Lentiviral transfection is a method for introducing genetic material into cells. It involves the use of lentiviruses, a type of retrovirus, to deliver the desired DNA into the target cells. Lentiviral transfection has a unique advantage over other transfection methods, in that it can infect both dividing and non-dividing cells, making it useful for a variety of applications.
The history of lentiviral transfection dates back to the discovery of retroviruses in the 1970s. Over time, researchers have developed various methods to modify the genetic material of these viruses to make them safer and more effective as a delivery system.
The importance of lentiviral transfection lies in its ability to efficiently introduce genes into cells, allowing for the study of gene function and the development of new therapies. In particular, it has proven valuable for applications in gene therapy, where the delivery of therapeutic genes can be used to treat genetic diseases. Lentiviral transfection has also been used in the fields of neuroscience, stem cell biology, and vaccine development, among others.
This approach can be used to generate lentivirus from a lentiviral vector transfected into Lenti-X 293T cells via a polyethyenimine (PEI) transfection strategy. This approach is adaptable for packaging various cell lines or transfection reagents. Once created, lentivirus can be utilised in a variety of downstream applications, including the production of stable cell lines.
Workflow Timeline
- Seed 293T packaging cells on day 0
- Evening of Day 1: Transfect packaging cells
- Day 2 (morning): 18 hours after transfection. Replace used media with new media.
- Day 3-4 (am): Harvest virus
Equipment
- Biosafety cabinet
- Pipetman
- Pipettors
- Incubator
- pH meter
- Stir plate
- Magenetic Stir Bar
Reagents
- DMEM high glucose
- L-alanyl-L-glutamine (or alternative stable glutamine)
- Heat-inactivated FBS
- Low serum medium such as Opti-MEM or Opti-Pro SFM
- Chloroquine diphosphate, 25 µM
- PEI, 1 mg/mL
- Microcentrifuge tubes
- 10 cm tissue culture dishes
- Pipettes
- Pipette tips
- Hydrochloric acid
- Sodium hydroxide
- 0.22 μm polyethersulfone (PES) filter
- 0.45 μm PES filter
- Syringes for filtering
Reagent Preparation
- DMEM Complete: 10% v/v FBS and 4 mM L-alanyl-L-glutamine. 55 mL of heat-inactivated FBS and 11 mL of 200 mM L-alanyl-L-glutamine are added to a 500 mL container of DMEM high glucose. Keep at 4 °C.
- Pro-Tip Different FBS brands and quantities can either stimulate or prevent transfection. Test numerous FBS brands and quantities to discover one that is compatible with your protocols. FBS can be obtained already inactivated by heat, or it can be inactivated in the laboratory by heating to 56 °C for 30 minutes.
- 25 mM chloroquine diphosphate
- In 10 mL of sterile water, disperse 0.129 grammes of chloroquine diphosphate salt.
- Using a 0.22 μm filter, sanitise a substance.
- Aliquot 50-100 μL and store at -20 ℃.
- Prior to use, aliquots may be frozen and kept at 4 °C. 1-2 months after thawing, aliquots must be discarded.
- 1 mg/mL PEI, linear MW 25,000 Da
- 100 mg of powder should be dissolved in 100 mL of deionized water.
- Slowly add hydrochloric acid while stirring until the solution clears.
- Check the solution’s pH level.
- To get a pH of 7.0, utilise hydrochloric acid or sodium hydroxide. Usually, the solution will be basic, necessitating an initial correction with hydrochloric acid.
- After 10 minutes, verify the pH of the solution to ensure that it has not changed.
- The solution is filtered by a 0.22 μm membrane.
- Aliquot 500-1000 μL into sterile tubes.
- Maintain the tubes at -80 °C.
- The solution can be stored at 4 °C for up to two months after defrosting. After two months, dispose of the tube and thaw a fresh supply.
- For each fresh batch of 1 mg/mL PEI and each cell line, the ideal mass DNA:mass PEI ratio must be found empirically.
Considerations Before You Start
- To get a high viral titer, the health of the packaging cell line is essential.
- 3 times per week, 293T cells must be split:
- Monday: Plate 1×106 cells in 15 mL of DMEM in a T75 flask.
- Wednesday: Plate 1×106 cells in 15 mL DMEM in a T75 flask.
- Friday: Plate 8×105 cells in 15 mL of DMEM in a T75 flask.
- Do not add pen-strep to media outlets.
- Utilize cells less than passage 15 for viral generation.
Lentiviral Transfection Protocol
- Plant 3.8×106 293T packaging cells per plate in full DMEM in 10 cm tissue culture plates.
- Incubate the cells for 20 hours at 37 °C and 5% CO2.
- Add 10 mL of fresh DMEM complete containing 25 μM cloroquine diphosphate and incubate for approximately five hours.
