What is Immunoelectrophoresis?
Immunoelectrophoresis is a biochemical approach for protein separation and characterization that is based on electrophoresis and antibody-antigen interactions. It uses immunodiffusion and electrophoresis techniques to identify and analyse specific antigens within a protein mixture.
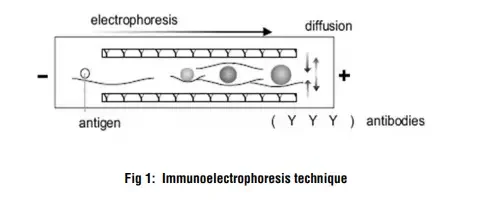
The immunoelectrophoresis procedure begins with electrophoresis, which separates the antigen mixture into its distinct components. This is accomplished by inserting the antigens into wells cut in a gel matrix and applying an electric field, which causes the antigens to migrate according to their charge. The gel acts as a medium for the antigens to pass through.
Following the electrophoresis stage, a trough perpendicular to the antigen transport direction is carved into the gel. The trough is then filled with antibodies specific to the antigens of interest. These antibodies migrate laterally within the gel and come into contact with the migrating antigens. When antibodies bind to their target antigens, a lattice forms, causing immune complexes to precipitate. This lattice development and precipitation allows the nature of the antigens present in the initial mixture to be determined.
The term “immunoelectrophoresis” was coined by Grabar and Williams in 1953, and since then, different variants of the technique have been developed and utilized. Some of the notable variants include:
- Immunoelectrophoretic analysis: This method, also known as one-dimensional immunoelectrophoresis, follows the approach introduced by Grabar. It involves the separation of antigens in a single dimension, followed by the application of antibodies to identify and characterize the antigens.
- Crossed immunoelectrophoresis: This technique, also known as two-dimensional quantitative immunoelectrophoresis, was developed by Clarke and Freeman. It involves performing two separate electrophoresis steps at right angles to each other. The first electrophoresis separates the antigens, and the second electrophoresis involves the application of antibodies to form immune complexes and facilitate their identification.
- Rocket immunoelectrophoresis: This method, also known as one-dimensional quantitative immunoelectrophoresis, was introduced by Laurell. It utilizes a rocket-shaped gel to measure the concentration of a specific antigen in a sample. The height of the rocket-shaped precipitation arc correlates with the concentration of the antigen.
- Fused rocket immunoelectrophoresis: This variant, developed by Svendsen and Harboe, combines the principles of rocket immunoelectrophoresis and fused rocket electrophoresis. It allows for the quantification of both antigen and antibody concentrations simultaneously.
- Affinity immunoelectrophoresis: This method, developed by Bøg-Hansen, employs antibodies coupled to solid supports such as agarose beads. The immobilized antibodies selectively bind to the corresponding antigens, enabling their separation and identification.
Immunoelectrophoresis has been widely used in biochemical research and clinical diagnostics for several decades. It offers a versatile tool for the separation, quantification, and characterization of proteins based on their antigenic properties.
Immunoelectrophoresis Definition
Immunoelectrophoresis is a biochemical technique that combines electrophoresis and antibody-antigen reactions to separate and identify proteins based on their antigenic properties.
Objectives of Immunoelectrophoresis
The objectives of immunoelectrophoresis include:
- Separation of proteins: Immunoelectrophoresis allows the separation of proteins based on their charge and size. This helps in identifying and analyzing individual protein components within a mixture.
- Identification of antigens: By utilizing specific antibodies, immunoelectrophoresis enables the identification and characterization of antigens present in a protein mixture. This information is crucial for understanding the composition and properties of the sample.
- Quantification of antigens: Immunoelectrophoresis can be used to estimate the concentration of specific antigens in a sample by measuring the height or intensity of precipitate arcs formed during the reaction. This quantitative aspect is valuable in various research and diagnostic applications.
- Study of antibody-antigen interactions: Immunoelectrophoresis provides insights into the interactions between antibodies and antigens. It helps in evaluating the specificity and affinity of antibodies towards their target antigens, contributing to the understanding of immune responses and immune-related disorders.
- Characterization of immune complexes: By visualizing the lattice formation and precipitation of immune complexes, immunoelectrophoresis assists in characterizing the nature and properties of these complexes. This information is useful for studying immune reactions and diagnosing immune-related diseases.
