What is Micropropagation?
- Micropropagation is a specialized method of plant propagation that uses tissue culture techniques to multiply plants in large quantities. It involves the asexual reproduction of plants in a laboratory setting, and its primary objective is crop improvement and rapid vegetative propagation. This process ensures that plants maintain genetic uniformity since they are all clones of the original plant.
- The procedure involves growing plant tissues, typically from the apical meristem, under sterile, controlled conditions. The apical meristem, a region of actively dividing cells, is used because it is virus-free and has high regenerative potential. Due to the use of the apical meristem, micropropagation is often referred to as meristem culture or mericlones. This ensures that each plant grown from the tissue will be genetically identical to the original, providing uniform traits in the new plants.
- The word “clone” in the context of micropropagation refers to a group of plants that have been reproduced vegetatively from a single parent plant. These plants are genetically identical, as they are derived from the same original source, and do not represent individual variations but are simply transplanted parts of the same genetic structure.
- One significant advantage of micropropagation is its efficiency. In a relatively short amount of time and with limited space, a vast number of plants can be produced. For example, the axillary bud proliferation technique can result in a tenfold increase in shoot production within a month. Given six months, it’s possible to produce up to a million plants from just one initial tissue sample, offering significant benefits for commercial agriculture and plant conservation efforts.
- Micropropagation also allows for the cultivation of a wide variety of plant species, including angiosperms, gymnosperms, and pteridophytes. In vitro culture of these plants can lead to the formation of new buds, shoots, embryoids, or entire plants, making it a versatile tool for researchers and horticulturists alike.
Micropropagation Definition
Micropropagation is a method of rapidly multiplying plants by growing plant tissues, typically from the apical meristem, in sterile, controlled conditions to produce genetically identical clones. It is used for large-scale plant production, crop improvement, and maintaining genetic uniformity.
Stages of Micropropagation – Steps of Micropropagation
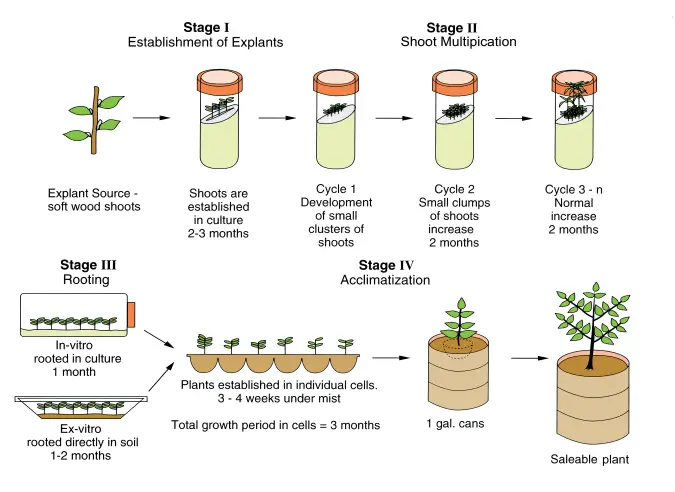
Micropropagation is a technique used for the rapid multiplication of plants through tissue culture methods. It consists of several stages, each with distinct processes and functions that ensure the successful development of plantlets from small tissue samples. The stages outlined by Murashige in 1974 provide a framework for understanding how micropropagation is conducted from start to finish.
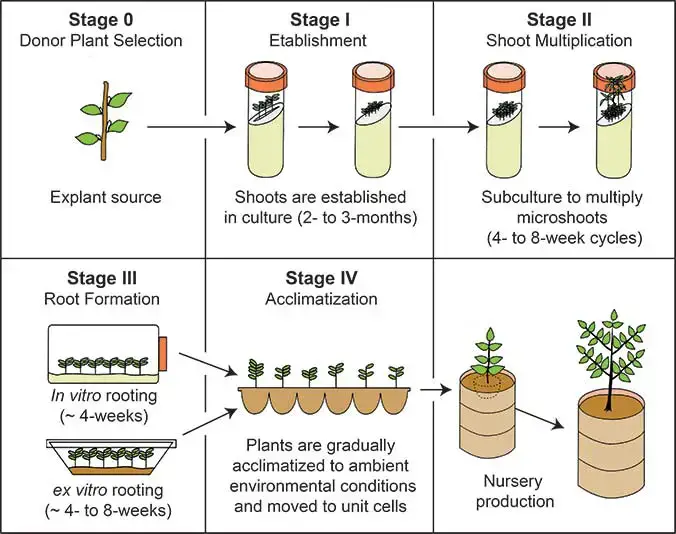
- Stage 0: Selection of Stock Plant
This preliminary step involves selecting a healthy, disease-free stock plant, from which explants—small tissue sections used for culture—can be taken. The health and quality of the stock plant are crucial, as it influences the success of the subsequent stages. - Stage I: Initiation of Culture
- Explant Selection: Small sections of the apical meristem, typically around 5-10 mm in size, are carefully excised from the healthy stock plant. This is done under sterile conditions using a sterile blade to minimize contamination risks.
