What is a Bioreactor?
- A bioreactor, also known as a fermentation vessel, is a device or system designed to cultivate and grow biological cells, tissues, or organisms under controlled conditions. It serves as a vessel-like apparatus that provides a stable environment for microorganisms to flourish and maintains a steady balance in the biochemical processes carried out by these microorganisms to create desired substances.
- The history of bioreactors can be traced back to the early 20th century when the first stirred-tank bioreactors were developed. These early bioreactors were relatively simple in design, consisting of a tank with a stirrer to mix the contents. They were primarily used for the cultivation of microorganisms.
- In the following decades, bioreactor technology advanced significantly with the introduction of new types of bioreactors, such as airlift and bubble column bioreactors. These innovations improved oxygen transfer and mixing within the bioreactor, allowing for the cultivation of more oxygen-sensitive organisms.
- During the 1970s and 1980s, the use of bioreactors in the production of pharmaceuticals and other high-value products began to increase. Biotechnology companies developed new types of bioreactors, including perfusion bioreactors and hollow fiber bioreactors. These advancements enabled the large-scale cultivation of cells and tissues.
- In recent years, the field of bioreactor technology has continued to evolve with the introduction of microfluidic bioreactors and 3D bioprinting bioreactors. Additionally, advanced technologies like automation and process control have been integrated into bioreactor systems, enhancing their efficiency and effectiveness. These advancements have made bioreactors more widely used and accessible in various industries.
- The importance of bioreactors lies in their ability to efficiently produce a wide range of products. They are utilized in industries such as food production, pharmaceuticals, biofuels, and bioplastics. Bioreactors can produce enzymes, proteins, microbial biomass, vaccines, biofuels like ethanol and biodiesel, as well as other valuable substances.
- Furthermore, bioreactors are valuable in tissue engineering, where they provide a controlled environment for growing replacement tissues and organs for medical use. This controlled environment allows for the production of high-quality and consistent products.
- In addition to industrial applications, bioreactors are also used in research and development. They provide a controlled system for studying the growth and behavior of cells and organisms under different conditions, contributing to advancements in various scientific fields.
- In summary, bioreactors have played a crucial role in many industries, enabling efficient and effective production of a wide range of products. They have evolved over time in terms of design, capabilities, and applications, and continue to contribute to advancements in various fields of science and technology.
Definition of Bioreactor
A bioreactor is a device or system used to cultivate and grow biological cells, tissues, or organisms under controlled conditions.
Principle of Bioreactor
- The principle of a bioreactor revolves around providing an ideal environment for microorganisms to grow and produce metabolites through biotransformation and bioconversion processes. The bioreactor serves as the core component of any biochemical process.
- To ensure optimal growth and productivity, bioreactors can be engineered or manufactured according to the specific requirements of the microorganisms being used. These reactors are designed to create a controlled and favorable environment that supports the biological processes taking place within them.
- The primary function of a bioreactor is to facilitate the transformation of biological-based materials into desired products. This includes the production of various enzymes and the execution of bio-catalysis processes. By carefully controlling factors such as temperature, pH, aeration, agitation, nutrient feeding, and sterility, bioreactors create an environment conducive to the growth and metabolic activity of the microorganisms.
- Through the proper design and operation of a bioreactor, it is possible to maximize the yield and efficiency of the biochemical processes being carried out. This principle is applicable across a wide range of applications, from industrial production of enzymes and bio-catalysis to pharmaceutical manufacturing and biotechnological research.
- In summary, the principle of a bioreactor lies in providing an optimal environment for microorganisms to grow, thrive, and carry out biochemical processes for the production of desired products. By controlling the variables within the reactor, it becomes possible to harness the potential of biological-based materials and achieve efficient biotransformation and bioconversion processes.
An ideal Bioreactor Should Have Following Qualities
An ideal bioreactor should possess the following qualities:
- Aseptic Operation: The bioreactor vessel should be capable of operating aseptically for a few days. This ensures that the growth of microorganisms within the bioreactor remains free from contamination, allowing for the production of desired products without interference from unwanted organisms.
- Proper Agitation and Aeration: The bioreactor should provide efficient and effective agitation and aeration mechanisms. Agitation ensures proper mixing of cells and the growth medium, while aeration supplies oxygen for aerobic fermentations. These processes are vital for promoting optimal growth and metabolic activity of the microorganisms.
- Minimal Power Consumption: The bioreactor should be designed to minimize power consumption while maintaining optimal performance. This helps reduce energy costs and enhances the economic feasibility of bioreactor operations.
- Temperature and pH Control: The bioreactor should offer precise control over temperature and pH. Microorganisms have specific temperature and pH requirements for optimal growth and product formation. By maintaining these parameters within the desired ranges, the bioreactor provides an ideal environment for the microorganisms to thrive.
- Sampling Facilities: The bioreactor should have provisions for easy and convenient sampling. Regular sampling allows for monitoring and analysis of the growth and productivity of the microorganisms, enabling process optimization and quality control.
- Low Evaporation Losses: The bioreactor should minimize losses from evaporation during the fermentation process. Excessive evaporation can lead to a loss of valuable products and adversely affect process efficiency and productivity.
- Minimal Labor Requirement: The bioreactor should require minimal labor for production cleaning, harvesting, and maintenance. This reduces labor costs and streamlines the overall operation of the bioreactor system.
- Smooth Internal Surfaces: The internal surfaces of the bioreactor should be smooth to prevent the accumulation of microorganisms or debris, ensuring easy cleaning and maintenance.
- Containment: The bioreactor should be designed to prevent the leakage of viable cells from the fermenter or downstream equipment. This ensures the integrity of the production process and prevents potential contamination.
- Aseptic Protection: Aseptic operations in the bioreactor require protection from contamination. Proper measures should be in place to prevent the entry of unwanted microorganisms, ensuring the purity and quality of the cultured cells or organisms.
Bioreactor Design
- Bioreactor design plays a crucial role in the efficient operation and productivity of the bioprocess. The design and mode of operation are influenced by factors such as the organism being produced, the optimal conditions required for desired product formation, the value of the product, and the scale of production.
- A well-designed bioreactor can contribute to improved productivity, higher quality products, and lower production costs. To achieve this, a bioreactor typically consists of various features and systems. These may include an agitator system for proper mixing, an oxygen delivery system for aerobic processes, a foam control system to manage foam formation, and systems for temperature and pH control. Additionally, the bioreactor may have sampling ports for monitoring and analysis, as well as systems for cleaning and sterilization. Charging and emptying lines are also present for adding and removing materials from the reactor.
- When selecting materials for bioreactor construction, several important properties need to be considered. The material should be non-corrosive to ensure the integrity and longevity of the bioreactor. It should not introduce any toxic substances into the fermentation media, which could harm the microorganisms or affect product quality. The material should be able to withstand the steam sterilization process commonly used in bioreactors. Furthermore, it should be capable of tolerating high pressure and resisting changes in pH.
- The sizes of bioreactors can vary widely depending on the specific application. Bioreactors are available in a range of sizes to accommodate different scales of production. These sizes can range from small-scale fermenters for microbial cells (few mm3) to shake flasks (100-1000 ml), laboratory-scale fermenters (1-50 L), pilot-scale bioreactors (0.3-10 m3), and even large-scale plant bioreactors (2-500 m3) for industrial production.
- In summary, bioreactor design is a critical aspect of bioprocess engineering. It involves incorporating various features and systems to create an environment that promotes efficient growth and product formation. The choice of materials for construction and the selection of an appropriate size depend on the specific requirements of the application, ensuring optimal performance and successful bioproduction.
Important factors need to be consider in designing Bioreactors
Several important factors need to be considered when designing bioreactors, taking into account the specific characteristics of the biochemical processes involved:
- Product Characteristics: The value and volume of the product being produced influence the design requirements. Low-value and large-volume alcohol-based beverages may require simpler fermenters without the need for aseptic conditions. High-value and low-volume products, on the other hand, often require more complex processes and aseptic conditions to maintain product quality.
- Substrate and Product Levels: The levels of substrates (starting substances) and products in the reaction mixture need to be carefully controlled. Inadequate levels of substrates or the presence of excessive products can hinder the process. Maintaining optimal conditions for cell development, intracellular enzymes, and product formation, such as providing proper nutrition, salts, oxygen, and maintaining suitable temperature, reactant concentration, and pH within a narrow range, is crucial.
- Substances, Inhibitors, and Effectors: Specific substances, inhibitors, effectors, and metabolic products can have an impact on the rate and nature of the reactions and intracellular regulation. These factors need to be considered in the design to ensure optimal process performance.
- Unconventional Substrates and Contaminants: Microorganisms used in bioreactors can metabolize unconventional substrates or even contaminants present in raw materials, such as cellulose, minerals, starch, waste, and air pollution. Designing bioreactors that can handle such substrates, including highly viscous mediums, is important for efficient and effective bioprocessing.
- Microorganism Characteristics: Unlike isolated enzymes and chemicals, microorganisms can adjust the structure and function of their enzymes in response to process conditions. This adaptability can impact their productivity and selectivity. Additionally, microorganisms are susceptible to mutations, which can occur under certain conditions, necessitating careful design considerations.
- Environmental Influences: Microorganisms are often vulnerable to high shear stress, as well as chemical and thermal influences. Designing bioreactors that minimize these stresses and provide a stable and controlled environment is crucial for maintaining optimal microbial activity.
- Reaction Systems: Bioreactors typically involve gas-liquid-solid systems, with the liquid phase being predominantly aqueous. Designing appropriate mixing, aeration, and separation mechanisms is necessary to ensure efficient mass transfer and reaction kinetics.
- Dynamics and Growth: Continuous bioreactors, in particular, can exhibit complex dynamic behavior due to the continuous flow and varying conditions. Additionally, during biochemical conversion, the mass of microbial cells can grow, leading to effects such as growth on walls, flocculation, and autolysis. These growth-related phenomena need to be considered in bioreactor design.
Taking these factors into account when designing bioreactors helps ensure optimal performance, productivity, and product quality in biochemical processes.
Fermenter Design
A good fermenter must have the following features: Heat and oxygen transfer settings Sterilization processes and foam control, a fast and thorough cleaning system A proper monitoring and control system.
- Traditional designs are open-circular or rectangular vessels constructed from stone or wood.
- The majority of fermentations are conducted in close systems to prevent contamination.
- It should be constructed of non-toxic and corrosion-resistant materials.
- Small fermenters with a capacity of just a few liters are made of glass or stainless steel.
- Pilot scales and a variety of production vessels are constructed from stainless steel, with polished internal surfaces.
- Large fermenters are usually constructed of mild steel, and then lined with plastic or glass to cut down on costs.
- If an aseptic process is required the pipelines that transport inoculum, air and ingredients for fermentation have to be sterilized, normally with steam.
- The majority of vessel cleaning processes are now automated with spray jets and are referred to as Cleaning in Place (CIP). It is located inside the vessel.
- The pipework must be designed to limit the chance of microbial contamination. There shouldn’t be joints in the horizontal direction, or any unnecessary pipes and stagnant spaces that are dead where substances can gather; otherwise, the result could be ineffective sterilization.
- Typically, fermenters with a capacity of 1000 liters capacity are equipped with an outer jacket. larger vessels come with internal coils.
- Safety and pressure gauges valves should be used, (required during sterilization and operation).
- To transfer media, pumps are employed. Centrifugal pumps (generate high shear forces and provide a routes for easy contaminations) magnetically coupled jet and the peristaltic pumps.
- Alternative methods for liquid transfer include gravity feeding or vessel pressure
- In ferments operating at high temperatures or that contain volatile compounds A sterilizable condenser could be needed to stop the loss of evaporation.
- Fermenters are usually operated with positive pressure to stop the entry of contaminants.
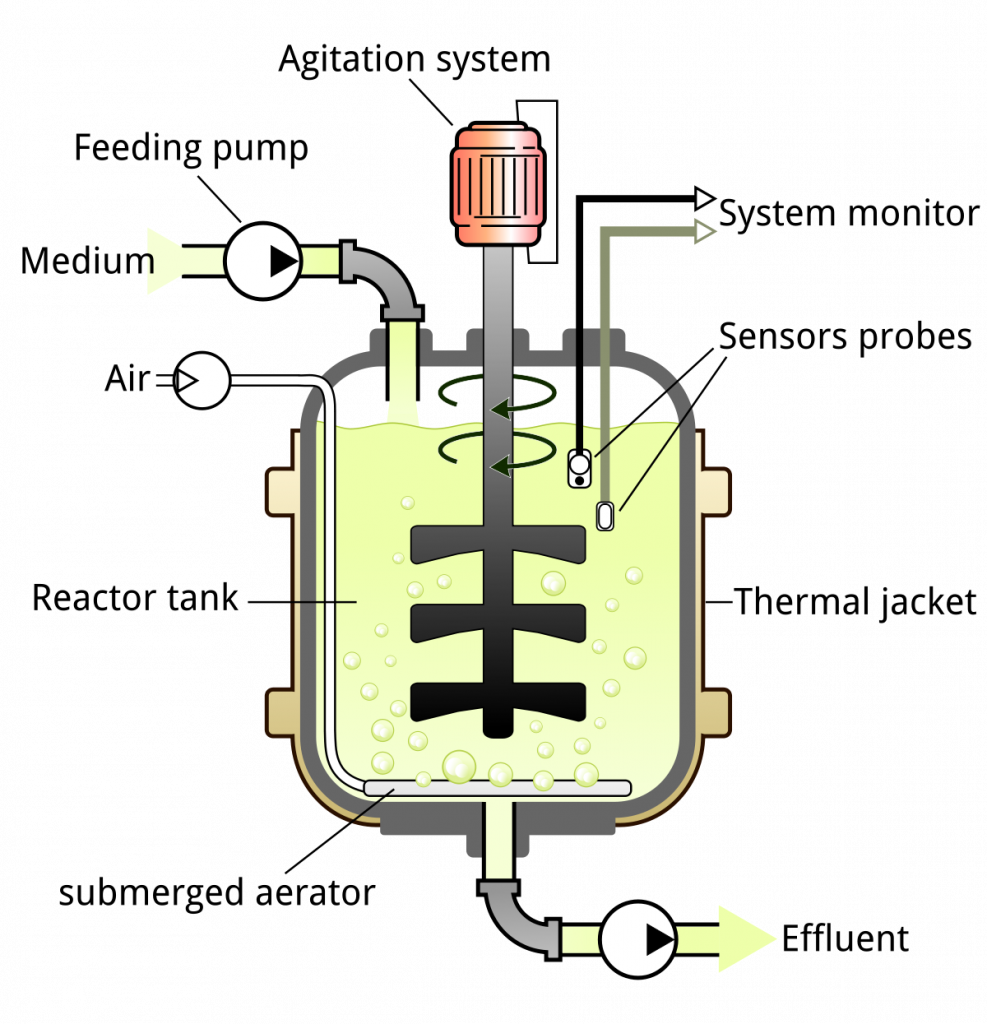
Parts of the bioreactor and their function
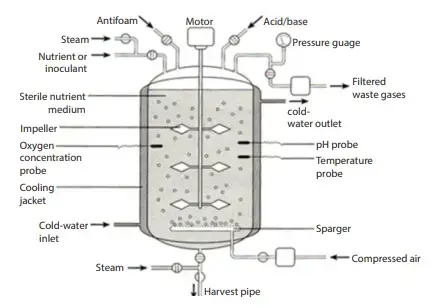
1. Fermenter Vessel/Vessel
- A fermenter vessel, also known as a fermentor, is a large cylindrical container that is closed at the top and bottom and connected with various pipes and valves. The design of the vessel is intended to allow for work under controlled conditions in bioprocesses.
