glucose Permease:
- Facilitates the transport of glucose across cell membranes, ensuring cellular energy supply.
- Ion Channels and Gates:
- These proteins regulate the passage of ions across cellular membranes, maintaining cellular homeostasis and facilitating signal transduction.
- Gap Junction Proteins:
- These proteins form intercellular channels, allowing direct communication between adjacent cells.
- G Protein-Coupled Receptors (e.g., Beta-adrenergic receptor):
- These are a large family of receptors that, upon ligand binding, activate intracellular G proteins, initiating various cellular responses.
- Seipin:
- Involved in lipid droplet formation, seipin plays a role in lipid metabolism and storage.
Quiz Practice
Which of the following is NOT a function of integral membrane proteins?
a) Facilitating cell-cell adhesion
b) Acting as receptors for hormones
c) Synthesizing ATP
d) Storing genetic information
Which structural motif in integral proteins allows them to span the entire lipid bilayer?
a) Beta sheet
b) Alpha helix
c) Lipid anchor
d) Beta barrel
Which integral protein is involved in the phototransduction pathway in the retina?
a) Insulin receptor
b) Glycophorin
c) Rhodopsin
d) Band 3
Which of the following proteins is responsible for the exchange of chloride and bicarbonate ions across the plasma membrane?
a) CD36
b) Glucose Permease
c) Band 3
d) Seipin
Which type of integral protein facilitates the transport of glucose across cell membranes?
a) Ion channels
b) Glucose Permease
c) Gap junction proteins
d) G protein-coupled receptors
Which structure is formed when multiple beta sheets extend through the membrane, creating a pore?
a) Alpha helix
b) Beta sheet
c) Beta barrel
d) Lipid anchor
Which of the following is NOT a type of cell adhesion molecule (CAM)?
a) Integrins
b) Cadherins
c) Rhodopsins
d) Selectins
Which integral protein is involved in lipid uptake, inflammation, and angiogenesis?
a) CD36
b) Seipin
c) Glycophorin
d) Insulin receptor
Which of the following proteins is a light-sensitive receptor protein enabling vision?
a) Insulin receptor
b) Rhodopsin
c) Band 3
d) CD36
Which structural feature of integral proteins allows them to be embedded within the plasma membrane without spanning it entirely?
a) Alpha helix
b) Beta barrel
c) Lipid anchor
d) Beta sheet
FAQ
What are integral membrane proteins?
Integral membrane proteins are proteins that are permanently embedded within the plasma membrane of cells. They play crucial roles in various cellular functions due to their specific functional domains.
How do integral proteins differ from peripheral proteins?
Integral proteins are embedded within the lipid bilayer of the cell membrane, often spanning its entire width. In contrast, peripheral proteins are loosely attached to the surface of the membrane and can be easily removed without disrupting the membrane.
What are the primary functions of integral membrane proteins?
Integral membrane proteins serve various functions, including transporting molecules across the cell membrane, acting as receptors for signal transduction, facilitating cell adhesion, and participating in enzymatic activities.
How are integral proteins anchored to the membrane?
Integral proteins are anchored to the membrane through hydrophobic interactions with the lipid bilayer. Some also possess lipid anchors, which are hydrophobic moieties covalently attached to the protein.
What is the significance of the alpha helix and beta barrel structures in integral proteins?
The alpha helix and beta barrel are common structural motifs in integral proteins that allow them to span the lipid bilayer. The hydrophobic amino acids in these structures interact with the hydrophobic core of the membrane, stabilizing the protein within the bilayer.
Are all integral proteins transmembrane proteins?
No, while all transmembrane proteins are integral proteins, not all integral proteins span the entire membrane. Some may only interact with one face of the membrane.
How do integral proteins facilitate cell communication?
Many integral proteins act as receptors, detecting extracellular signals and initiating intracellular signaling cascades. This allows cells to respond to external stimuli and communicate with neighboring cells.
Why are integral proteins challenging to study?
Due to their hydrophobic nature and strong association with the lipid bilayer, integral proteins are difficult to extract and purify without disrupting their native conformation. This poses challenges for structural and functional studies.
Can integral proteins move within the membrane?
Yes, many integral proteins exhibit lateral mobility within the lipid bilayer, allowing them to interact with other membrane components and perform their functions.
What role do integral proteins play in diseases?
Dysfunctions or mutations in integral proteins can lead to various diseases. For instance, mutations in ion channel proteins can result in neurological disorders, while alterations in receptor proteins can lead to cancers or metabolic diseases.
References
- Whitelegge JP. Integral membrane proteins and bilayer proteomics. Anal Chem. 2013 Mar 5;85(5):2558-68. doi: 10.1021/ac303064a. Epub 2013 Feb 19. PMID: 23301778; PMCID: PMC3664232.
- https://aklectures.com/lecture/lipids-proteins-and-the-cell-membrane/integral-and-peripheral-membrane-proteins
- https://www.biologyonline.com/dictionary/integral-protein
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/integral-membrane-protein
- https://www.sciencedirect.com/topics/neuroscience/integral-membrane-protein
- https://biologydictionary.net/integral-protein/