- Diagnostic applications: Immunoelectrophoresis has been widely used in clinical laboratories for the detection and diagnosis of various diseases, including immunodeficiency disorders, autoimmune diseases, and certain types of cancers. It provides valuable information about the presence and characteristics of specific antigens in patient samples.
Overall, the objectives of immunoelectrophoresis revolve around the separation, identification, quantification, and characterization of proteins and their interactions with antibodies. It plays a vital role in research, diagnostics, and understanding immune-related processes.
Principle of Immunoelectrophoresis
The principle of immunoelectrophoresis is based on the combination of electrophoresis and antibody-antigen reactions to separate and identify protein components within a mixture. The process involves several key steps.
- Electrophoresis: An electric current is applied to a slide or gel layered with a matrix, such as agarose or polyacrylamide. The antigen mixture is placed into wells cut into the gel. As the current is applied, the antigens migrate through the gel matrix according to their charge and size. Electrophoresis separates the mixture into individual antigen components.
- Antibody-antigen reaction: After the electrophoresis step, specific antisera or antibodies are placed in troughs parallel to the direction of electrophoretic migration. These antisera contain antibodies that are specific to the target antigens of interest.
- Diffusion: Once the antisera are added, diffusion is allowed to occur. The antibodies in the troughs move toward the separated antigen components that have migrated through the gel. The diffusion process facilitates the interaction between the antibodies and antigens.
- Formation of precipitin lines: As the antibodies encounter their respective antigen components, a reaction occurs, resulting in the formation of precipitin lines. Each precipitin line corresponds to the reaction between an individual protein and its specific antibody. The precipitin lines typically become visible within 18-24 hours after the diffusion step.
By observing the formation and positioning of the precipitin lines, it is possible to identify and characterize the antigen components present in the original mixture. Each precipitin line represents a specific protein-antibody reaction and indicates the presence of a particular antigen in the sample.
The principle of immunoelectrophoresis relies on the movement of antigens through the gel matrix under the influence of an electric field, followed by their interaction with specific antibodies leading to the formation of visible precipitin lines. This technique allows for the separation, identification, and characterization of proteins based on their antigenic properties, contributing to various research and diagnostic applications.
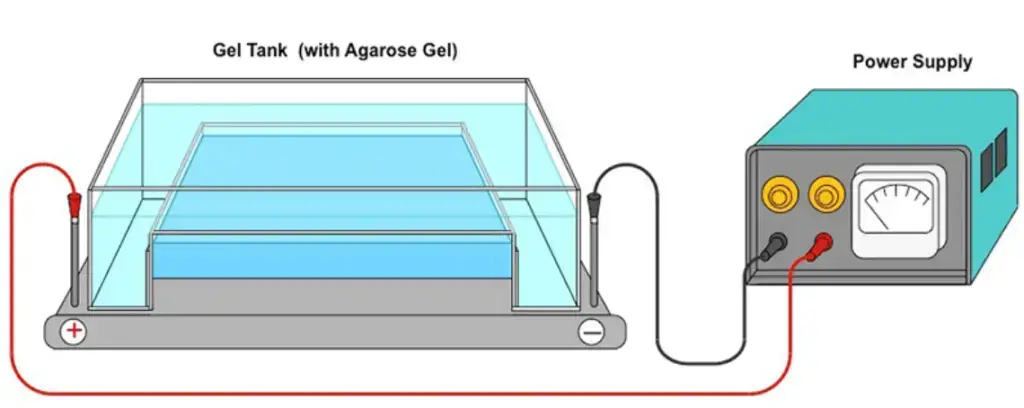
Material Required
To perform immunoelectrophoresis, several materials and reagents are required. These include:
- Glass wares: Conical flask, measuring cylinder, and beaker are commonly used glassware items in the laboratory for preparing and handling solutions during the immunoelectrophoresis procedure.
- Reagents: a. Distilled water: High-quality distilled water is necessary for preparing solutions and reagents used in immunoelectrophoresis. b. Alcohol: Usually, ethanol or isopropyl alcohol is used for cleaning and sterilizing laboratory equipment and glassware.
- Incubator (37°C): An incubator set at a temperature of 37°C is essential for providing the optimal conditions required for the incubation of antigen-antibody reactions during immunoelectrophoresis.
- Microwave or Bunsen burner: A microwave or Bunsen burner is used for heating and melting agarose or other gel materials used for creating the gel matrix in the electrophoresis process.