- There are two main types of fermenter vessels used: glass vessels and stainless steel vessels. Glass vessels are typically used in small-scale industries. They are non-toxic and resistant to corrosion. The advantage of using glass vessels is that they provide transparency, allowing for easy observation and study of the internal reactions occurring within the vessel. Sterilization of glass vessels is typically performed using an autoclave. These vessels are relatively small, usually measuring around 60 centimeters in size.
- Stainless steel vessels, on the other hand, are commonly used in large-scale industrial applications. They are designed to withstand high pressure and resist corrosion. Stainless steel vessels are favored for their durability and ability to handle the rigorous demands of large-scale fermentations. The sterilization process for stainless steel vessels is typically performed in situ, meaning within the vessel itself.
- The vessel is carefully designed to minimize maintenance and ensure cleanliness during operation. The interior of the vessel is smooth to prevent the accumulation of residues or contaminants. Additionally, the vessel is constructed using cost-effective materials that deliver optimal performance.
- In summary, fermenter vessels play a vital role in facilitating controlled bioprocesses. Glass vessels are preferred for small-scale industries due to their non-toxicity, corrosion resistance, and ease of study. Stainless steel vessels are commonly used in large-scale industries, offering robustness, pressure resistance, and corrosion resistance. Both types of vessels are designed to support efficient and clean operations under controlled conditions.
2. Heating and Cooling Apparatus
Heating and cooling apparatus play a crucial role in maintaining optimal temperature conditions during fermentation in a fermentor vessel. Here is some information regarding these apparatus:
- Cooling Jacket: The exterior of the fermentor vessel is equipped with a cooling jacket, which seals the vessel and allows for the circulation of cooling water. The cooling jacket is necessary for sterilizing the nutrient medium and removing the heat generated during fermentation. It helps regulate and control the temperature inside the vessel.
- Heat Supply: To provide heat within the fermentor vessel, thermostatically controlled baths or internal coils are commonly used. Thermostatically controlled baths ensure precise temperature control by immersing the vessel in a temperature-controlled bath. Internal coils, on the other hand, transfer heat directly to the fermentor by circulating hot water or steam through the coils.
- Heat Removal: Excess heat generated during fermentation needs to be removed to maintain the desired temperature. Silicone jackets are often employed for heat removal. These jackets, placed around the vessel, are designed to dissipate excess heat and maintain temperature control. They are equipped with double-silicon mats with heating wires sandwiched in between. If the vessel exceeds its capacity and the silicone jacket surface becomes covered, internal coils may be used to circulate cold water and help remove the excess heat.
Maintaining precise temperature conditions is crucial in bioprocessing and fermentation, as it directly influences the growth and metabolic activity of microorganisms. The heating and cooling apparatus in a fermentor vessel enable the creation of optimal temperature environments for efficient and controlled fermentation processes.
3. Sealing Assembly
The sealing assembly in a fermenter is an important component that ensures proper agitation and prevents leakage of the stirrer shaft. Here is some information about the sealing assembly:
- Purpose of Sealing Assembly: The sealing assembly is used to seal the stirrer shaft in order to maintain proper agitation within the fermenter. It prevents any leakage of the shaft and helps maintain aseptic conditions during operation.
- Types of Sealing Assembly:
- a) Packed Gland Seal: This type of seal consists of multiple asbestos or non-asbestos packing rings that surround the stirrer shaft. These rings are compressed by a gland, which is pushed against the shaft. The packed gland seal provides a reliable seal and can be adjusted as needed. Periodic inspection and replacement of the packing rings are required to ensure effective sealing and to prevent heat absorption.
- b) Mechanical Seal: A mechanical seal consists of two main components – a stationary part and a rotating part. The stationary part is typically located within the bearing, while the rotating part is attached to the shaft. These two components are held together by springs, creating a seal that prevents leakage. In some cases, stem condensate is used to cool and lubricate the mechanical seals during operation.
- c) Magnetic Drives: Magnetic drives offer an alternative to conventional seals. They utilize magnets to transmit the rotational motion from an external drive to the stirrer shaft without the need for a physical shaft seal. There are two types of magnets involved – a driving magnet and a driven magnet. The driving magnet is positioned on the exterior of the fermenter’s head plate and connected to the drive shaft. The driven magnet is located on the opposite side of the shaft, secured within bearings on the interior face of the head plate. This arrangement allows for the transfer of motion without the need for a direct physical connection.
The choice of sealing assembly depends on factors such as the process requirements, aseptic conditions, and maintenance considerations. Each type of seal has its own advantages and considerations in terms of reliability, adjustability, and longevity.
In summary, the sealing assembly in a fermenter is responsible for sealing the stirrer shaft to ensure proper agitation. Different types of sealing assemblies, including packed gland seals, mechanical seals, and magnetic drives, are utilized depending on the specific requirements of the fermentation process. Proper selection and maintenance of the sealing assembly are essential for efficient and leak-free operation of the fermenter.
4. Baffles
Baffles are important components incorporated into fermenters to serve multiple purposes, such as preventing the formation of a vortex and improving aeration within the fermenter. They are typically made of metal strips and are attached radially to the wall of the fermenter. Here is some more information about baffles:
- Vortex Prevention: The primary function of baffles is to prevent the formation of a vortex or swirling motion in the liquid inside the fermenter. A vortex can interfere with proper mixing and agitation of the fermentation broth. By strategically placing the baffles, they disrupt the circular flow patterns and promote more uniform mixing and circulation of the liquid.
- Aeration Improvement: Baffles also play a role in improving aeration within the fermenter. They help to distribute the incoming air or oxygen evenly throughout the liquid medium, ensuring that the microorganisms receive adequate oxygen for growth and metabolism. By enhancing the aeration process, baffles contribute to the optimal functioning of the fermentation process.
- Baffle Design: Baffles are typically constructed as flat metal strips that are attached to the inner wall of the fermenter. The number, size, and positioning of the baffles depend on the specific requirements of the fermentation process. The arrangement of the baffles is designed to create a desired flow pattern that promotes efficient mixing and aeration.
- Impact on Mixing and Mass Transfer: The presence of baffles in the fermenter improves mixing and enhances mass transfer processes. They help to break up any stagnant regions within the fermentation broth, ensuring that nutrients, gases, and metabolites are well distributed throughout the liquid medium. This promotes the growth and activity of the microorganisms and facilitates efficient biochemical reactions.
- Baffle Material: Baffles are commonly made of materials such as stainless steel or other corrosion-resistant metals. These materials are suitable for the harsh and demanding conditions of the fermentation process, ensuring longevity and resistance to corrosion.
In summary, baffles are integral components of fermenters that serve to prevent vortex formation and improve aeration within the vessel. By disrupting swirling motion and promoting even distribution of air or oxygen, baffles contribute to efficient mixing, mass transfer, and optimal growth of microorganisms during fermentation.
5. Impeller
Impellers play a crucial role in the operation of fermenters by ensuring the uniform suspension of microbial cells in various nutrient media. They consist of blades attached to a motor on the lid of the fermenter. Here is some information about impellers:
- Suspension of Microbial Cells: The primary function of impellers is to create agitation and mixing within the fermentation vessel, ensuring that the microbial cells are uniformly suspended in the nutrient medium. This promotes efficient interaction between the cells and the nutrients, facilitating optimal growth and metabolic activity.
- Design and Construction: Impellers are typically composed of blades that are attached to a central hub. The impeller assembly is connected to a motor located on the lid of the fermenter. The number, shape, and size of the blades can vary depending on the specific requirements of the fermentation process.
- Bubble Reduction and Distribution: Impeller blades also play a critical role in reducing the size of air bubbles introduced during aeration and distributing them uniformly throughout the fermentation media. This helps to enhance oxygen transfer and promote better gas-liquid mass transfer within the vessel.
- Types of Impellers: Various types of impellers are used in fermenters, and their selection depends on factors such as the characteristics of the microorganism, the properties of the nutrient medium, and the desired process outcomes. Two common types of impellers used in fermenters are:
- a. Disc Turbines: These impellers consist of a series of flat, disc-shaped blades. They are known for their efficient gas-liquid mixing and are particularly effective in high-viscosity media. The disc turbines create strong radial flow patterns, ensuring good agitation throughout the vessel.
- b. Variable Pitch Open Turbine: This type of impeller features blades with a variable pitch angle. The varying pitch helps to generate better flow and shear rates, improving mixing efficiency. Variable pitch open turbines are often used when a more intense mixing action is required.
Impellers are an essential component of fermenters, providing the necessary agitation and mixing to maintain uniform suspension of microbial cells and promote efficient nutrient utilization. Their design and selection depend on the specific process requirements and characteristics of the fermentation system. By reducing bubble size and ensuring uniform distribution, impellers contribute to optimal gas-liquid mass transfer and overall process performance.
6. Sparger
A sparger plays a vital role in ensuring proper aeration within a fermentation vessel by introducing sterile air. It is a system that helps in promoting the growth and metabolism of microorganisms during fermentation. Here is some information about spargers:
- Aeration and Oxygen Supply: The primary function of a sparger is to provide a controlled and sterile air supply to the fermentation vessel. The sparger pipes are equipped with small holes, typically measuring 5-10 mm in diameter, through which pressurized air is released into the vessel. This air supply facilitates the transfer of oxygen to the microbial culture, supporting their respiration and metabolism.
- Types of Spargers: There are three main types of spargers used in fermentation processes:
- a. Porous Sparger: This type of sparger consists of a porous material, such as sintered metal or ceramic, which allows air to pass through evenly distributed pores. The porous structure helps in creating fine air bubbles, which enhances the contact between air and the fermentation medium, leading to efficient oxygen transfer.
- b. Nozzle Sparger: Nozzle spargers utilize a system of nozzles or orifices to release pressurized air into the fermentation vessel. These nozzles are strategically positioned to ensure proper distribution of air throughout the medium. Nozzle spargers are known for providing strong agitation and effective mixing, resulting in improved oxygen transfer.
- c. Combined Sparger-Agitator: In some cases, the sparger is integrated with an agitator system within the fermentation vessel. This combined sparger-agitator design helps in achieving both aeration and agitation simultaneously. The sparger component introduces air, while the agitator ensures proper mixing and distribution of the air bubbles, maximizing oxygen transfer.
Spargers are essential for maintaining adequate oxygen levels in the fermentation process, which is critical for the growth and metabolic activity of microorganisms. By releasing pressurized air through small holes or pores, spargers facilitate proper aeration and promote efficient oxygen transfer within the fermentation vessel. The selection of the sparger type depends on factors such as the specific fermentation process, vessel design, and desired aeration characteristics.
7. Feed Ports
Feed ports play a crucial role in the operation of a fermentor by allowing the addition of nutrients, acids, alkalis, or other substances necessary for the fermentation process. Here is some information about feed ports:
- Nutrient Addition: Feed ports are used to introduce nutrient solutions into the fermentor. These solutions contain essential components required for microbial growth and metabolism. The nutrients can include sugars, salts, vitamins, and other compounds necessary for the specific fermentation process.
- Acid and Alkali Addition: In addition to nutrients, feed ports also enable the controlled addition of acid or alkali solutions to the fermentor. This helps in regulating the pH of the fermentation medium, as maintaining the optimal pH range is crucial for the growth and activity of microorganisms.
- Construction: Feed ports are typically designed as tubes made of silicone or other suitable materials that are compatible with the fermentation process. Silicone is commonly used due to its flexibility, chemical resistance, and ability to withstand sterilization processes.
- In-situ Sterilization: Before the addition or removal of products or nutrients through the feed ports, in-situ sterilization is performed. This sterilization process ensures that the added substances are free from contamination and do not introduce any unwanted microorganisms into the fermentor. Sterilization can be achieved through methods such as autoclaving, heat treatment, or chemical disinfection, depending on the specific requirements and characteristics of the fermentation process.
Feed ports provide a controlled and sterile pathway for the addition of nutrients, acid/alkali solutions, or other substances to the fermentor. They are designed to maintain aseptic conditions and prevent contamination during the fermentation process. By enabling precise control over the nutrient composition and pH of the fermentation medium, feed ports contribute to the successful and efficient progress of the fermentation process.
8. Foam Control
Foam control is a critical aspect of fermentor operation to prevent excessive foam formation, which can lead to contamination and interfere with the proper functioning of the fermentation process. Here is some information about foam control:
- Importance of Foam Control: Excessive foam in a fermentor can cause problems such as blockage of air filters, loss of valuable product, and contamination of surrounding equipment. Therefore, it is necessary to minimize foam formation to maintain optimal fermentation conditions.
- Foam-Sensing Unit: A foam-sensing unit is employed to detect the presence of foam in the fermentor. It typically consists of sensors or probes that monitor the foam level. These sensors can be designed to detect changes in electrical conductivity, capacitance, or optical density caused by the presence of foam.
- Control Unit: The foam-sensing unit is connected to a control unit, which receives signals from the sensors and regulates the foam-controlling device. The control unit uses the foam level information to adjust the operation of the foam-controlling device and maintain the desired foam level in the fermentor.
- Foam-Control Device: The foam-control device is usually mounted on top of the fermentor, with an inlet that connects to the fermentor. It can take various forms depending on the specific application. Common foam-control devices include foam breakers, foam traps, or foam skimmers. These devices are designed to break down foam bubbles or capture and remove foam from the fermentor, thus preventing excessive foam buildup.
By detecting the presence of foam and adjusting the operation of the foam-controlling device, foam control units help maintain the foam level within acceptable limits. This ensures a clean and efficient fermentation process while minimizing the risk of contamination. Effective foam control contributes to the overall productivity, stability, and success of the fermentation operation.