- Sterilization of Explants: The chosen explants are sterilized to remove potential contaminants. Sterilization agents such as 70% alcohol, sodium hypochlorite, or calcium hypochlorite are used for 5-10 minutes. However, care must be taken not to expose the explants for too long, as overexposure can kill the cells. After sterilization, the excess agent is removed by thoroughly washing the explants several times with distilled water.
- Transfer to Nutrient Media: Once sterilized, the explants are transferred to a nutrient-rich medium, initiating sterile culture.
- Stage II: Shoot Proliferation
- Inoculation into Culture Medium: The sterilized explants are placed into a cultivation medium, such as the Murashige and Skoog (MS) medium. This medium provides the necessary nutrients for growth.
- Incubation: The explants are incubated at an optimal temperature range of 20-25°C for around two months. During this period, shoots begin to develop from the explants.
- Multiple Shoot Induction: The initial shoots can then be transferred to another medium specifically designed to induce the formation of multiple shoots. This step allows for the proliferation of several shoots from a single explant, increasing the plant yield.
- Stage III: Rooting of Shoots
- Separation and Transfer to Rooting Medium: In this stage, individual shoots are separated and transferred to a medium that promotes root formation. The development of roots is necessary for the eventual survival of the plant.
- Formation of Plantlets: By the end of this stage, fully developed plantlets with both roots and shoots are formed, typically contained within a test tube.
- Stage IV: Acclimatization
- Preparing for Soil Growth: Before the plantlets can be transferred to soil, they must be acclimatized to ensure they can survive outside the controlled environment of the laboratory. This is critical because plantlets grown in sterile, nutrient-rich environments may not immediately adapt to external soil conditions, which can lead to desiccation or failure to thrive.
- Acclimatization Process: The plantlets are gradually exposed to less favorable conditions while still in the controlled environment. This process, lasting around two weeks, allows the plantlets to adjust from a dependent, lab-based nutrition system to independent growth in soil. Functional leaves and roots are induced to enhance their ability to survive outside.
Types of Micropropagation
Micropropagation is a versatile method of plant reproduction that encompasses various techniques tailored to specific plant species and desired outcomes. The classification of micropropagation methods can be broadly divided into three primary groups, each with distinct processes and applications that facilitate plant regeneration.
- Group I: Meristem Culture
- This method focuses on the utilization of meristems to enhance axillary bud proliferation. It involves the multiplication of existing meristems through various techniques, allowing for the efficient generation of new plants.
- One common approach is the excision of apical shoots from the parent plant, commonly referred to as meristem and shoot tip culture. This technique exploits the regenerative capabilities of the apical meristem, which can produce multiple shoots.
- Additionally, this group includes the multiplication of existing meristems located within axillary shoots. Following the removal of the explants from the parent plant, these axillary buds proliferate, leading to the generation of new plant material through single node and axillary bud culture.
- Group II: Callus Culture
- Callus culture involves the formation of a mass of undifferentiated cells known as callus, which can subsequently develop into individual plant organs, such as shoots and roots. This process is especially useful when preformed meristems are absent from the explant.
- Organogenesis is a key feature of this method, where shoots and roots arise directly from the explant tissue or through de novo development from the callus. The ability of the callus to differentiate into organized structures provides flexibility in generating new plant material.
- Group III: Embryo Culture
- This technique is centered on the formation of a bipolar structure that includes both shoot and root meristems, a process termed somatic embryogenesis. It can occur from either the direct origin of embryonic tissues from the explant or from de novo formation following the induction of callus.
- In embryo culture, the explant is cultivated in a specialized embryo-inducing medium designed to promote the formation of embryos. Once developed, these embryos are then transferred to rooting and shooting media to facilitate the growth of complete plants.
Advantages of Micropropagation
Micropropagation presents numerous advantages in the realm of plant propagation and cultivation, making it a preferred method in both commercial agriculture and research settings. This technique, involving the rapid multiplication of plants under controlled conditions, offers distinct benefits that significantly enhance plant production efficiency and quality.