- Electrophoresis unit: An electrophoresis unit is required to run the electrophoretic separation of antigens in the gel matrix. It typically consists of a power supply, buffer chambers, and electrode connections.
- Vortex mixer: A vortex mixer is used for efficient mixing and resuspension of reagents and samples.
- Spatula: A spatula is used for transferring solid reagents or scraping materials during the preparation of solutions or gels.
- Micropipettes and tips: Micropipettes and disposable tips are used for accurate and precise measurement and transfer of small volumes of reagents and samples.
- Gel cutter: A gel cutter is used to precisely cut out specific regions of the gel after the immunoelectrophoresis procedure for further analysis or characterization.
- Moist chamber: A moist chamber, typically a box lined with wet cotton, is used to maintain the humidity around the gel during the incubation period, preventing dehydration of the gel and ensuring optimal conditions for antigen-antibody reactions.
These materials and reagents are essential for the successful execution of immunoelectrophoresis. It is important to ensure that all equipment and glassware are clean and sterile to avoid contamination and accurate measurement and handling of reagents to obtain reliable results.
Procedure of Immunoelectrophoresis
The procedure of immunoelectrophoresis involves several steps to separate and identify specific antigens within a protein mixture. Here is a step-by-step overview of the procedure:
- Preparation of agarose gel: An agarose gel is prepared on a glass slide placed in a horizontal position. The gel provides a matrix for the electrophoretic separation of antigens.
- Well formation: Using a sample template, wells are carefully created in the application zone of the gel. These wells will be used to load the samples and controls.
- Sample preparation: The sample is diluted by mixing 20 μl of antigen solution with 10 μl of protein diluent solution. This dilution helps ensure proper migration and detection of the antigens.
- Sample application: Using a 5 μl pipette, 5 μl of the diluted sample and corresponding control are applied across each respective slit (Control slit and Sample slit) on the gel. This ensures that the antigens from the sample and control are properly positioned for electrophoresis.
- Electrophoresis: The gel, with the loaded samples on the cathodic side, is placed into the electrophoresis chamber. Electrophoresis is run for approximately 20 minutes at 100 volts. The electric current separates the antigen components based on their charge and size, causing them to migrate through the gel.
- Addition of antiserum: After the completion of electrophoresis, 20 μl of the corresponding antiserum (specific antibodies) is added to troughs created in a moist chamber. The gel is placed horizontally in the moist chamber and incubated for 18-20 hours at room temperature. During this incubation, the antiserum diffuses laterally and reacts with the separated antigens.
- Drying the gel: Once the incubation period is complete, the agarose gel is carefully removed from the moist chamber and placed in a horizontal position. Blotter sheets are used to dry the gel, removing excess moisture.
- Gel washing: The gel is soaked in saline solution for 10 minutes to remove any remaining contaminants or unwanted substances. This washing step is repeated twice more to ensure thorough cleaning of the gel.
- Drying and staining: The gel is dried at a temperature below 70°C to remove residual moisture. It can then be stained with a protein staining solution for approximately 3 minutes. After staining, the gel is decolorized by immersing it in distaining solution baths for 5 minutes.
- Evaluation of results: Once the gel is dry and the staining process is complete, the results of the immunoelectrophoresis can be evaluated. The precipitin lines formed indicate the specific protein-antibody reactions, allowing for the identification and characterization of the antigens present in the original sample.
By following this step-by-step procedure, immunoelectrophoresis enables the separation, identification, and evaluation of specific antigens within a protein mixture.
Results of Immunoelectrophoresis
The results of immunoelectrophoresis are obtained by analyzing the formation and characteristics of precipitin arcs or lines that indicate the interaction between antigens and antibodies. Here are some key aspects to consider when interpreting the results:
- Elliptical precipitin arcs: The presence of elliptical precipitin arcs on the gel indicates successful antigen-antibody interactions. These arcs represent the regions where antigens and antibodies have come together, forming a lattice structure and resulting in the precipitation of immune complexes. The elliptical shape of the arcs is typically observed due to the diffusion of antibodies and antigens during the incubation period.
- Absence of precipitate: If no precipitate is formed or observed, it suggests that there was no specific reaction between the antigens and antibodies. This could indicate the absence of the target antigen in the sample or insufficient concentration of antibodies to detect the antigen.