9. Valves
Valves play a crucial role in controlling the flow and movement of liquids in a fermentor vessel. They are used to regulate the passage of fluids, allowing for precise control over the fermentation process. Here are some key points about valves in fermentors:
- Purpose of Valves: Valves are employed to control the flow rate, direction, and pressure of liquids within the fermentor. They enable the adjustment and isolation of various process streams, facilitating efficient operation and maintenance of the fermentation system.
- Types of Valves:
- Globe Valve: Globe valves are commonly used in fermentors for their ability to provide precise flow control. They feature a spherical-shaped body with an internal baffle and a movable plug that regulates the flow through the valve.
- Butterfly Valve: Butterfly valves are characterized by a disc-shaped closure element that rotates within the valve body to control the flow. They are often used for larger pipe diameters and provide quick and easy shutoff.
- Ball Valve: Ball valves have a spherical closure element (a ball) with a hole in the center. When the ball is aligned with the flow path, the valve is open, and when rotated 90 degrees, the valve is closed. Ball valves offer fast operation and tight sealing.
- Diaphragm Valve: Diaphragm valves use a flexible diaphragm as the closure element. By compressing or releasing the diaphragm, the flow is regulated. These valves are suitable for controlling corrosive or abrasive fluids.
- Safety Valve: A safety valve is an essential component in the air and pipe layout of the fermentor. It is designed to automatically release excess pressure to prevent over-pressurization and ensure the safety of the system and personnel.
- Functionality and Control: Valves can be manually operated or controlled automatically through pneumatic or electric actuators. Automated valve systems allow for precise and remote control of the fermentation process, enhancing operational efficiency and accuracy.
- Installation and Maintenance: Valves are strategically placed within the fermentor system to facilitate fluid flow and ensure ease of access for maintenance and repair. Proper installation, regular inspection, and maintenance are necessary to ensure valves function effectively and avoid any potential leaks or malfunctions.
In summary, valves in a fermentor vessel enable precise control over the movement of liquids, allowing for optimal operation and regulation of the fermentation process. By selecting the appropriate valve type and ensuring proper installation and maintenance, operators can maintain efficient flow control and enhance the overall performance of the fermentor system.
10. Aeration System
The aeration system plays a crucial role in a fermentor, especially in aerobic fermentation processes. Here is some information about the aeration system:
- Importance of Aeration: In aerobic fermentation, microorganisms require oxygen for their growth and metabolic processes. Without proper aeration, the bacteria or yeast cells would not be able to grow and digest the substrate effectively. Insufficient oxygen supply can lead to lower production of desired products and higher levels of byproducts like carbon dioxide.
- Aeration Devices: The fermentor’s aeration system typically consists of two separate devices, namely an impeller and a sparger. The impeller, often a propeller-like device, helps to stir and mix the gas bubbles within the liquid culture medium. This promotes the dispersion of oxygen throughout the fermentation broth. The sparger, on the other hand, introduces oxygen into the liquid by releasing air or oxygen bubbles through fine pores or nozzles.
- Mixing and Oxygen Availability: Stirring in the fermentor serves two important purposes. Firstly, it facilitates the mixing of gas bubbles with the liquid culture medium, ensuring better contact between the oxygen and microorganisms. This maximizes the oxygen transfer efficiency. Secondly, stirring also helps to mix the microbial cells throughout the liquid culture medium, ensuring that they have equal access to nutrients and oxygen. This promotes uniform growth and metabolic activity of the microorganisms.
- Selecting a Reliable Aeration System: Choosing a suitable and reliable aeration system is crucial to ensure proper oxygen availability and aeration throughout the fermentation process. The system should be capable of providing adequate oxygen supply for the desired microbial growth and product formation.
In summary, the aeration system is an essential component of a fermentor, particularly in aerobic fermentation processes. It ensures proper oxygen supply for the microorganisms’ growth and metabolism, leading to efficient production of desired products. The combination of an impeller and a sparger in the aeration system helps to mix gas bubbles, distribute oxygen, and provide uniform nutrient access for microbial cells.
11. Controlling devices for environmental factors
- Bioprocess industries have always struggled with controlling devices. Bioreactor design must consider many parameters such as temperature, pH, dissolved oxygen and carbon dioxide concentrations. These should all be controlled at certain levels during the process. This will control growth, reduce contamination, improve production rate and increase product-quality.
- This will allow you to better control the environment in a bioreactor.
- These devices will enable us to monitor the temperature, carbon dioxide, oxygen concentration, and pH of the reactor at any time.
- We also want to offer an interface that allows users to program parameters such as the amount of nutrients provided and the rate at which these are added.
- Many devices can be used to regulate environmental elements such as temperature, oxygen concentrations, pH, cell mass and essential nutrients levels.
12. Fermenter using Computer
- Fermentors can be paired with semi-automatic and automated computers to improve process efficiency, data collection, and monitoring.
- Students will have more information because computers are used in fermenters. The output of each fermentation chamber will be visible to students. Students will be able view temperature and progress of each fermenter, keep track of activity and compare results between batches. This will help them understand how microbiology works at every stage of the process.
- Although the fermenter’s computer cannot be used continuously, some users claim that it can do a decent job maintaining temperature stability if it is switched off between batches.
- The fermenter is a computer-controlled device that monitors fermentation activity and automatically adjusts pH levels. It also pumps CO2 into the mixture to maintain a constant level.
Bioreactor Types
The different types of fermentors such as;
- Continuous Stirred Tank Bioreactors
- Bubble column bioreactors
- Air-lift bioreactors
- Packed Bed Reactors
- Fluidized Bed Bioreactor
- Photobioreactor
- Membrane Bioreactor
- Rotary Drum Bioreactor
- Mist Bioreactor
- Immobilized cell bioreactor
- Activated sludge bioreactor
- Immersed membrane bioreactor
- Reverse membrane bioreactor
1. Continuous Stirred Tank Bioreactors
- A continuous stirred tank fermentor (CSTF) is a commonly used type of bioreactor in industrial applications. It consists of a cylindrical vessel with a central shaft controlled by a motor, supporting one or more agitators or impellers. The combination of the sparger and impellers allows for efficient distribution of gases throughout the vessel, promoting optimal conditions for microbial growth and fermentation.
- The CSTF offers several advantages in bioprocessing. It can be operated continuously, allowing for a steady supply of nutrients and efficient removal of products. Temperature control is easily achieved in the fermentor, ensuring optimal conditions for microbial activity. The construction of a CSTF is cost-effective, making it a practical choice for large-scale production. It is relatively easy to operate, resulting in lower labor costs, and the vessel is easy to clean, facilitating maintenance and preventing contamination.
- The cylindrical vessel of a CSTF typically has a height-to-diameter ratio (aspect ratio) ranging from 3 to 5. Baffles are often installed inside the vessel to prevent swirling and vortices. These baffles protrude from the vessel walls and their width is generally one-tenth or one-twelfth of the tank diameter. However, in animal cell culture applications, which are more sensitive to turbulence, vessels are usually unbaffled to minimize potential cell damage.
- The number and positioning of impellers in a CSTF depend on the aspect ratio of the vessel. Typically, the bottom impeller is located about one-third of the tank’s diameter above the bottom. Additional impellers are spaced between one and two diameters apart. The impeller types used vary depending on the application. Gas dispersion impellers like Rushton disc turbines and concave bladed impellers have a diameter of approximately one-third of the vessel diameter. For highly viscous mycelial broths, larger hydrofoil impellers with diameters of 0.5 to 0.6 times the tank diameter are used as they provide excellent bulk mixing.
- In animal cell culture vessels, a single, large-diameter, low-shear impeller such as a marine propeller is commonly employed. Gas is introduced into the liquid through a sparger, either a perforated pipe ring with a slightly smaller diameter than the impeller or a single-hole sparger. The impeller speed in vessels larger than 50 liters generally does not exceed 120 revolutions per minute, especially in animal or plant cell culture. For microbial cultures, except for mycelial and filamentous cultures, higher stirring rates are used, while keeping the impeller tip speed below 7.6 m/s to prevent damage to certain mycelial fungi.
- To ensure effective gas dispersion, the superficial aeration velocity (gas flow rate divided by the vessel’s cross-sectional area) should remain below the flooding point of the impeller. Flooding occurs when the impeller receives more gas than it can disperse effectively, resulting in poor mixing. Typically, the superficial aeration velocity does not exceed 0.05 m/s.
- Stirred tanks, such as the CSTF, are widely utilized in the production of antibiotics and organic acids due to their versatility, efficient mixing capabilities, and ease of operation. They offer a reliable and scalable solution for various bioprocesses in the pharmaceutical, chemical, and biotechnology industries.
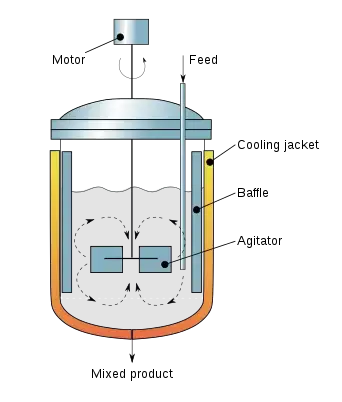
Features of Stirred Tank Bioreactors
Stirred tank bioreactors, also known as stirred tank fermenters, are bioreactors that are designed to hold and mix a liquid culture of microorganisms or cells. They typically consist of a cylindrical tank with a stirrer or impeller to mix the contents and provide oxygen for respiration. Some key features of stirred tank bioreactors include:
- Agitation: The stirrer or impeller is used to mix the culture and provide oxygen for respiration. The type and speed of agitation can be adjusted to optimize growth conditions.
- Temperature control: The temperature of the culture is usually maintained at a specific value by heating or cooling the tank.
- pH control: The pH of the culture is usually maintained at a specific value by adding acid or base as needed.
- Aeration: Oxygen is supplied to the culture either through the stirrer or by bubbling air or oxygen through the culture.
- Sterilization: The bioreactor and its associated equipment can be sterilized to prevent contamination of the culture.
- Monitoring and control: Various sensors and control systems are used to monitor and control the conditions inside the bioreactor.
- Scalability: Stirred tank bioreactors can be scaled up or down depending on the desired production volume.
Working Mechanism of Stirred Tank Bioreactors
- In bioreactors with stirred tanks, it is possible to add air into the medium under pressure using an instrument called a sparger.
- The sparger could be a ring with a number of holes or a tube having only one orifice.
- The sparger in conjunction together with the impellers (agitators) allows for a better gas distribution throughout the vessel.
- The bubbles produced by the sparger are crushed down to smaller ones through impellers and scattered across the medium.
- This creates an even and uniform environment within the bioreactor. This allows the bioprocess to run efficiently.
- The bioprocess continues to produce the desired end product through the vent.
Advantages of Stirred Tank Bioreactors
- Continuous operation.
- Excellent temperature control.
- It is easy to adapt easily to easily adapt to.
- Control of parameters is good and also the environmental conditions.
- The simplicity of construction 6. Flexible and low operating (labor) costs and investment requirements.
- Clean and easy to maintain.
- can handle the highest concentrations thanks to its high heat transfer.
- Efficacious gas transfer to developing cells, and mixing of contents.
Disadvantages of Stirred Tank Bioreactors
- The requirement for bearings and shaft seals.
- Limitation of size by motor size as well as shaft length and weight.
- The problem of foaming can be a major one.
- Power consumption is increased because of the Mechanical pressure pumps.
Application of Stirred Tank Bioreactors
- The most effective continuous methods to date have relied on the yeast and bacteria where the most desired products are cells.
- Production of the primary metabolites, enzymes and amino acids.
- The process of producing alcohol(product evidently linked with growing or energy-producing mechanisms).
- The most popular is the process of activated sludge employed in the wastewater treatment industries.
2. Bubble column bioreactors
- A bubble column fermentor is a cylindrical vessel used in various biochemical and chemical processes, including fermentation and biological wastewater treatment. It is a simple and cost-effective reactor design that facilitates gas-liquid contact and mass transfer.
- The bubble column fermentor consists of a cylindrical vessel equipped with a gas sparger, typically located at the base of the column. The sparger introduces air or gas into the liquid phase through perforated pipes, perforated plates, or metal micro-porous spargers. The rheological properties of the fluid and the gas flow rate play a significant role in mixing and other performance factors.
- To enhance mass transfer and modify the vessel’s design, internal devices such as horizontal perforated plates, vertical baffles, and corrugated sheet packings can be installed. These internal structures promote better gas-liquid mixing and improve overall reactor performance.
- The aspect ratio of a bubble column, which is the ratio of its height to diameter, typically ranges from 4 to 8. However, the column diameter has minimal impact on reactor behavior, except for axial mixing, which improves with increasing vessel diameter.
- Gas flow rate and rheological properties of the fluid are key factors influencing oxygen transfer, mixing efficiency, and overall performance. Increasing the gas flow rate enhances mass and heat transfer as well as the predominant shear rate within the column. However, the highest aeration velocity in bubble columns rarely exceeds 0.1 m/s.
- The liquid flow rate does not significantly affect the gas-to-liquid mass transfer coefficient as long as the surface liquid velocity remains below 0.1 m/s. This makes bubble columns suitable for aerobic fermentations and the biological treatment of wastewater, particularly when dealing with less viscous fluids.
- Bubble column fermentors are favored for their simplicity in construction, ease of maintenance, and low operating costs. They find applications in various industries, including biochemical, chemical, and petrochemical sectors, where gas-liquid contact and mass transfer are crucial for the desired processes.