- Mass Propagation:
Micropropagation allows for the mass propagation of genetically identical plants, or clones. For instance, while traditional vegetative propagation might yield around 10,000 plants per year from an initial cutting, micropropagation can produce over 1,000,000 plants from a single explant within the same timeframe. This remarkable increase in plant production is a significant advantage for nurseries and agricultural producers. - Space Efficiency:
The method requires minimal space to establish cultures. With an area of approximately 1 m² in a culture room, it is possible to produce between 20,000 and 100,000 plantlets annually. This spatial efficiency enables growers to maximize production in limited areas. - Disease-Free Plantlets:
One of the most critical benefits of micropropagation is the ability to produce disease and virus-free plantlets. By utilizing meristem tip culture, which targets shoot apices known to be low in viral concentration, growers can successfully cultivate plants free from many pathogens, including viruses. This technique is particularly effective for species such as potato, chrysanthemum, and sweet potato, which may be systemically infected. - Rapid Production of Slow-Propagating Species:
Micropropagation can significantly accelerate the production of plants that typically propagate slowly, such as bulbous crops and Narcissus. This capability is essential for meeting market demands efficiently. - Vegetative Propagation of Sterile Hybrids:
The technique facilitates the vegetative propagation of sterile hybrids, enabling seed production in crops where conventional methods may fail. For example, cabbage can be successfully propagated vegetatively to ensure a reliable seed supply. - Speed and Flexibility:
In vitro micropropagation techniques are favored over traditional methods of asexual propagation due to their efficiency and adaptability. A small amount of plant tissue is sufficient to generate millions of clonal plants within a year, which contrasts sharply with the lengthy processes associated with conventional propagation methods. This advantage is crucial for rapidly increasing new cultivars, particularly in tree species that would otherwise take years to propagate. - International Exchange of Plant Materials:
The sterile nature of in vitro cultures allows for the safe international exchange of plant materials, minimizing the risk of disease introduction. This capability reduces or eliminates quarantine periods, promoting the global movement of genetic resources. - Year-Round Production:
The year-round proliferation of invitro stocks is another advantage of micropropagation. Growers can produce ornamental, fruit, and tree species irrespective of seasonal limitations, ensuring a continuous supply. - Germplasm Conservation:
Micropropagation is vital for germplasm conservation, which is crucial for plant breeding programs. The preservation of genetic materials ensures availability when needed. Meristem cells, due to their stability and viability, are excellent candidates for cryopreservation, allowing for long-term storage without the risks associated with seed longevity. - Production of Artificial Seeds:
The concept of artificial or synthetic seeds, created through somatic embryogenesis, has gained popularity. These encapsulated embryos aim to mimic true seeds, offering an alternative for plant propagation that combines the advantages of both traditional seeds and in vitro techniques. Two types of synthetic seeds, hydrated and desiccated, have been developed, broadening the scope of micropropagation applications.
Disadvantages of Micropropagation
Micropropagation, while a valuable technique in plant propagation, is accompanied by several limitations that can impact its effectiveness and application. Understanding these constraints is essential for researchers and educators aiming to leverage micropropagation in agricultural and horticultural practices.
- High Costs and Facility Requirements:
Micropropagation demands expensive laboratory equipment and sophisticated facilities, which can pose a barrier to entry for many growers. Additionally, skilled manpower is necessary to operate these facilities effectively, increasing operational costs. - Contamination Risks:
Despite rigorous sterilization and precautionary measures, contamination by various pathogens remains a significant concern. Such contamination can lead to substantial losses within a short time frame, underscoring the need for stringent aseptic techniques. - Genetic Variability:
Genetic variability is a pronounced issue in certain culture systems. While shoot tip cultures typically exhibit stability, those utilizing adventitious shoots or callus multiplication can demonstrate significant genetic variability. Sources of this variability may include chimeral breakdown, aberrant cell division, epigenetic effects, and pre-existing genetic variability within the source plant. - Vitrification:
During repeated cycles of in vitro shoot multiplication, cultures can exhibit a condition known as vitrification or hyperhydration, characterized by water-soaked, translucent leaves. This condition results from morphological, physiological, and metabolic derangements that occur during intensive multiplication in culture. It leads to a decline in growth rates, necrosis, and, in some cases, the eventual death of the shoots. Preventive measures to mitigate hyperhydration include increasing agar concentration, overlaying media with paraffin, using desiccants like CuSO₄, improving aeration through bottom cooling, and adjusting cytokinin levels or nutrient concentrations in the medium. - Non-Autotrophic Conditions:
Plants in culture are not autotrophic and rely on the nutrient-rich media provided to them. This dependency can create challenges when transitioning the plants to field conditions, as they may lack the necessary adaptations to survive independently. - Adaptation Challenges:
Plants produced via micropropagation often require a period of acclimatization before they can be transferred to field conditions. This step is crucial because micropropagated plants are frequently poorly adapted to the natural environment, which can lead to high mortality rates if not properly managed. - Limited Applicability:
Not all crops can be effectively propagated through micropropagation techniques. For example, species like cowpea exhibit high recalcitrance, making regeneration through in vitro culture challenging. Similarly, the regeneration of adult woody plants via micropropagation is often difficult. - Root Induction Issues:
Achieving successful root induction remains a challenge for many species within micropropagation systems. The variability in rooting success can hinder the overall efficiency of the propagation process. - Inconsistent Growth Rates:
Each explant may demonstrate different in vitro growth rates and maturation times, leading to a lack of uniformity in plant development. This inconsistency can be particularly problematic for flowering plants, where uniformity is often desired for commercial production.
- https://www.onlinebiologynotes.com/micropropagation-stages-types-applications-and-limitations/
- https://biologyreader.com/micropropagation-technique.html
- https://egyankosh.ac.in/bitstream/123456789/86108/1/Unit-12.pdf
- https://www.microscopemaster.com/micropropagation.html
- https://en.wikipedia.org/wiki/Micropropagation