- To complete 10 mL of DMEM, add 10 μL of 25 mM chloroquine diphosphate.
- Combine the following three transfection plasmids:
| Reagent | Amount per 10 cm dish* |
|---|---|
| psPAX2 | 1.3 pmol |
| pMD2.G | 0.72 pmol |
| Transfer Plasmid* | 1.64 pmol |
| OptiPro SFM to total volume | 500 μL |
*Plasmid concentrations and ratios should be optimized for each transfer plasmid.
Pro-Tip Endotoxins can hinder transfection; consequently, endotoxin removal must be incorporated into the plasmid DNA purification process. Plasmid DNA should be propagated in an endonuclease-negative E. coli strain, such as NEB stable, for optimal quality.
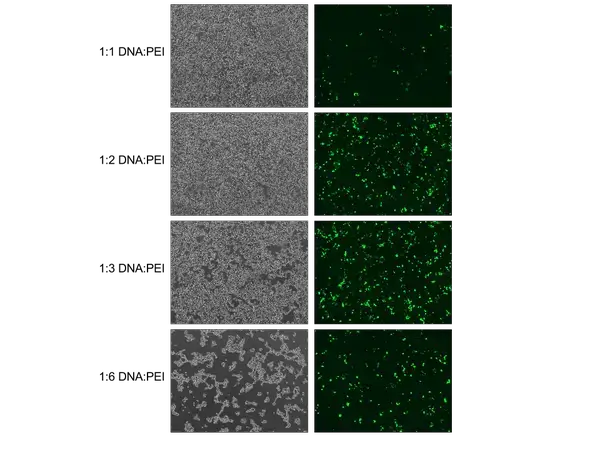
- Dilute the aforesaid 500 μL mixture into 500 μL PEI-OptiPro SFM with sufficient PEI such that the ratio of μg DNA to g PEI is 1:3 (a total of 1000 μL per 10 cm dish).
- This would be 83.4 μL of 1 mg/mL PEI in 416.6 μL of OptiPro SFM per 10 cm plate when using transfer plasmid pHAGE TRE dCas9-KRAB (plasmid DNA total of 27.8 μg).
- Refer to the following table for potential ratios to test:
| Ratio of DNA:PEI | μg of DNA | μL of 1 mg/mL PEI |
|---|---|---|
| 1:1 | 18.9 | 18.9 |
| 1:2 | 18.9 | 37.8 |
| 1:3 | 18.9 | 56.7 |
| 1:4 | 18.9 | 75.6 |
| 1:5 | 18.9 | 94.5 |
| 1:6 | 18.9 | 113.4 |
- Add the diluted PEI to the diluted DNA with care. Drop by drop, add the diluted PEI while gently agitating the diluted DNA. 15-20 minutes of incubation at room temperature.
- Transfer the transfection mixture with caution to the Lenti-X 293T packaging cells. Drop by drop, add the transfection mixture, taking care not to disturb the cells.
- The cells should be incubated for 18 hours, or until the following morning.
- The next morning, thoroughly aspirate the media. Replace the medium with 15 mL of full DMEM medium.
- Perform an incubation on the cells.
- At 48, 72, and 96 hours after transfection, virus can be harvested as individual harvests or as a combined harvest in which all individual harvests are pooled. Transfer harvested medium to a polypropylene storage tube and store at 4 °C between harvests if pooling harvests.
- At ~500 g for 5 minutes, centrifuge the viral supernatant to pellet any packing cells acquired during harvesting.
- Apply a 0.45 micron PES filter to the supernatant.
- The viral supernatant may be held at 4 °C for several hours, but it must be aliquoted, flash-frozen in liquid nitrogen, and stored at -80 °C as soon as possible to prevent titer loss.
References
- https://pharm.ucsf.edu/xinchen/protocols/lv-transfection
- https://www.creative-biogene.com/support/lentivirus-transduction-protocol.html
- https://www.addgene.org/protocols/lentivirus-production/
- https://www.sigmaaldrich.com/IN/en/technical-documents/protocol/genomics/advanced-gene-editing/lentiviral-transduction
- https://www.mdanderson.org/documents/core-facilities/Functional%20Genomics%20Core/Lentivirus%20production%20protocols.pdf
- https://cdn.origene.com/assets/documents/lentiviral/how_to_use_lentivirus_a_short_protocol.pdf
- https://biochem.slu.edu/faculty/gonzalo/wp-content/uploads/2019/03/Retroviraltransduction.pdf
- https://www.bio-protocol.org/exchange/preprintdetail?type=3&id=241
- https://www.salk.edu/wp-content/uploads/2015/11/3rdgenLentiTransfectionprotocol.pdf