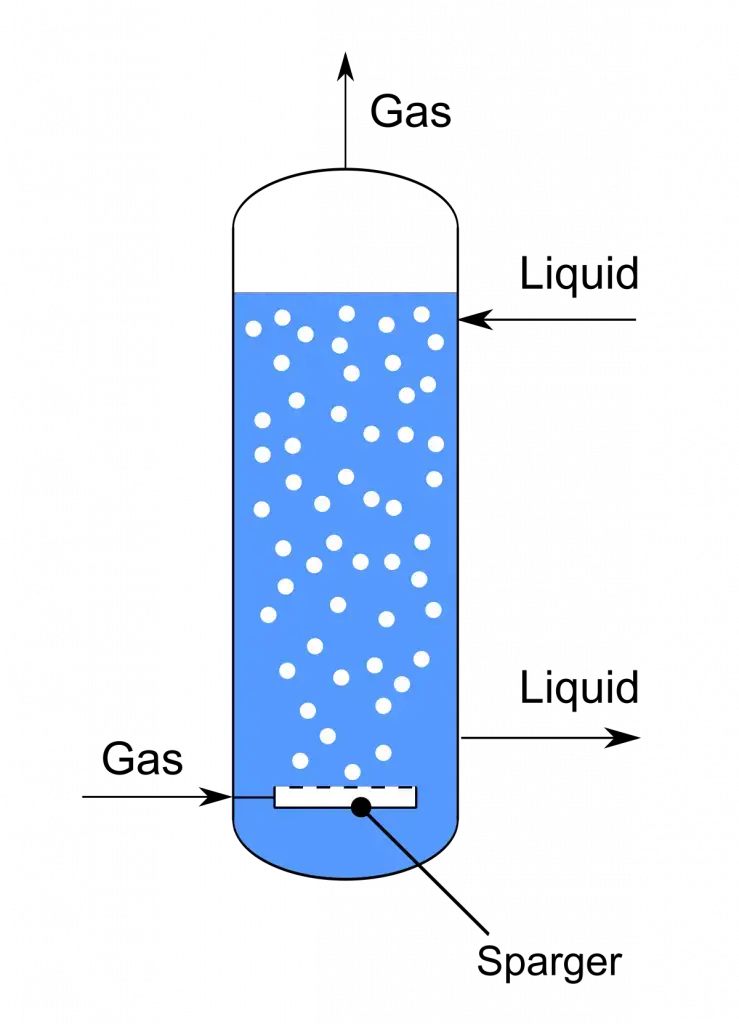
Features of Bubble column bioreactors
- The ratio for height-to-diameter is usually between 4-6.
- Gas is sucked at the bottom by perforated pipes or plates , or metal spargers with porous materials.
- O2 transfer, mixing , and other performance parameters are affected mostly by the gas flow rate as well as the rheological characteristics of the gas.
- Mixing and mass transfer could be improved by putting perforated plates, or baffles with vertical sides within the vessel.
- Doesn’t contain any draft tubes.
Mechanisms of Bubble column bioreactors
- In the bioreactor bubble column the gas or air is introduced into the bottom of the column by perforated pipes, plates, or through metal micro porous spargers. This creates an unstable stream that allows gas exchange.
- The flow rate of gas or air affects the performance factors O2 transfer mixing.
- The bubble column bioreactors can be equipped with perforated plates for improved the efficiency.
- The reactants are compressed by a finely dispersed catalyst , and so create the product using the process of fermentation.
Advantages of Bubble column bioreactors
- High volumetric efficiency and outstanding heat management.
- Greater utilization of the plate’s area as well as flow distrubution.
- Self-regulating.
Disadvantages of Bubble column bioreactors
- Inefficient compared to other bioreactors.
- Doesn’t have draft tube
- A higher consumption of catalysts that the bed fixed
- Installation costs are higher, and the design is difficult to create
Applications of Bubble column bioreactors
- The reactor is used extensively for the cultivation of herring-sensitive organisms. E.g. Plant cells and mould
- Chemical and pharmaceutical production.
- Also, for fermentation processes.
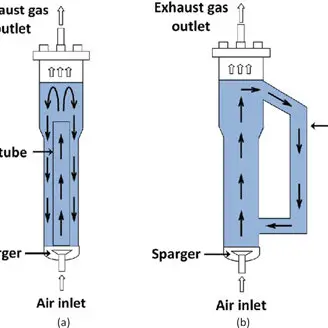
3. Air-lift bioreactors
- An airlift fermentor, also known as an airlift bioreactor or tower reactor, is a type of bioreactor used for gas-liquid or gas-liquid-solid processes. It utilizes a two-zone system to enhance circulation, oxygen transfer, and force equalization within the reactor.
- In an airlift bioreactor, the fluid volume is divided into two interconnected zones. One zone, known as the riser, is not sparged with gas, while the other zone, called the downcomer, is sparged with gas. The riser and the downcomer can be separate vertical pipes joined at the top and bottom to form an external circulation loop. The ratio of cross-sectional areas between the riser and downcomer affects gas-to-liquid mass transfer performance and is typically between 1.8 and 4.3.
- One advantage of airlift bioreactors is their simplicity of design. They do not contain any moving parts or agitators, making them easy to sterilize and maintain. They also have low energy requirements and are cost-effective compared to other types of bioreactors. Airlift bioreactors are commonly used in aerobic bioprocessing, where controlled liquid flow is achieved in a recycling system using pumps.
- The gas-liquid dispersion in the riser flows upward due to its lower bulk density, while in the downcomer, it flows downward. This circulation is driven by the difference in gas holdup between the two zones. Airlift bioreactors are known for their energy efficiency and are particularly suitable for shear-sensitive cultures, making them ideal for the mass production of biopharmaceutical proteins derived from delicate animal cells.
- Airlift devices are also utilized in applications such as high-rate biotreatment of wastewater, production of insecticidal nematode worms, and low-viscosity fermentations. They offer comparable heat and mass transfer capacities to other systems and outperform bubble columns in suspending solids.
- The liquid circulation rate in an airlift bioreactor is influenced by the gas injection rate. Increasing the height of the airlift device proportionally increases the rate of liquid circulation. Gas-liquid separators are sometimes employed in the head zone of the reactor to limit or eliminate gas carryover to the downcomer. Well-designed separators improve liquid circulation by enhancing the driving force for circulation, compensating for any increased flow resistance caused by the separator.
- Overall, airlift fermentors provide efficient gas-liquid mixing, favorable mass transfer characteristics, and low shear environments, making them valuable tools in various bioprocessing applications.

Features of Air-lift bioreactors
- Two zones are separated The zone that is sparged is referred to as the riser and the zone that is fueled by no gas is called the downcomer.
- The density in the region of riser is less than in the downcomer area which causes the circulation (so the circulation will be enhanced when there is less or no gas in the region down).
- For maximum mass transfer the riser-to-downcomer cross-sectional area ratio should fall between 1.8 to 4.3.
- The rate of circulation of liquid increases by an increase in the square of an airlift system. Thus the reactors are built with large aspect ratios.
- A gas-liquid separator located in the head-zone could reduce gas carry-over to the downstream and, consequently, improve the capacity of the

Mechanisms of Air-lift bioreactors
- The performance of the bioreactors with airlift depend on pumping (injection) by air as well as the circulation of liquid.
- It differs than that of the Stirred tank bioreactor, which requires the heating coat or plate around the tank to create a an insulated bioreactor. It is obvious to see that Airlift bioreactor is more efficient in removing heat in comparison to the Stirred tank.
Two-stage airlift bioreactors
- Two-stage airlift bioreactors are utilized for the formation of temperature-dependent batches of substances.
- Cells that are growing from an individual bioreactor (maintained at 30degC) are transferred to another bioreactor (at temperature of 42degC).
- There is a need for the airlift bioreactor with two stages as it is difficult to quickly raise the temperature from 30degC up to 42degC in an identical vessel.
- Each of the bioreactors is equipped with valves and are connected via pumps and transfer tubes.
- The cells are produced within the bioreactor, and the bioprocess itself is carried out in the second one.
Advantages of Air-lift bioreactors
- Highly efficient in terms of energy efficiency and productivity. are similar to stirred tank bioreactors.
- Simple design, no moving parts or an agitator to ensure lower maintenance and less chance of a defect.
- Easier sterilization (no agitator shaft parts)
- Low energy requirement vs. stirred tank clearly doesn’t require energy for the moving components (agitator shaft).
- More efficient heat removal vs. stirred tank In the Airlift bioreactor, there is no need for the heat plate in order to control the temperature since the Draught-Tube that is within the bioreactor is able to function as an the internal exchanger of heat.
Disadvantages of Air-lift bioreactors
- More air flow and higher pressures are needed.
- The agitation in the Airlift bioreactor is controlled by the supply air . This allows it to regulate the supply air, and the required pressure.
- the greater pressure of air required, then more energy consumption required and more costs must be paid.
- Ineffectively break the foam when foaming takes place.
- There aren’t any bubble breakers, there aren’t any blades used to break the bubbles that result from in the supply of air (sparger).
Applications of Air-lift bioreactors
- The reactor is commonly used in the culture of shear sensitive organisms.
- Airlift bioreactors are commonly employed for aerobic bioprocessing technology. They ensure a controlled liquid flow in a recycle system by pumping.
- Due to high efficiency, airlift bioreactors are sometimes preferred e.g., methanol production, waste water treatment, single-cell protein production.
4. Packed Bed Reactors
- A packed bed fermentor is a type of bioreactor that consists of a bed of solid particles with biocatalyst either on or within the matrix of solids. It can operate in two modes: submerged mode with or without aeration or trickle flow mode. Packed bed reactors, also known as fixed bed reactors, are commonly used in various chemical processing processes such as absorption, distillation, stripping, separation, and catalytic reactions.
- In packed-bed bioreactors, air is introduced through a sieve that supports the substrate. The reactor offers several advantages, including high conversion rates for the catalyst, ease of operation, low construction and operation costs, increased contact between reactants and catalyst, and the ability to work at high temperatures and pressures.
- The packed bed consists of solid particles, typically with limiting walls, and the biocatalyst is supported on or within a porous or nonporous solid matrix. The solids can be compressible polymeric materials or harder substances. A circulating fluid containing nutrients continuously flows across the bed to provide necessary nutrients to the immobilized biocatalyst. Metabolites and byproducts are released into the fluid and then drained away. The flow of fluid may be upward or downward, but downward flow is more common due to gravity.
- The depth of the bed is determined by various parameters, including the density and compressibility of the solids, the need to maintain a specific minimum level of key nutrients like oxygen (O2) throughout the depth, and the required flow rate for a given pressure drop. As the bed depth increases for a certain void volume (solids-free volume fraction), the gravity-driven flow rate across the bed decreases.
- As the fluid flows downward through the bed, nutrient concentrations decrease while metabolite and product concentrations increase. This creates a heterogeneous environment within the packed bed, but concentration fluctuations along the depth can be reduced by increasing the flow rate. pH gradients are possible if the reaction involves the consumption or generation of H+ or OH- ions. Controlling the pH through the addition of acid and alkali is challenging due to poor mixing in the packed bed.
- The concentration of biocatalyst in a given volume of the bed decreases as the voidage (void volume) increases. If the packing material is compressible, its weight may compress the bed if the packing height is not kept low. Flow through a compressed bed becomes problematic due to decreased voidage. Packed beds are commonly used as immobilized enzyme reactors, but only a portion of the biocatalyst is exposed to significant amounts of the product, which can impede its activity.

Features of Packed Bed Reactors
- A bed of particles are confined in the reactor. The biocatalyst (or cell) is immobilized on the solids which may be rigid or macroporous particles.
- A fluid containing nutrients flows through the bed to provide the needs of the immobilized biocatalyst. Metabolites and products are released into the fluid and removed in the outflow.
- The flow can be upward or downward. If upward fluid is used, the velocity can not exceed the minimum fluidization velocity.
Advantages of Packed Bed Reactors
- Higher conversion per unit mass of catalyst than other catalytic reactors
- Low operating cost.
- Continuous operation.
- No moving parts to wear out.
- Catalyst stays in the reactor 6. Reaction mixture/catalyst separation is easy
- Design is simple
- Effective at high temperatures and pressures
Disadvantages of Packed Bed Reactors
- Undesired heat gradients.
- Poor temperature control.
- Difficult to clean.
- Difficult to replace catalyst.
- Undesirable side reactions.
Application of Packed Bed Reactors
- These are used with immobilized or particulate biocatalysts.
- High conservation per weight of catalyst than other catalytic reactors. Thus mostly preferred fermentor.
- Used is waste water treatment.
5. Fluidized Bed Bioreactor
- A fluidized-bed fermentor is a type of bioreactor that utilizes a packed bed of smaller particles to enhance mass transfer, oxygen transfer, and nutrient distribution to the cells. This design overcomes issues commonly associated with packed bed reactors, such as clogging, high liquid pressure drop, channeling, and bed compaction.
- In a fluidized-bed fermentor, the catalyst is placed at the bottom of the reactor, and the reactants are pumped into the reactor through a distributor pump, causing the bed to become fluidized. The cells are immobilized on small particles that move with the fluid, allowing for improved contact between the cells and the surrounding liquid.
- These bioreactors are suitable for reactions involving fluid-suspended biocatalysts, such as immobilized enzymes, immobilized cells, and microbial flocs. The reactor operates by suspending or fluidizing the particles with an upward-flowing liquid stream. Geometrically, the fluidized-bed reactor resembles a bubble column, but with an enlarged top section to reduce the surface velocity of the fluidizing liquid, which allows the solids to settle and fall back into the narrower reactor column below. This ensures that the particles remain in the reactor while the liquid exits.
- To create a gas-liquid-solid fluid bed, the liquid fluidized bed can be sparged with air or another gas. In some cases, if the solid particles are too light, they may need to be artificially weighted by incorporating heavier materials, such as stainless steel balls, into the solid matrix.
- Denser solids improve mass transfer between the solid particles and the liquid phase by increasing the relative velocity between the phases. However, the density of the solid particles should not exceed that of the liquid to maintain fluidization.
- The introduction of gas into the liquid fluidized bed enhances turbulence and agitation, which can be particularly useful in calm liquid fluidized beds. However, with relatively light particles, the surface liquid velocity required to suspend the solids may be too high, leading to inadequate solid-liquid contact time for the desired reaction. In such cases, recycling the liquid may be necessary to achieve a sufficiently long cumulative contact time with the biocatalyst.
- The minimum fluidization velocity, also known as the superficial liquid velocity required to suspend solids from a settled condition, depends on various factors, including the density difference between the phases, particle diameter, and viscosity of the liquid.
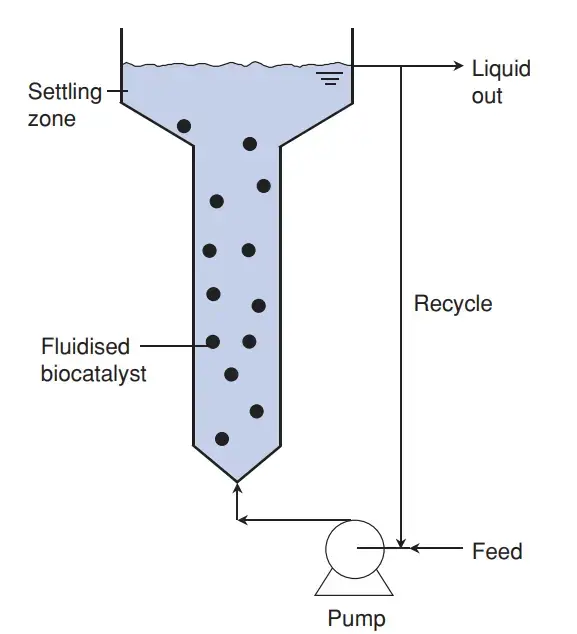
Features of Fluidized Bed Bioreactor
- Suitable for reactions involving a fluid-suspended particulate biocatalyst such as immobilized enzyme and cell particles.