- Identification of antigens: Different antigens (proteins) present in the sample can be identified based on various characteristics of the precipitation lines. These characteristics include:
- Intensity: The intensity of the precipitin lines can vary, indicating different concentrations or levels of the target antigens in the sample.
- Shape: The shape of the precipitin lines can provide additional information. For example, a sharp and well-defined line may indicate a strong antigen-antibody interaction, while a diffuse or smudged line could suggest weaker or multiple interactions.
- Position: The position of the precipitin lines on the gel can help identify specific antigens. By comparing the positions of the lines with known standards or controls, it is possible to determine the identity of the antigens present in the sample.
Analyzing the results of immunoelectrophoresis requires careful observation and comparison with appropriate controls and standards. The intensity, shape, and position of the precipitin lines provide valuable information about the presence, concentration, and characteristics of the antigens in the original sample. This information is essential for identifying and characterizing specific proteins and their interactions with antibodies, contributing to research, diagnostic, and immunological studies.

Note : For better precipitin lines incubate for longer period at 37oC
Advantages of Immunoelectrophoresis
Immunoelectrophoresis offers several advantages as a powerful analytical technique for the identification and characterization of antigens. Here are some key advantages of immunoelectrophoresis:
- High resolving power: Immunoelectrophoresis combines the separation of antigens by electrophoresis with immunodiffusion against an antiserum. This integration of techniques provides high resolving power, allowing for the separation and identification of different antigens based on their charge, size, and antigen-antibody interactions. It enables the detection of multiple antigens in a single sample.
- Identification of multiple antigens: One of the main advantages of immunoelectrophoresis is the ability to identify multiple antigens in serum or other biological samples simultaneously. By utilizing specific antisera against different antigens, multiple precipitin lines can be observed on the gel, each representing a distinct antigen-antibody interaction. This makes immunoelectrophoresis a valuable tool for detecting and characterizing a wide range of antigens in a single analysis.
- Sensitivity: Immunoelectrophoresis is a highly sensitive technique, capable of detecting small amounts of antigens in a sample. The interaction between antibodies and antigens leads to the formation of visible precipitin lines, even at low concentrations. This sensitivity makes it a valuable tool in diagnostic applications, where the detection of trace amounts of antigens is crucial for accurate diagnosis.
- Versatility: Immunoelectrophoresis can be applied to various sample types, including serum, plasma, and other biological fluids. It is widely used in medical laboratories and research settings for the identification and characterization of proteins and antibodies. The technique can also be adapted for different purposes, such as the study of immune responses, antigen profiling, and disease diagnosis.
- Cost-effective: Immunoelectrophoresis is a relatively cost-effective method compared to some other protein analysis techniques. It requires minimal equipment and reagents, making it accessible to laboratories with limited resources. Additionally, it allows for the analysis of multiple antigens in a single assay, reducing the need for separate tests and saving time and resources.
In summary, immunoelectrophoresis offers several advantages, including high resolving power, the ability to identify multiple antigens in a sample, sensitivity, versatility, and cost-effectiveness. These advantages have made it a valuable tool in various fields, including medical diagnostics, immunology research, and protein analysis, contributing to our understanding of antigens and their interactions with antibodies.
Disadvantages of Immunoelectrophoresis
While immunoelectrophoresis is a valuable analytical technique, it does have certain disadvantages that should be taken into consideration. Here are some of the main disadvantages associated with immunoelectrophoresis:
- Slower and less sensitive compared to Immunofixation electrophoresis: Immunoelectrophoresis is generally slower and less sensitive than newer techniques such as Immunofixation electrophoresis. Immunofixation electrophoresis offers higher sensitivity and the ability to detect smaller quantities of target proteins, making it a preferred choice in some diagnostic applications.
- Limited detection of small monoclonal M-proteins: Immunoelectrophoresis may fail to detect some small monoclonal M-proteins, which are abnormal proteins produced by certain plasma cell disorders such as multiple myeloma. The presence of rapidly migrating immunoglobulins in high concentrations can overshadow the presence of small M-proteins, making their detection challenging.
- Limited availability of specific antibodies in food analysis: The use of immunoelectrophoresis in food analysis is limited by the availability of specific antibodies. Developing specific antibodies against food antigens can be challenging, which restricts the application of immunoelectrophoresis in this field. Alternative techniques such as enzyme-linked immunosorbent assays (ELISAs) or molecular-based methods are often preferred for food analysis due to their wider range of available antibodies and higher sensitivity.