- Similar to the bubble column reactor except that the top section is expanded to reduce the superficial velocity of the fluidizing liquid to a level below that needed to keep the solids in suspension.
- Consequently, the solids sediment in the expanded zone and drop back, hence the solids are retained in the reactor whereas the liquid flows out.
- The properties include:
- Extremely high surface area contact between fluid and solid per unit bed volume
- High relative velocities between the fluid and the dispersed solid phase.
- High levels of intermixing of the particulate phase.
- Frequent particle-particle and particle-wall collisions.

Mechanism of Fluidized Bed Bioreactor
- For an efficient operation of fluidized beds, gas is spared to create a suitable gas-liquid-solid fluid bed.
- It is also necessary to ensure that the suspended solid particles are not too light or too dense (too light ones may float whereas to dense ones may settle at the bottom), and they are in a good suspended state.
- Recycling of the liquid is important to maintain continuous contact between the reaction contents and biocatalysts. This enable good efficiency of bioprocessing.
Advantages of Fluidized Bed Bioreactor
- Uniform Particle Mixing
- Uniform Temperature Gradients
- Ability to Operate Reactor in Continuous State
Disadvantages of Fluidized Bed Bioreactor
- Increased Reactor Vessel Size
- Pumping Requirements and Pressure Drop
- Particle Entrainment
- Lack of Current Understanding
- Erosion of Internal Components
- Pressure Loss Scenarios
Application of Fluidized Bed Bioreactor
- These reactors can utilize high density of particles and reduce bulk fluid density.
- Fluidized beds are used as a technical process which has the ability to promote high levels of contact between gases and solids.
- In a fluidized bed a characteristic set of basic properties can be utilized, indispensable to modern process and chemical engineering
- The food processing industry: fluidized beds are used to accelerate freezing in some individually quick frozen (IQF) tunnel freezers.
- The fluid used in fluidized beds may also contain a fluid of catalytic type.
- Fluidized beds are also used for efficient bulk drying of materials.
- Fluidized bed technology in dryers increases efficiency by allowing for the entire surface of the drying material to be suspended and therefore exposed to the air.
6. Photobioreactor
- A photobioreactor is a specialized unit used for fermentation that is designed to be illuminated either by direct sunlight or artificial light sources. These bioreactors are typically constructed with materials such as glass or transparent plastic, and they consist of tubes or flat panels that serve as light-receiving systems.
- In photobioreactors, the medium is circulated through solar receivers using centrifugal pumps or airlift pumps. These reactors are commonly operated in continuous mode at temperatures ranging from 25 to 40 °C. They are primarily used for the photosynthetic cultivation of microalgae and cyanobacteria to produce valuable products like astaxanthin and β-carotene.
- Photosynthetic cultures require a source of light, either natural or artificial. While artificial illumination is prohibitively expensive, outdoor photobioreactors are the more feasible option for large-scale production. Open ponds and raceways are often utilized for cultivating microalgae, especially in wastewater treatment processes. However, when mono-septic cultures are required, hermetically sealed photobioreactors must be employed.
- Photosynthesis can only occur at relatively shallow depths because it relies on light. Algal ponds are typically no deeper than 0.15 meters. Excessive light can lead to photoinhibition, so a slight reduction in light intensity can actually increase the rate of photosynthesis. As the cell population increases, the self-shading effect of cells further restricts light penetration.
- In addition to light, photosynthetic algae cells require a carbon source, typically in the form of carbon dioxide. Closed photobioreactors designed for monoculture consist of arrays of glass or transparent plastic tubes. These tubes may be positioned horizontally or organized vertically as long rungs on a ladder-like structure. Alternatively, a continuous single-run tubular loop form or a spirally looped tube around a vertical cylindrical support can be used. In smaller-scale operations, thin flat or inclined panels may also be employed in addition to tubes.
- The solar receiver in a photobioreactor is composed of an array of tubes or a flat panel. Various mechanisms, such as centrifugal pumps, positive displacement mono pumps, Archimedean screws, and airlift devices, are used to circulate the culture through the solar receiver. Airlift pumps are particularly advantageous as they do not contain mechanical components, are easy to operate aseptically, and are suitable for shear-sensitive applications.
- The flow within the solar receiver tubes or panels should be sufficiently turbulent to facilitate the periodic movement of cells from the darker, less illuminated interior to the regions closer to the walls. The velocity of the flow should be maintained at a level that prevents the sedimentation of cells. Typically, the average linear velocity across the receiver tubes ranges from 0.3 to 0.5 m/s. Scaling up a tubular solar receiver by solely increasing the tube diameter is not feasible due to the need to maintain sufficient sunlight penetration. In general, the tube diameter should not exceed 6 cm. The penetration of light is influenced by factors such as biomass density, cellular shape and color, as well as the absorption properties of the cell-free culture media.
Advantages of Photobioreactor
- Higher productivity
- Large surface-to-volume ratio
- Better control of gas transfer.
- Reduction in evaporation of growth medium.
- More uniform temperature. Powerpoint Templates

Disadvantages of Photobioreactor
- Capital cost is very high.
- The productivity and production cost in some enclosed photobioreactor systems are not much better than those achievable in open-pond cultures.
- The technical difficulty in sterilizing Powerpoint Templates
Application
- The main applications of photobioreactors are in photosynthetic processes, involving vegetable biomass growth or microalgae growth under restricted conditions.
7. Membrane Bioreactor
- A membrane bioreactor (MBR) is a system that integrates traditional wastewater treatment processes with membrane filtration. This combination allows for the removal of organic compounds, suspended solids, and high nutrient levels from the wastewater.
- In an MBR, membranes are submerged within an aerated biological reactor. These membranes have pore sizes ranging from 0.035 microns to 0.4 microns, which enables them to effectively filter out contaminants from the wastewater. By using pure oxygen in the bioreactor, the efficiency of the system is enhanced, resulting in even higher rates of biological treatment and providing compact control of chemical oxygen demand (COD) and microorganisms.
- MBRs have been in use since the 1990s and have proven to be effective in wastewater treatment. They offer several advantages over conventional treatment systems, such as improved removal of organic and suspended solid matter, as well as high nutrient removal.
- However, one of the main challenges associated with MBRs is membrane fouling. Over time, the membranes can become fouled or clogged with solids and organic matter, which reduces their filtration efficiency. Efforts are being made to address this issue and develop improved membrane materials and cleaning techniques.
- For MBRs to be widely adopted in large-scale wastewater treatment applications, it is crucial to reduce the cost of the membranes. As the technology continues to advance and economies of scale are achieved, the price of membranes is expected to decrease, making MBRs more economically viable for widespread use in wastewater treatment processes.

Advantages
- The loss of enzyme is reduced.
- Enzyme lost by denaturation can be made up by periodic addition of enzyme.
- Substrate and enzyme can be easily replaced.
Applications
- The use has widely extended and is rapidly growing both in research and commercial applications.
- Several variations of MBR systems have evolved and presently, an MBR system is widely used in treatment of waste water from several sources.
8. Rotary Drum Bioreactor
- A rotary drum bioreactor is a type of bioreactor that consists of a horizontally rotating drum, which may or may not be equipped with a paddle mixer. The drum rotates at a slow speed to ensure proper mixing of the fermentation substrate. When scaling up this bioreactor, certain assumptions need to be made to facilitate the process.
- The bioreactor itself has a cylindrical shape with a length (L) and a diameter (D). It is partially filled with the fermentation substrate. During the fermentation process, the solid materials within the bioreactor undergo degradation. However, for scaling-up purposes, it is assumed that only the density of the bed is affected by this degradation.
- In terms of the gas phase within the bioreactor, it is assumed that the dry gas volume remains constant in the headspace. Additionally, the gas flow rates are assumed to remain the same between the inlet and the outlet of the bioreactor.
- It is also assumed that the solid particles and gas phase within the bioreactor are in equilibrium in terms of moisture and temperature. Furthermore, the diffusion from the axis of the drum is considered to be negligible.
- These assumptions help in simplifying the modeling and scaling-up process of rotary drum bioreactors, allowing for better understanding and prediction of their performance in larger-scale applications.
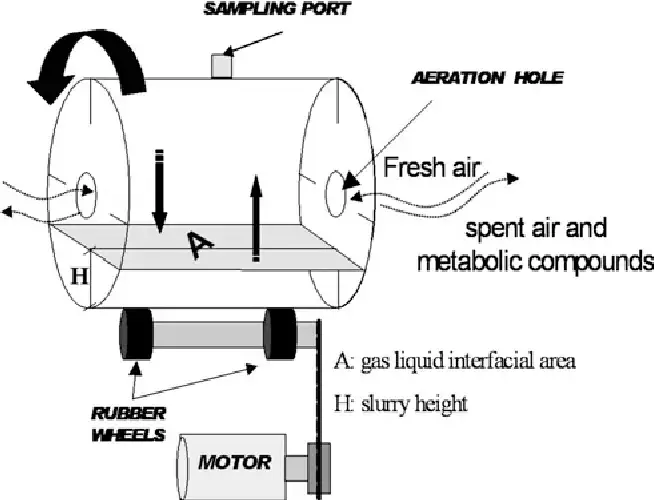
Advantages of Rotary Drum Reactor
- High oxygen transfer.
- Good mixing facilitates better growth and impart less hydrodynamic stress.
Disadvantages of Rotary Drum Reactor
- Difficult to scale up.
9. Mist Bioreactor
- A mist bioreactor is a type of hydraulically-driven bioreactor specifically designed for root cell cultures, particularly in plant cell cultures. Its main characteristic is the use of a disposable bag, which can be either single-use or multi-use, where the roots of the plants are immobilized and aerated on a frame.
- The mist bioreactor operates by creating a fog or mist of the culture medium, which is then distributed around the supporting frame of the root system. This fog is generated using either a two-component jet or an ultrasonic atomizer. The mist or fog provides a highly oxygenated environment for the roots, facilitating their growth and development.
- One of the notable features of mist bioreactors is the use of disposable bags. These bags can be easily replaced between experiments or batches, reducing the risk of contamination and simplifying the cleaning and sterilization processes. The disposable nature of the bags also allows for convenient scaling up of the bioreactor system.
- Former ROOTec, a company known for its mist bioreactor systems, has produced some of the largest disposable mist bioreactors available, with a capacity of up to 60 liters. These larger systems provide greater flexibility and capability for large-scale plant cell culture applications.
- Mist bioreactors offer advantages such as efficient aeration, ease of operation, and the ability to maintain a controlled and sterile environment for root cell cultures. They are particularly useful in research and industrial settings where the production of plant-derived compounds or the propagation of plant cells is required.

Advantages of Mist Bioreactor
- High oxygen transfer.
- Hydrodynamic stress elimination.
- Low production cost.
Disadvantages of Mist Bioreactor
- Mesh trays and cylindrical stainless steel meshes are required.
10. Immobilized cell bioreactor
- An immobilized cell bioreactor (ICR) is a bioreactor system that operates based on the principle of immobilization, which involves confining cells within a specific space to limit their mobility.
- The immobilization of cells in an ICR is driven by interactions such as hydrogen bonding, hydrophobic interactions, and the formation of salt bridges between the cells and the adsorbent material. Immobilization can generally be categorized into two types: passive and active.
- In passive immobilization, cells naturally adhere to a solid matrix, forming a biofilm. Active immobilization, on the other hand, involves the use of physical or chemical methods to induce immobilization, including techniques like attachment, entrapment, gathering, and confinement.
- Managing immobilized cells within the bioreactor can be challenging because they are not freely suspended in the liquid or gas phase. However, the performance of immobilized cells can be improved through appropriate reactor design.
- Designing a good immobilized cell bioreactor requires considering various criteria. Minimizing shear forces is crucial, as they can disrupt the immobilized cells. The reactor should have a capacity to hold a maximum amount of immobilized particles. Effective heat and mass transfer must be ensured to support cell metabolism and product formation.
- One common method of immobilization involves using sodium alginate (typically at a 2 percent concentration) to create cell beads. The substrate or nutrient is introduced into the reaction vessel containing the immobilized cells, leading to interactions that result in the production of desired products and byproducts.
- ICRs often employ a two-stage configuration, consisting of an enricher stage and a stripper stage. The enricher stage facilitates the growth and production phase, while the stripper stage is used to remove and treat excessive byproducts.
- After the fermentation process is complete, the inner and outer surfaces of the immobilized cell beads can be examined. It is often observed that initially, the cells reside in the inner part of the beads, but over time, they migrate and locate themselves on the outer surface of the beads.
- ICRs find applications in fermentation industries where it is beneficial to separate the production and growth phases, allowing for efficient control and optimization of the process.

Advantages
- The harvesting of the product you want is a breeze and requires any effort if the substances are released in the medium
Disadvantages
There are some restrictions in the ICR like;
- A limitation in mass transfer due to the intraparticle diffusion resistance, which restricts the ability of the substrate to get to cells. This type of problem occurs in aerobic reactions, where there is an oxygen shortage to cells, resulting in lower reactor performance.
- Another issue is inhibition of the product in which the concentration of the product is reduced within the inner core and, consequently, the rate of reaction is also decreased.
11. Activated sludge bioreactor
- An activated sludge bioreactor is a wastewater treatment system that utilizes a process known as the activated sludge process. In this bioreactor, the proportion of microbes, oxygen, and substrate are carefully controlled to facilitate efficient treatment of the wastewater.
- The bioreactor consists of a homogeneous tank where the wastewater feed is dispersed throughout the tank to ensure uniform conditions. This promotes the growth and activity of the microbial community responsible for wastewater treatment. The microbes in the activated sludge bioreactor feed on the organic pollutants present in the wastewater, effectively breaking them down and removing them from the water.
- In some variations of the activated sludge bioreactor, such as the plug flow system, the reactor is designed with an extended channeled inlet. This configuration helps restrict excessive microbial growth and improves the settling characteristics of the sludge, facilitating the separation of treated water from the sludge.
- Another variation of the activated sludge bioreactor is the step feed reactor. In this system, sewage is injected at multiple points in the aeration tank, creating a stepwise introduction of the wastewater. The food-to-microbes (F/M) ratio in step feed reactors is typically higher compared to plug flow systems. This higher F/M ratio promotes enhanced microbial activity and organic matter removal.
- Additionally, oxidation ditches are another type of activated sludge-based bioreactor. This system employs a modified activated sludge treatment process with extended solids retention times (SRTs) to effectively eliminate biodegradable organic matter. Oxidation ditches are equipped with an aeration rotor or brushes to ensure sufficient circulation and aeration within the reactor. The reactor configuration in oxidation ditches is similar to that of a whole mix reactor.
- Overall, activated sludge bioreactors are widely used in wastewater treatment plants to effectively treat organic pollutants present in the wastewater. The controlled conditions and microbial activity in these bioreactors help in achieving efficient and reliable wastewater treatment.
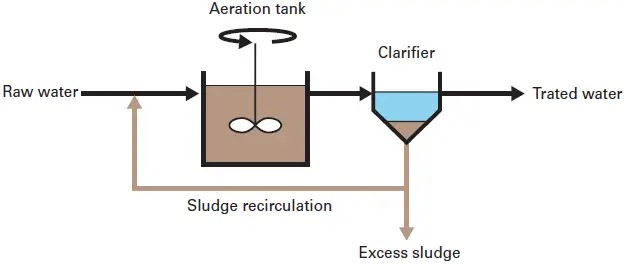
Mechanism/Process of activated sludge reactor
The mechanism or process of an activated sludge reactor involves several steps in the treatment of wastewater:
- Influent Introduction: Wastewater containing organic matter is pumped into the aeration tank of the activated sludge system. The wastewater serves as the influent or feedstock for the process.
- Aeration and Mixing: Once in the aeration tank, the wastewater is mixed with a suspension of microorganisms known as activated sludge. The aeration process supplies oxygen to the microorganisms, creating aerobic conditions necessary for their metabolic activity. Mixing ensures uniform distribution of microorganisms and oxygen throughout the tank.
- Metabolism of Organic Matter: The microorganisms in the activated sludge metabolize the organic matter present in the wastewater as their food source. Through biochemical reactions, they break down complex organic compounds into simpler forms, such as carbon dioxide (CO2) and water (H2O). This metabolic process releases energy that the microorganisms utilize for their growth and maintenance.
- Solid-Liquid Separation: After the wastewater has undergone the treatment process, it enters the next stage, which involves the separation of solid sludge from the treated liquid. This separation process can be achieved through various methods, such as sedimentation or filtration. The treated liquid, known as the effluent, is separated from the settled or filtered sludge.
- Sludge Disposal: The separated sludge, also called excess sludge or waste sludge, is further processed for disposal. It may undergo additional treatments, such as thickening or dewatering, to reduce its volume and moisture content. The ultimate disposal method for the sludge can vary depending on regulations and specific treatment plant practices. Common disposal options include incineration, land application, or further treatment for beneficial reuse.
- Sludge Recirculation: In order to maintain the population of microorganisms in the system, a portion of the settled sludge is returned to the aeration tank. This process, known as sludge recirculation or return activated sludge, replenishes the microbial population and ensures a continuous supply of active microorganisms for wastewater treatment.
By repeating this cycle of influent introduction, aeration and mixing, metabolism of organic matter, solid-liquid separation, sludge disposal, and sludge recirculation, the activated sludge reactor achieves effective removal of organic pollutants from wastewater and produces a treated effluent that meets regulatory standards.
Application
- The reactor is employed in the treatment of wastewater and sewage.
- This particular reactor is used to produce biofuels such as biogas, bioethanol and so on. such as biofuels that are made by milk-based waste.
Advantage
- The reactor can be operated with high organic loading rates.
Limitation
- This reactor is a major consumer of energy and also capital.
- The operating expenses are high.
11. Immersed membrane bioreactor
- Immersed membrane bioreactors (IMBRs) are a type of membrane bioreactor that combines suspended growth bioreactor principles with membrane separation to achieve enhanced wastewater treatment. In IMBRs, the filtration process and the biological treatment occur simultaneously, synergistically improving the overall treatment efficiency.
- The core component of an IMBR is the filtration system, which includes membranes that are immersed or submerged within the biomass. These membranes can be placed directly in the bioreactor or in a separate tank specifically designed for membrane filtration. The membranes used in IMBRs can be of different types, such as hollow fibers, flat sheets, or a combination of both.
- The filtration process in IMBRs is facilitated by applying a vacuum or suction on the interior of the membranes. This creates a pressure gradient that allows the separation of solids, particulate matter, and microorganisms from the treated wastewater. The membranes act as a physical barrier, allowing only the permeate (filtered liquid) to pass through while retaining the biomass and other solids.
- To maintain the effectiveness of the membranes and prevent fouling, an online backwash system is typically integrated into the IMBR. This system periodically cleans the membrane surface by reversing the flow or applying pulses of air or water to dislodge accumulated solids or biofilm, minimizing membrane fouling and maximizing filtration efficiency.
- Aeration is also essential in IMBRs to ensure proper mixing and prevent fouling. The introduction of air into the bioreactor creates air scouring, which helps to dislodge any particles or biomass adhering to the membrane surface. Aeration also provides oxygen to the microorganisms within the system, supporting their metabolic activity and promoting effective biological treatment.
- IMBRs are commonly utilized in large-scale and medium-sized wastewater treatment facilities due to their high treatment capacity and efficiency. The use of hollow fiber membranes is prevalent in these systems, as they offer a large surface area for filtration, facilitating the separation process and accommodating higher volumes of wastewater.
- Overall, immersed membrane bioreactors combine the advantages of suspended growth bioreactors and membrane separation technologies, enabling efficient and reliable wastewater treatment by achieving simultaneous biological treatment and membrane filtration.
Process of immersed membrane bioreactor
The process of an immersed membrane bioreactor (IMBR) involves several key components and considerations to ensure its effective operation and wastewater treatment. The five fundamental components of the IMBR process are:
- Membrane and its structure: The membrane plays a crucial role in the IMBR as it acts as a physical barrier that allows for the separation of solids and microorganisms from the treated wastewater. The membrane’s structure, such as hollow fibers or flat sheets, influences its permeability and filtration efficiency. Proper maintenance of the membrane is essential to prevent fouling and ensure its long-term performance.
- Feedwater and pretreatment: The quality and properties of the feedwater, which refers to the wastewater being treated, are important considerations in the IMBR process. Proper pretreatment is often necessary to remove large particles, debris, and substances that could potentially cause fouling or damage to the membrane. Pretreatment processes may include screening, sedimentation, or other filtration techniques to improve the feedwater quality.
- Aeration of the biomass and membrane: Aeration is a critical aspect of the IMBR process as it provides oxygen to the microorganisms within the system. Proper aeration promotes the growth and activity of the biomass, ensuring efficient biological treatment. Aeration also helps prevent membrane fouling by creating turbulence and promoting the removal of accumulated solids or biofilm from the membrane surface.
- Sludge withdrawal and residence time: Sludge withdrawal refers to the removal of excess biomass or solids from the IMBR system. It is necessary to maintain a balance between biomass growth and removal to prevent excessive accumulation and potential adverse effects on the system’s performance. The residence time, or the duration that the wastewater and biomass spend in the reactor, is carefully controlled to optimize treatment efficiency.
- Bioactivity and nature of biomass: The bioactivity and nature of the biomass within the IMBR are essential factors in achieving effective wastewater treatment. The microorganisms present in the biomass play a crucial role in biodegradation and the removal of organic contaminants from the wastewater. Factors such as the microbial community composition, diversity, and metabolic activity influence the overall treatment performance of the IMBR.
By carefully managing these five components, an immersed membrane bioreactor can effectively treat wastewater by combining biological treatment with membrane filtration. The membrane’s permeability, feedwater quality, aeration, sludge withdrawal, and biomass characteristics all contribute to the overall efficiency and success of the IMBR process.
Advantage
- Low energy consumption
- lower capital costs.
Application
- This kind of bioreactor is mainly used to treat textile and tannery wastes along with wastewaters and for the reuse of aquaculture wastes.
12. Reverse membrane bioreactor
- A reverse membrane bioreactor (rMBR) is a unique approach that combines traditional membrane bioreactor technology with cell encapsulation methods. In this system, cells are separated from the feed and enclosed within a membrane, which is then fixed within the reactor. The basic principle of the rMBR is similar to an immersed membrane reactor, where the membrane is submerged in the reactor. However, in the rMBR, microorganisms are contained within membrane layers, creating a sachet-like structure.
- There are two types of membrane configurations commonly used in rMBRs: integrated permeate channels (IPC) and packed columns. The choice of configuration depends on the desired product and the associated byproducts. For instance, a multilayer membrane column configuration is utilized for the production of biogas, while an IPC membrane configuration is employed for ethanol production. Synthetic membranes are commonly used to allow specific nutrients to flow through, mimicking the natural separation of cytoplasm and other cellular components.
- The primary objective of enclosing cells within the membrane layers is to increase cell density and tolerance. For example, the concentration of yeast cells can be increased to as high as 309 grams/L. However, such high cell densities can result in increased stress due to limited nutrient availability. As a response to this stress, the cells express stress-related genes, enhancing their resilience and adaptability.
- The use of a reverse membrane bioreactor offers several advantages. By encapsulating cells within the membrane, their density and overall productivity can be significantly increased. Additionally, the enclosed environment provides protection against external contaminants and helps maintain optimal conditions for cell growth and metabolism. The rMBR technology has potential applications in various fields, including biofuel production, pharmaceutical manufacturing, and bioremediation.
- Overall, the reverse membrane bioreactor provides a novel approach to enhance cell density, productivity, and stress tolerance by encapsulating cells within membranes, enabling efficient bioprocessing and expanding the possibilities for various biotechnological applications.

Mechanism
In general, in rMBRs, the diffusion mechanism is carried out in three distinct stages.
- The dispersion of the substrate from the feed side of the membrane’s surface, and the reverse process to the product
- the transport of substances (substrate or metabolites) through the membrane and
- the dispersal of feed and products on the cell’s side via biofilm.
The rate at which compounds diffuse through the membrane is influenced by different parameters, including hydrophilicity, tortuosity and porosity and concentration gradient etc.
Cultivation principles
1. Submerged cultivation
- Submerged cultivation, also known as submerged fermentation, is a process where a microbial culture is inoculated into a liquid medium to produce the desired product. There are two main types of fermentation processes: aerobic and anaerobic fermentation.
- In submerged fermentation, the entire working volume of the bioreactor is mixed and aerated to supply the producer cells (microorganisms) with the necessary nutrients and oxygen (in aerobic processes). This makes the process cost-effective and creates optimal conditions for the accumulation of a large volume of actively growing biomass and the production of the desired product.
- Submerged fermentation played a significant role in the history of biotechnology, particularly in the production of penicillin during World War II. By transitioning from surface fermentation in flasks and bottles to submerged fermentation, the production of penicillin increased rapidly to meet the urgent demand.
- Submerged fermentation can be either aerobic or anaerobic. Aerobic fermentation, which involves the incorporation of oxygen into the liquid medium, is used for the production of antibiotics and enzymes. On the other hand, anaerobic fermentation is employed for the production of substances such as butanol, where the presence of oxygen inhibits the process. Some fermentation processes, like ethanol production, involve facultative anaerobic organisms that can switch between aerobic and anaerobic modes.
- Submerged fermentation processes can be modified based on the desired end product. The product can be biomass, ferments, or low-molecular-weight molecules such as ethanol, methanol, and organic acids. Metabolites can be classified as primary or secondary. Primary metabolites directly contribute to normal growth and reproduction, such as lactic acid and various amino acids. Secondary metabolites, such as antibiotics and atropine, are produced during or near the end of the stationary phase and do not affect growth or reproduction.
- Different fermentation processes can be distinguished based on technology and product type. Biomass, high-molecular-weight molecules like enzymes, and low-molecular-weight metabolites can be targeted. The timing and addition of inducers and precursors depend on the desired product. Secondary metabolites are often produced in stressful conditions, requiring the addition of inducers and precursors.
- Submerged fermentation is well-suited for microorganisms that require high levels of moisture, such as yeasts and bacteria. It also simplifies product purification. Various types of bioreactors, including stirred tank, bubble column, airlift, photobioreactors, and membrane bioreactors, can be used for conducting submerged fermentations, depending on the specific application.
2. Solid state fermentation
- Solid state fermentation (SSF) is a process in which microorganisms grow and metabolize on a solid substrate with limited or no free water. The substrate provides enough moisture to support the growth and metabolism of the microorganisms. SSF is commonly used in the production of various fermented foods such as bread, meat, cheese, pickles, and yogurt.
- One of the advantages of SSF is its ability to utilize agro-industrial residues and by-products to obtain a wide range of products including enzymes, organic acids, food fragrances, biopesticides, mushrooms, pigments, xanthan gum, and plant hormones, among others. SSF is also favored due to its simple equipment requirements and straightforward bioreactor construction. However, scaling up SSF processes can be challenging as precise monitoring and control of environmental conditions for microorganisms is difficult.
- SSF processes are typically longer compared to other fermentation methods due to the slower growth rate of microorganisms on solid substrates. There are certain processes that can only be successfully executed using SSF. For example, some fungi require SSF for sporulation as they cannot sporulate on liquid media.
- Various types of bioreactors can be used for SSF, including horizontal drums, trays, packed beds, and bench-scale bioreactors. Each type has its own advantages and may be selected based on the specific requirements of the process.
- In summary, solid state fermentation is a microbial fermentation process that occurs on solid substrates with limited water availability. It is utilized for the production of fermented foods and a wide range of products using agro-industrial residues. While SSF has certain limitations, it offers unique advantages and plays a vital role in various industrial applications.
3. Immobilization
- Immobilization refers to the process of binding an enzyme or other biological entity to an insoluble carrier while preserving its catalytic activity. This technique is employed in various applications where it is necessary to ensure the absence of enzyme residues to prevent immunological reactions.
- The immobilization of enzymes offers several advantages, including enhanced stability and the ability to reuse the enzyme over an extended period. It allows for continuous or repeated use of a single batch or series of commercial biocatalysts. Immobilization involves physically separating the biocatalyst from the solvent in such a way that the substrate and reaction products can freely pass between the liquid and solid phases.
- Both enzymes and whole cells can be immobilized. Immobilization of cells has advantages, such as in ethanol production, where the cells can be reused and have a longer lifespan. By immobilizing cells in gels, such as alginate, collagen, chitosan, agarose, or cellulose, the cells can be retained while allowing small molecules to diffuse through the gel pores, facilitating substrate delivery and removal of metabolic byproducts.