Despite these limitations, it’s important to note that immunoelectrophoresis continues to be a valuable tool in various fields, including medical diagnostics, research, and protein analysis. It provides useful information on antigen-antibody interactions and the characterization of proteins. However, in certain situations where higher sensitivity or specific antibody availability is crucial, alternative techniques may be preferred over immunoelectrophoresis.
Application of Immunoelectrophoresis
Immunoelectrophoresis has a wide range of applications in various fields, particularly in medical diagnostics and immunology. Here are some key applications of immunoelectrophoresis:
- Identification and quantification of proteins: Immunoelectrophoresis is a valuable tool for identifying and approximately quantifying various proteins present in serum or other biological samples. It allows for the separation and visualization of different proteins based on their antigen-antibody interactions, enabling the analysis of complex protein mixtures.
- Diagnosis of gammopathies: Immunoelectrophoresis is commonly used in patients suspected of having monoclonal or polyclonal gammopathies. It helps in the detection and characterization of abnormal proteins, including myeloma proteins, in human serum. By analyzing the pattern of protein bands, it aids in the diagnosis and evaluation of conditions such as multiple myeloma and other disorders affecting the immune system.
- Monitoring antigen and antibody purity: Immunoelectrophoresis is useful for monitoring the purity of antigens and antibodies. It can identify a single antigen in a mixture of antigens, ensuring the specificity and quality of the components used in various immunological assays and experiments.
- Qualitative analysis of M-proteins: Immunoelectrophoresis is an older but still relevant method for qualitatively analyzing M-proteins in serum and urine. M-proteins are abnormal monoclonal proteins associated with conditions such as multiple myeloma and related disorders. Immunoelectrophoresis helps in the identification and characterization of these proteins, aiding in the diagnosis and evaluation of disease states.
- Evaluation of therapeutic response and immune system disorders: Immunoelectrophoresis plays a role in diagnosing and evaluating therapeutic responses in various disease states affecting the immune system. It can provide valuable insights into the production and distribution of immunoglobulins, aiding in the diagnosis of conditions such as hypogammaglobulinemia or hypergammaglobulinemia.
Overall, immunoelectrophoresis has a significant impact on protein identification, immunology, and medical diagnostics. Its applications range from protein analysis and monitoring purity to the diagnosis and evaluation of immune-related disorders. While newer techniques have emerged, immunoelectrophoresis continues to play a valuable role in specific clinical and research settings.
Interpretation
Interpretation of immunoelectrophoresis results involves observing the formation of precipitin lines and ensuring proper handling of samples and equipment. Here are some key points for interpretation:
- Formation of precipitin lines: The presence of precipitin lines indicates the interaction between antibodies and specific antigens. A single continuous precipitin line denotes the homogeneity of the antiserum to the antigen, indicating a specific antibody-antigen reaction.
- Heterogeneity of antiserum: The presence of more than one precipitin line indicates the heterogeneity of the antiserum to the antigen. Multiple lines suggest the presence of different immunodominant epitopes, indicating the non-identical nature of these epitopes.
- Proper loading of samples: It is important to load samples directly into the wells and troughs without spilling to the sides. Inadequate filling of the wells and troughs can result in the absence of precipitin lines or improper loading of the samples, leading to inaccurate results.
- Moist chamber maintenance: The moist chamber used during incubation should have enough moist cotton to prevent the agarose gel from drying. Drying of the gel can affect the diffusion of antibodies and antigens, compromising the formation of precipitin lines.
- Sufficient electrophoresis distance: The antigen should travel at least 3/4th of the gel during electrophoresis to ensure sufficient separation and migration. Insufficient electrophoresis distance may result in incomplete separation and affect the interpretation of results.
- Proper pouring of gel: When pouring the gel, it is important to place the glass plate on a flat surface and avoid disturbing it once the gel is poured. Uneven pouring can lead to variations in the gel thickness, which can affect the migration and resolution of antigens during electrophoresis.
By considering these factors and following proper procedures, accurate interpretation of immunoelectrophoresis results can be achieved, leading to valuable insights into the antibody-antigen interactions and the characterization of proteins.