- Various techniques for immobilization have been developed, including stable porous gels, hydrogels, solid macroporous carriers, and immobilization in different types of bioreactors. These techniques find applications in industries such as food, dairy, and pharmaceutical production, where the target substance needs to be isolated and purified without the presence of biological impurities.
- The use of immobilized biological entities in industrial settings offers economic benefits, including standardized biosynthetic conditions and increased production efficiency. Immobilized systems have a longer lifespan and require fewer raw materials per unit of output.
- However, there are challenges associated with immobilized cell bioreactors, such as the potential for undesirable side reactions due to multiple catalytically active enzymes present in the cells. The cell membrane may also act as a diffusion barrier, limiting productivity. Additionally, controlling the physiological condition of microorganisms in immobilized cell bioreactors can be challenging, leading to process variability and limited adaptability.
- Various types of bioreactors can be utilized for immobilized cells, including stirred tank reactors, fixed bed reactors, fluidized bed reactors, moving bed reactors, and packed bed reactors. The choice of bioreactor depends on the specific requirements of the immobilization process and the desired outcome.
Operation modes in Bioreactor
1. Batch
Batch culture is a closed system in which the growth rate of biomass goes toward zero due to substrate depletion and inhibitor buildup. These systems are perpetually unstable.
Microorganisms undergo six growth phases during batch cultivation.
- The lag phase or induction period begins when the microbe is inoculated into the nutritional medium and is the period of their adaption. During this phase, the micromolecular and macromolecular components of the microbial culture undergo reorganisation, as well as the creation or inhibition of enzymes or structural components of the cell. Depending on external variables, the duration of this phase can range from one hour to three or more hours. During this phase, the cell mass may alter without the cell count changing.
- The lag period is followed by an exponential growth phase. This is a stage of fast biomass and reaction product buildup.
- The phase of linear growth is defined by balanced growth in the steady state, that is, the growth rate remains constant during the cultivation process, and the chemical composition of the culture medium changes as nutrients are absorbed and metabolic products are created. As a result, the microorganisms’ surrounding habitat is continually changing, yet the development rate does not depend on the amounts of nutrients.
- The phase of linear growth is replaced by the phase of growth retardation, during which the growth rate of the culture declines to zero.
- Furthermore, the culture can enter a somewhat stable stationary phase, with the rate of microbe death being compensated by the rate of biomass gain.
- With the depletion of the nutritional medium (substrate) and the accumulation of growth-inhibiting products, considerable physiological changes (lysis) occur in the culture, and the so-called phase of culture withering begins.
2. Fed-batch
- Fed-batch is based on the addition of a substrate containing a growth-limiting nutrient to a culture. By altering the feeding method, the cell development and fermentation process can be managed.
- In a bioreactor, fed-batch is typically initiated with a batch fermentation phase until the consumption of one or more substrates and/or inducers. Using various feeding regimes, the new medium can be added.
- During the course of the procedure, only a fixed or variable volume of a new medium or substrate can be added as feeding. During the fed-batch phase, this feeding might be continuous, exponential, or in pulses over a short or long duration.
- When the goal product is positively correlated with microbial growth, fed-batch fermentation is particularly advantageous for bioprocesses aiming for high biomass concentration or high yield. A typical fed-batch method for producing a product consists of three steps.
- First, in batch mode, the biomass grows to a concentration that allows the process to continue with a limitation on the substrate (without the accumulation of the substrate in the medium). The second stage is biomass cultivation, in which a substrate that stimulates rapid development of the culture is added to the medium (glucose, sucrose or glycerin).
- When a biosynthesis inducer is added to the medium, the last stage of product synthesis (recombinant protein or other substances) is initiated. This could be an activator of gene transcription (such as IPTG) or an auto-inducer substrate (for example, methanol for P. pastoris or lactose for E coli).
- The most straightforward method of feeding strategy is the calculation of a time-dependent, modified feeding profile. Typically, this feeding profile is determined using mathematical models. To avoid catabolism repression and prevent the repressive effects of excessive substrate concentrations, the operator must regulate the feeding regime. Controlling fed-batch is hard due to the lack of good on-line biomass and substrate concentration measuring techniques.
- By utilising customised feeding profiles, the operator must always modify these profiles during a fed-batch. Due to the impossibility of delivering entirely repeatable fermentations, this is the case. At some phases of the fermentation process, automatic feeding based on the values of the dissolved oxygen (DO) sensor is feasible.
- However, there are various issues here. When reasonably high biomass densities are reached, it is also impossible to perform this regulation in its best form.
- Model-based fed-batch control is capable of resolving the aforementioned issue. The solutions are not yet available for purchase. Typically, model-based control systems are designed for particular applications. Attempts have been made to design systems with wider application.
- In general, model-based control operates as follows: During the fermentation process, samples are collected so that the software can be updated with the most recent findings of tests on biomass and substrates.
- Following the input of test results, the software performs an automatic comparison between these findings and the outputs of calculations based on the mathematical model in use. If variations exceed the established standards, the software calculates a new feeding profile.
- The amended feeding profile is automatically sent to the process controller PLC, and the substrate feeding is then adjusted to correspond with the new profile (up to a following update). The mathematical model is implemented as a PC software that is connected to the PLC via an OPC server.
3. Continuous
- Continuous cultivation is distinguished by the constant addition of fresh nutrient medium to the bioreactor and the constant selection of a suspension or a spent medium. Continuous culture is an open system that strives to achieve a dynamic balance.
- Thus, it is possible to maintain constant environmental conditions within the growing medium. If the product’s demand potential is sufficient, continuous cultivation may be utilised. The second typical application of continuous cultivation is in the treatment of wastewater using wastewater as the in-flow substrate.
- If a consistent amount of product is required, continuous cultivation is utilised. As a result of the incorporation of additional devices into the bioreactor connection schema, it is structurally more complicated and necessitates additional automatic control.
- In a batch culture, conditions are constantly changing: the culture’s density increases and the substrate concentration decreases.
- However, it is frequently required that cells can remain in the exponential growth phase for an extended period of time at a constant substrate concentration and under other unaltered conditions.
- This can be accomplished by continuously introducing a new nutrient solution into a vessel containing a cell culture while simultaneously removing a suitable amount of cell suspension.
- Chemostat and auxostat cultivation methods are commonly used in microbiological research.
- The chemostat method of cell cultivation is based on the use of a bioreactor into which a nutrient medium is supplied at a constant rate and, simultaneously (for example, drainage according to the level), the cell suspension is removed.
- Likewise, the volume of the grown suspension remains constant. The concentration of the substrates governs the growth of the culture within the chemostat. The system’s stability is dependent on this limitation of the growth rate by the concentration of one of the necessary substrates.
- Auxostats are closed-loop systems governed by feed-back regulation of some state variable, such as biomass, substrate concentration, or pH. Auxostats can be classified as turbidostats, nutristats, or pH-auxostats depending on their operational control principle.
- In a turbidostat, an optical density (turbidity) controller adjusts the feed rate to maintain a constant biomass concentration over time. Under nutrient-rich conditions, the turbidostat facilitates growth rates close to the maximum.
- The application problem of turbidostat is associated with some technical difficulties of sensor readings, such as fouling of the sensor due to microbial growth on its surface and signal transfer disturbances caused by air bubbles or coloured and particulate media.
- Nutristat’s operation is predicated on the measurement and control of substrate concentration through substrate feeding. The application of nutristat is constrained by the absence of suitable analytical tools for the on-line measurement of the majority of relevant substrate concentrations.
- The pH-auxostat is based on the measurement of pH, which is frequently correlated with the rate of biomass production, but is easier to measure and regulate than turbidity and substrate concentrations.
- In a titration mode, the signal from the pH sensor is used to control the medium in-flow so that the addition of fresh medium restores the pH to the setpoint and the same amount of medium is removed from the bioreactor in out-flow. The pH-auxostat method is applicable to microorganisms whose growth results in changes in the medium’s pH.

What are Aerobic and anaerobic bioreactors?
Aerobic reactors
- Aerobic reactors are used for processes that require oxygen to support the growth and metabolism of microorganisms. The efficiency of product conversion or degradation in an aerobic reactor depends on factors such as bubble size and the rate of gas-to-liquid mass transfer.
- Maintaining optimal oxygen solubility is crucial for aerobic reactors, as the presence of salt can inhibit oxygen’s solubilizing property. Simple aerobic bioreactors can be created using aerated lagoons or oxidation ponds for waste storage in an open environment. Another approach involves using a rotating disc containing microbes as a biofilm for periodic churning.
- Several types of aerobic bioreactors are commonly used in industrial applications. Stirred tank bioreactors, airlift bioreactors, and inverse fluidized bed bioreactors are among them. Stirred tank reactors are the most common type of aerobic reactor, where air is sparged from the bottom to provide oxygen to the microorganisms.
- In airlift bioreactors, mixing is achieved through gas turbulence, resulting in a higher oxygen transfer coefficient compared to stirred tank reactors. Air is injected from the bottom of the reactor, creating a rotating motion of the reactor’s contents that maximizes gas transfer.
- The inverse fluidized bed reactor (FBR) is used for wastewater treatment. In this type of reactor, inert particles coated with biofilm serve as the solid phase, oxygen or air supply acts as the gaseous phase, and wastewater is the liquid phase. The gas flows in the opposite direction of the liquid, increasing the mass transfer rate and facilitating easy re-fluidization of the bed.
- Aerobic reactors offer efficient oxygen supply and are suitable for various applications that require aerobic microbial processes. The choice of reactor type depends on factors such as oxygen solubility, mixing efficiency, and the specific requirements of the process or application.
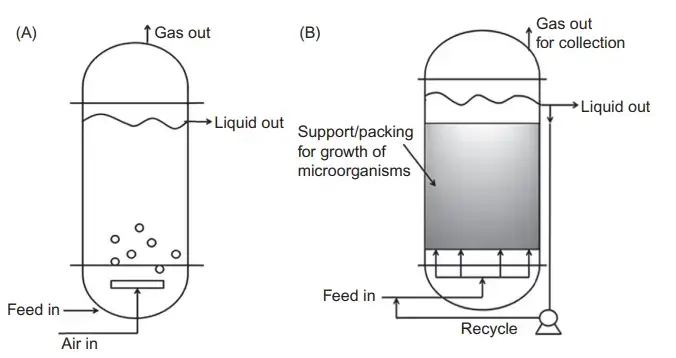
Anaerobic reactors
- Anaerobic reactors are similar to aerobic reactors, but they operate under specific conditions to maintain an anaerobic environment. These reactors have a simple design, high loading capacity, and can handle high levels of hazardous and organic chemicals.
- Specialist bacteria called methanogens play a crucial role in anaerobic processes. They have the ability to form immobilized granules that settle to the bottom as sludge, which is the fundamental principle utilized in anaerobic bioreactors. One example of an anaerobic reactor operating on this principle is the Upflow Anaerobic Sludge Blanket (UASB) reactor. In this type of reactor, an anaerobic fluidized bed is created where the microbial biomass forms a biofilm on carrier particles and is fluidized using the energy from the feed stream. The substrate diffuses through the biofilm, undergoes transformation into volatile fatty acids and CH4 (methane), and then diffuses out of the bulk liquid. As the biofilm expands, the particles become larger and leave the reactor, resulting in a decrease in particle density and concentration.
- Another type of anaerobic reactor is the membrane reactor. It can be designed in different forms. In one configuration, enzymes are suspended in the reactor, and the mixture is withdrawn along with the enzymes and passed through a membrane. The enzymes are retained by the membrane, while the products are collected as permeate. Alternatively, the membrane filter can be immersed inside the reactor, allowing the permeate to be devoid of enzymes, which are retained within the reactor. Membrane bioreactors (MBRs) produce less sludge compared to conventional bioreactors, which is their primary advantage. However, they also have drawbacks such as membrane fouling and high operational expenses.
- Anaerobic reactors offer efficient treatment of organic and hazardous compounds, and they play a vital role in processes such as wastewater treatment and biogas production. The choice of anaerobic reactor depends on factors such as the specific application, substrate characteristics, and desired performance.
Difference between bioreactor and fermenter
Bioreactor and fermenter are similar names, however there is a significant distinction between them. Bioreactor is frequently associated with the cultivation of mammalian, plant, and stem cells.
Fermenter is employed when the application involves the culture of bacteria, yeast, or fungi. It would not be incorrect to use the term bioreactor in these situations, however only the term bioreactor is used when discussing cell cultivation. The term fermenter refers to reactors in which fermentations, metabolic processes that cause chemical transformations in organic substrates by the action of enzymes, are conducted. The primary distinction between bioreactors used for cell and microbe cultivation is the mixing and aeration requirements, as well as the ratio of height to diameter H/D. The aeration rate for microbe cultivation is between 0.5 and 3 vvm (volume of cultivation media/volume of air flow per minute). Cell cultures require gentle mixing and an aeration rate between 0.01-0.1 vvm. The ideal H/D ratio of the vessel for microbe cultivation is 3:1, but it is 2:1 for cell cultures.
| Bioreactor | Fermenter |
|---|---|
| Associated with cultivation of mammalian, plant, and stem cells | Used for culture of bacteria, yeast, or fungi |
| Primarily used for cell cultivation | Primarily used for fermentations |
| Mixing and aeration requirements differ | Mixing and aeration requirements differ |
| Aeration rate for microbe cultivation: 0.5-3 vvm | Aeration rate for fermentations: 0.5-3 vvm |
| Aeration rate for cell cultures: 0.01-0.1 vvm | Aeration rate for cell cultures: 0.01-0.1 vvm |
| Ideal height to diameter (H/D) ratio for microbe cultivation: 3:1 | Ideal H/D ratio for microbe cultivation: 3:1 |
| Ideal H/D ratio for cell cultures: 2:1 | Ideal H/D ratio for cell cultures: 2:1 |
What is the Difference between conventional membrane bioreactor and reverse membrane bioreactor?
Conventional membrane bioreactor
The design of the traditional membrane bioreactor is either internal or external. The pressure gradient functions as an accelerating force. The mechanism for mass transfer occurs via the process of convection as well as diffusion. Microbial or other types in living cells get fed with the feed , and they are able to freely circulate within the medium.