Precautions of Immunoelectrophoresis
Precautions need to be taken during the immunoelectrophoresis experiment to ensure accurate and reliable results. Here are some key precautions to consider:
- Familiarize yourself with the procedure: Before starting the experiment, thoroughly read and understand the entire procedure. This helps ensure that you follow the correct steps and avoid any mistakes or omissions.
- Wear gloves: Always wear gloves while performing the immunoelectrophoresis experiment to maintain aseptic conditions and prevent contamination.
- Proper preparation of reagents: Follow the instructions carefully when preparing reagents such as 1X TAE (Tris-acetate-EDTA) and agarose gel. Use sterile distilled water and accurately measure the quantities to ensure the correct concentrations.
- Clean glass plates: Wipe the glass plates with cotton and clean them with alcohol to remove any grease or contaminants. This step ensures even spreading of the agarose gel and helps prevent irregularities in the gel surface.
- Neatly cut wells and troughs: Cut the wells and troughs in the gel neatly, without rugged margins. Clean and well-defined wells and troughs facilitate the proper loading of samples and antiserum.
- Temperature control: Allow the agarose gel to cool to 55°C before adding the antiserum. Higher temperatures can lead to the inactivation of antibodies, affecting the antigen-antibody reactions.
- Maintain a humid atmosphere: Ensure that the moist chamber contains enough wet cotton to maintain a humid environment. This helps prevent the drying of the agarose gel during incubation and ensures optimal diffusion of antibodies and antigens.
By following these precautions, you can minimize the risk of contamination, maintain the integrity of reagents, and create suitable conditions for accurate immunoelectrophoresis results.
FAQ
What is immunoelectrophoresis?
Immunoelectrophoresis is a biochemical method that combines electrophoresis and immunodiffusion to separate and characterize proteins based on their electrophoretic mobility and reaction with antibodies.
What is the principle of immunoelectrophoresis?
Immunoelectrophoresis involves the separation of antigens by electrophoresis, followed by reaction with specific antibodies. The formation of precipitin lines indicates the presence of antigen-antibody interactions.
What are the advantages of immunoelectrophoresis?
Immunoelectrophoresis has high resolving power and can identify multiple antigens in a sample. It is useful for protein identification, immunodominant epitope determination, and monitoring antigen-antibody purity.
How does immunoelectrophoresis compare to immunofixation electrophoresis?
Immunoelectrophoresis is slower, less sensitive, and more difficult to interpret compared to immunofixation electrophoresis. Immunofixation electrophoresis is more suitable for detecting small monoclonal M-proteins.
What are the applications of immunoelectrophoresis?
Immunoelectrophoresis is used for protein identification and quantization, detecting gammopathies, analyzing complex protein mixtures, diagnosing immune-related diseases, and evaluating therapeutic responses.
What are the materials required for immunoelectrophoresis?
The materials include glassware (conical flask, measuring cylinder, beaker), reagents (distilled water, alcohol), and equipment (incubator, electrophoresis unit, vortex mixer, micropipettes, gel cutter).
What is the procedure for immunoelectrophoresis?
The procedure involves preparing an agarose gel, loading samples and controls, performing electrophoresis, adding specific antiserum, incubating, drying the gel, staining, and evaluating the results.
How do you interpret immunoelectrophoresis results?
The presence of precipitin lines indicates antigen-antibody interactions. A single continuous line suggests homogeneity of the antiserum, while multiple lines indicate heterogeneity and the presence of different immunodominant epitopes.
What precautions should be taken during immunoelectrophoresis?
Precautions include wearing gloves, following the procedure carefully, properly preparing reagents, maintaining temperature control, cutting wells and troughs neatly, and ensuring a humid atmosphere in the moist chamber.
Are there limitations to immunoelectrophoresis?
Immunoelectrophoresis may fail to detect small M-proteins and has limited use in food analysis due to the availability of specific antibodies. Other advanced techniques like immunofixation electrophoresis or more sensitive immunoassays may be preferred in certain cases.
References
- Csako, G. (2018). Immunoelectrophoresis: A Method with Many Faces. Electrophoretic Separation of Proteins, 249–268. doi:10.1007/978-1-4939-8793-1_21
- https://en.wikipedia.org/wiki/Immunoelectrophoresis
- https://himedialabs.com/TD/HTI005.pdf
- http://lnmuacin.in/studentnotice/2020/Immunoelectrophoresis.pdf
- http://www.ndvsu.org/images/StudyMaterials/Micro/Immunoelectrophoresis-test.pdf