Reverse membrane bioreactor
For RMBR, it’s an type of bioreactor with an embedded structure in which the concentration gradient functions as a force driving the process and the mechanism for mass transfer occurs by diffusion. Living cells are not able to be fed alongside the feed. They have to be isolated and kept inside their membranes.
| Conventional Membrane Bioreactor | Reverse Membrane Bioreactor |
|---|---|
| Design: Internal or external | Design: Embedded structure |
| Pressure gradient functions as an accelerating force | Concentration gradient functions as a driving force |
| Mass transfer occurs via convection and diffusion | Mass transfer occurs by diffusion |
| Microbial or other living cells freely circulate within the medium | Living cells are isolated and kept inside their membranes |
| Cells can be fed alongside the feed | Cells cannot be fed alongside the feed |
Application example of bioreactors
The cultivation for acquiring vaccines against the SARS-CoV-2
Recent efforts have been focused on the development of a vaccine against the SARSCoV2 virus. Bioreactors are utilised in the manufacture of each and every vaccination. For instance, the procedure for developing two types of coronavirus vaccines that have already been clinically validated is as follows:
RNA vaccines are produced utilising a novel technique that was formerly exclusive to veterinary medicine. No RNA vaccination for human use has yet been licenced. This vaccine comprises a viral component that resembles the structure of human messenger RNA (mRNA). After entering human cells, the ribosomes use the mRNA to produce a viral protein. The protein in question promotes an immunological response in the human body, so creating natural resistance to the virus. Vaccine operations including Pfizer-BioNTech and Moderna utilise this technology.
For the production of these types of vaccines, a host organism that can cultivate huge quantities of viral mRNA is necessary. The Escherichia coli bacterium is the organism most commonly used for such applications. It is possible to develop the bacteria in bioreactors with a working volume of up to 10 m3 (and even bigger) because the procedure can be carried out in bioreactors made of stainless steel. This indicates that the procedure is easily scalable. Nonetheless, this type of cultivation method cannot be conducted in disposable (single-use) bioreactors, as they cannot typically offer the extensive mixing and aeration required for E. coli growth and mRNA generation. The other type of vaccinations utilise a weakened adenovirus that carries a specific viral protein for inducing an immune response.
These vaccines are examples of non-replicating viral vectors, as they employ an adenovirus shell encoding a SARSCoV2 protein and carrying DNA that encodes the protein. Vaccines against the coronavirus based on viral vectors are non-replicating, meaning they are incapable of creating new viral cells; instead, they produce just the antigen that induces a systemic immune response. This category of vaccines includes the COVID-19 vaccine by Oxford–AstraZeneca, Sputnik V (Russia), Convidicea (China), and Ad26.COV2.S by Johnson & Johnson.
For the production of these types of vaccinations, mammalian cell cultures are utilised. In such cultivations, single-use bioreactors can be utilised, allowing for comparatively straightforward bioreactor-based production facility growth. The highest working volume of reliable single-use bioreactors now available on the market is around 2,000 litres. However, announcements of bigger volume single-use bioreactors have been reported very lately. ABEC, a global provider of integrated solutions and services for biopharmaceutical manufacturing, recently announced the availability of single-use bioreactors with a maximum operating volume of 6,000 litres.
Applications of bioreactor
Bioreactors are used in a wide variety of applications, including:
- Industrial biotechnology: Bioreactors are used to produce a variety of bioproducts such as enzymes, antibiotics, and biofuels on a large scale.
- Cell culture: Bioreactors are used to culture and grow cells, such as stem cells, for research and therapeutic purposes.
- Tissue engineering: Bioreactors are used to create three-dimensional structures of living tissue for medical applications.
- Environmental biotechnology: Bioreactors are used to treat waste materials and pollutants, such as sewage and industrial waste, by using microorganisms to break down the contaminants.
- Food and beverage production: Bioreactors are used to ferment foods such as yogurt, cheese, beer, and bread.
- Pharmaceuticals: Bioreactors are used to produce vaccines, monoclonal antibodies, and other therapeutics.
- Research and Development: Bioreactors are used in research to study the behavior of cells, microorganisms and their metabolic pathways in different conditions.
Overall bioreactors are used in a wide range of fields, from the production of consumer goods to the development of new medicines, and from the treatment of waste to the production of biofuels.
| Type of bioreactor | Applications |
| Stirred tank fermenter | Stirred tank bioreactors (STBRs) are the reactors most widely employed for culturing of biological agents such as cells, enzymes, or antibodies. They are contactors where the well-mixed among phases is obtained mainly by internal mechanical agitation. Antibiotics, citric acid, Exopolysaccharides, cellulose, Chitinolytic enzymes, Laccase, Xylanase, Pectic, and pectate lyase, Tissue mass culture, Lipase, Polygalacturonases, Succinic acid |
| Bubble column fermentor | Bubble column reactors are extensively used in carrying out gas-liquid and gas-liquid-solid reactions in a variety of important industrial reactions, including hydrogenation, oxidation, hydroformylation, chlorination, bioreactions and so on. |
| Airlift fermentor | Antibiotics, Chitinolytic enzymes, Exopolysaccharides, Gibberelic acid, Laccase, Cellulase, Lactic acid, Polygalacturonases, Tissue mass culture |
| Fluid bed fermentor | Today, fluidized bed reactors are still used to produce gasoline and other fuels, along with many other chemicals. Many industrially produced polymers are made using FBR technology, such as rubber, vinyl chloride, polyethylene, styrenes, and polypropylene. |
| Packed bed fermentor | These bioreactors are widely applied for valorization of food, beverage, nutraceutical synthesis, as well as waste treatment. |
| Photobioreactor | A photobioreactor (PBR) refers to any cultivation system designed for growing photoautotrophic organisms using artificial light sources or solar light to facilitate photosynthesis. PBRs are typically used to cultivate microalgae, cyanobacteria, macroalgae, and some mosses. |
| Membrane bioreactor | Membrane bioreactors have been in use for a variety of applications during the last few decades. This ranges from food and biofuel production to amino acids, antibiotics, proteins, and fine chemicals manufacturing; the removal of pollutants; and wastewater treatment. |
| Rotary Drum Bioreactor | Rotating drums bioreactors were first utilized for the production of amylase in SSF by. Then, in the 1940s, rotating drum bioreactors were improved and applied in the commercial-scale production of penicillin. |
| Mist Bioreactor | for in vitro culture of differentiated tissue, in plant micropropagation, and in the culture of transformed (hairy) roots for secondary metabolite production. |
| Immobilized cell bioreactor | A variety of immobilized cell bioreactors have been developed to optimize the fermentation processes. Immobilized cells are currently being used industrially for vinegar, organic, and amino acid production, as well as in wastewater treatment. |
| Activated sludge bioreactor | municipal as well as industrial wastewater treatment. |
| Immersed membrane bioreactor | The EMBR is used to prevent contact between the SRB and wastewater. In EMBR, the wastewater is selectively passed over one surface of the membrane, while the microbial culture is maintained on the other side. |
Advantages of bioreactor
Bioreactors offer several advantages over traditional methods of growing microorganisms or cells, including:
- Controlled conditions: Bioreactors allow for precise control of temperature, pH, oxygen levels, and other environmental factors, which can greatly enhance the growth and productivity of the culture.
- Scalability: Bioreactors can be scaled up or down to meet the desired production volume, allowing for easy expansion or reduction of production.
- Automation: Bioreactors can be fully automated, allowing for precise control and monitoring of the culture without the need for constant human intervention.
- Sterility: Bioreactors can be sterilized to prevent contamination, which is important for industrial and medical applications.
- High productivity: Bioreactors can produce large amounts of cells, microorganisms or products per unit volume, that make it cost-effective.
- Repeatability: Bioreactors provide a controlled environment which allows for reproducibility of experiments and production.
- Versatility: Bioreactors can be used to grow a wide variety of microorganisms and cells, making them useful in many different fields.
- Bioreactors can be used to simulate the growth conditions of microorganisms in nature, and to study their behavior under different conditions.
Overall, bioreactors can be used to improve the efficiency, productivity, and repeatability of many industrial, medical and research processes, allowing for the production of high-quality, consistent products.
Disadvantages of bioreactor
- High cost: Bioreactors can be expensive to purchase and maintain, which can be a barrier for some companies or researchers.
- Technical complexity: Bioreactors can be difficult to operate and require specialized knowledge and skills to run effectively.
- Contamination risk: Bioreactors are closed systems, which can increase the risk of contamination if not properly sterilized and maintained.
- Scale-up challenges: Scaling up a bioreactor process from a small lab scale to a large-scale commercial operation can be challenging and may require significant optimization and experimentation.
- Limited product diversity: Bioreactors are typically optimized for specific types of products, such as proteins or enzymes, and may not be well-suited for producing other types of products.
- Environmental concerns: Bioreactors require a steady supply of energy, which can contribute to greenhouse gas emissions and other environmental impacts.
FAQ
What is a bioreactor?
A bioreactor is a device or system used for carrying out biological reactions or processes, typically involving living organisms such as cells, enzymes, or microorganisms. It provides a controlled environment for these biological entities to grow, reproduce, and produce desired products.
What is a bioreactor used for?
A bioreactor is an apparatus that allows bacteria growth and fermentation at controlled conditions. They are commonly used in industry to manufacture pharmaceuticals and foodstuffs such as beer and wine. It is an industrial device that uses microorganisms such as bacteria to break down organic materials into useful chemicals and energy, like hydrogen gas for fuel cells. Bioreactors can be found in wastewater treatment plants, producing pharmaceuticals, and food processing facilities.
How does a bioreactor work?
A bioreactor works by pumping nutrients into a solution of water and microorganisms, such as bacteria, algae, yeast, fungi, or protozoa. The organisms consume the nutrients until they reach a certain density, at which point they produce additional waste products. Bioreactors can be used to produce hydrogen for fuel cells, ethanol from sugarcane molasses, or synthetic chemicals. An example of a typical bioreactor setup would include: an air compressor, a reservoir of water containing microbes, a pump, a tube, diffuser plates that spread out the flow of nutrient solution, and monitoring equipment.
What does a bioreactor do?
A Bioreactor is used for culturing cells within the lab. The growth of cells in a bioreactor is much faster compared to those grown outside in Petri dishes or flasks. Also, the ability to control the temperature and pH level within a bioreactor enables scientists to study cell growth under controlled conditions.
What are the main types of bioreactors?
There are several types of bioreactors, including stirred tank bioreactors, airlift bioreactors, membrane bioreactors, packed bed bioreactors, fluidized bed bioreactors, and immobilized cell bioreactors. Each type has its own design and operating principles suitable for specific applications.
What are bioreactors used for?
Bioreactors have a wide range of applications in various fields such as pharmaceuticals, biotechnology, food and beverage production, wastewater treatment, biofuel production, and environmental remediation. They are used for processes like fermentation, cell culture, enzyme production, biodegradation, and bioconversion.
How do bioreactors control environmental conditions?
Bioreactors control environmental conditions by regulating factors such as temperature, pH, dissolved oxygen, nutrient concentration, agitation, and gas supply. Monitoring and adjusting these parameters ensure optimal growth and productivity of the biological organisms within the bioreactor.
What is the purpose of agitation in a bioreactor?
Agitation in a bioreactor promotes mixing of the culture medium, ensuring uniform distribution of nutrients and gases. It helps prevent concentration gradients, improves mass transfer rates, and enhances contact between the biological entities and the substrate, leading to more efficient reactions.
How is sterility maintained in a bioreactor?
Sterility is crucial in bioreactors to prevent contamination and maintain the purity of the desired culture or product. It is achieved through measures such as sterilization of equipment and media, aseptic handling techniques, air filtration, and continuous monitoring of microbial contamination.
How are nutrients supplied to the organisms in a bioreactor?
Nutrients required for the growth and metabolism of organisms in a bioreactor are supplied through the culture medium. The medium contains essential nutrients like carbon sources, nitrogen sources, vitamins, minerals, and trace elements. These nutrients support the biological processes occurring within the bioreactor.
What is scale-up in bioreactor technology?
Scale-up refers to the process of transitioning from small-scale laboratory bioreactors to larger production-scale bioreactors. It involves adapting the operating conditions, design parameters, and process control strategies to ensure successful and efficient operation at a larger scale, maintaining desired product yields and quality.
How is product separation achieved in bioreactors?
Product separation in bioreactors depends on the specific application and desired product. It can be achieved through methods such as filtration, centrifugation, sedimentation, chromatography, extraction, or utilizing specialized membrane systems. The chosen method depends on factors like product characteristics, desired purity, and economic feasibility.
What are the advantages of using bioreactors?
Bioreactors offer several advantages over traditional batch processes. They provide better control over process parameters, higher productivity, improved reproducibility, and the ability to operate continuously or semi-continuously. Bioreactors also allow for the production of complex products, enable scale-up to meet commercial demands, and offer a more sustainable approach to various biological processes.
References
- Principles and Applications of Fermentation Technology, Arindam Kuila, Vinay Sharma, DOI:10.1002/9781119460381
- Chisti, Y., & Moo-Young, M. (2003). Bioreactors. Encyclopedia of Physical Science and Technology, 247–271. doi:10.1016/b0-12-227410-5/00067-3
- Jaibiba, P., Naga Vignesh, S., & Hariharan, S. (2020). Working principle of typical bioreactors. Bioreactors, 145–173. doi:10.1016/b978-0-12-821264-6.00010-3
- Mist reactors: Principles, comparison of various systems, and case studies, January 2008
- Chisti, Y. (2006). Bioreactor design. In C. Ratledge & B. Kristiansen (Eds.), Basic Biotechnology (pp. 181-200). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511802409.009
- Working principle of typical bioreactors, P.JaibibaS.Naga VigneshS.Hariharan, https://doi.org/10.1016/B978-0-12-821264-6.00010-3
- Scaling-up and Modelling Applications of Solid State Fermentation and Demonstration in Microbial Enzyme Production Related to Food Industries: An Overview, 2016, DOI:10.1201/9781315368405-29
- Anthony H. Rose (1985). Principles of fermentation technology: by P. F. Stanbury and A. Whitaker, Pergamon Press, 1984. doi:10.1016/0167-7799(85)90016-2
- https://www.bioreactors.net/what-is-a-bioreactor
- https://microbenotes.com/bioreactor/
- https://www.infors-ht.com/en/blog/what-is-a-bioreactor-and-how-does-it-work/
- https://www.engr.colostate.edu/CBE101/topics/bioreactors.html
- https://www.researchgate.net/profile/Tomas-Branyik/publication/277047195/figure/fig1/AS:292716279414784@1446800407551/Schematic-representation-of-the-airlift-bioreactor.png
- https://www.slideshare.net/PoorvajaGanesan/bioreators
- http://a-z-singleuse.org/singleuse/en/Glossary/M/Mist+bioreactors.html#:~:text=are%20hydraulically%2Ddriven%20bioreactors%20for,and%20aerated%20on%20a%20frame.
- https://www.slideshare.net/kanishjay/bioreactors-18087061
- https://www.slideshare.net/ajithnandanam/types-of-bioreactors-fermenters