Microbiology laboratories require well-built rooms that are equipped with tools, glassware and equipment. Test tubes, culture tubes, Petri dishes and Erlenmeyer flasks are the most important types of glassware in a microbiological lab.
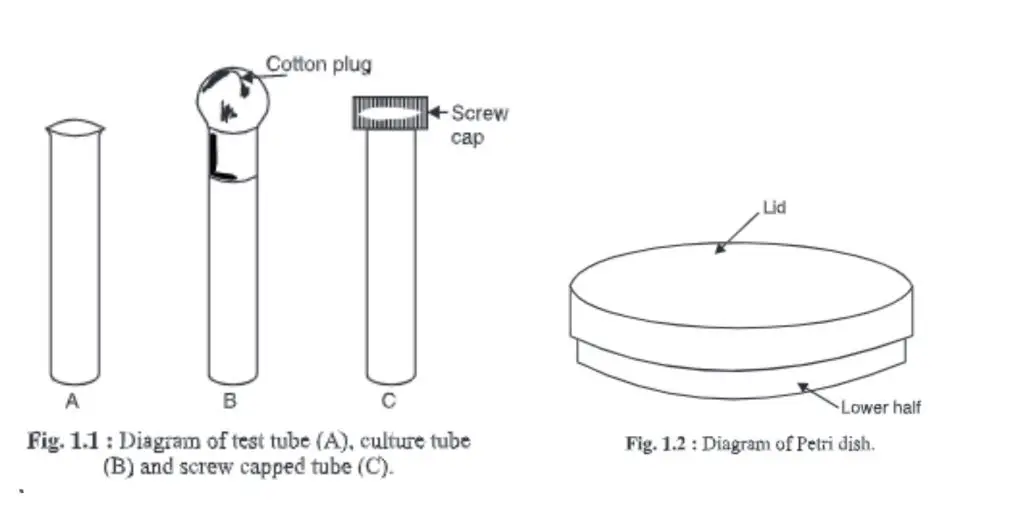
1. Test tube, culture tube and screw-capped tubes
- These glasses are made of glass with one side being closed and the other open.
- It is known as a test tube if its side wall curves outwardly from the end; it is also known as a culture tube. A screw-capped tube is characterized by a tube with a side wall that has screws so that he can be fitted e.g. : a screw-capped tube
- They are used in microbiological laboratories.
- Test tubes are used to test chemicals like pH. Culture tubes can be used to prepare agar slants or purify microorganisms. The open end of the tube is filled with a non-absorbent cotton plug.
- Sometimes, the microorganisms can be purified and kept in screw-capped tubes.
2. Petri dish
- This dish was created by R.J. Petri (a student of Robert Koch, a renowned bacteriologist) for the first time.
- It is made up of two small glass dishes: the lid or upper half and the bottom or lower half.
- These dishes can be used in any microbiological laboratory to isolate and cultivate different types of microorganisms.
- Its diameter can vary depending on the requirements.
- The bottom half of the sterilized Petri dish is covered with molten agar medium.
- Sterilize the Petri dishes by placing them in a Petri dish box and then in an oven/autoclave.
- Disposable sterile plastic Petri dishes can also be purchased for this purpose.
3. Pipette
- It is a graduated and cylindrical glass apparatus.
- The lower end, or the mouth piece, tapers. The middle portion of the mouth is larger or about the same size as its mouth end.
- It has the numbers 1, 2, and 10.
- There are different capacities such as 0, 0.5, and 1, respectively. 10 ml, etc. hence measures different quantities.
- It’s used to transfer liquid in appropriate containers.
- After being filled with cotton, it should be sterilized in an oven or autoclave.
- To ensure safety, liquid should be sucked using a pipette-sucker attached to the normal end.
- Sterile pipettes can be sterilized by placing them in a steal container (steal container). The steal container is then sterilized at 121C for 30 minutes.
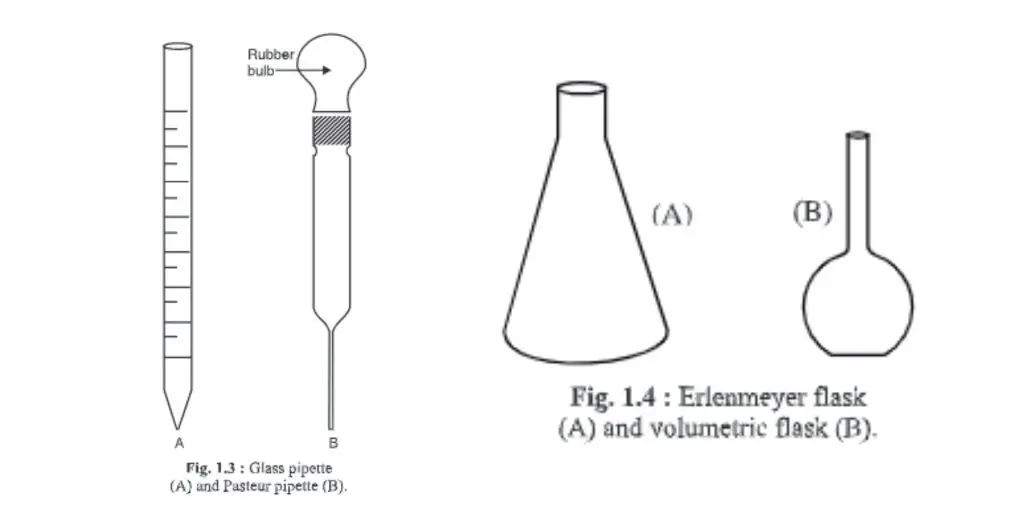
4. Pasteur pipette
- You can make the Pasteur pipette by using a hollow glass tube with a similar diameter to a standard or graduated pipette. One end of the tube is heated to melt the glass and form a narrow, similar end to a 10 ml pipette. A rubber bulb is attached to the other end.
- To prevent contamination, pipettes are often plugged with cotton before being sterilized.
- Pasteur pipettes can be used once, then disinfectant is applied to them.
5. Erlenmeyer flasks
- Erlenmeyer invented the flask for the first time. It is therefore called Erlenmeyer Flask.
- It has a narrow beak at the top and an opening at the bottom.
- Flasks come in different sizes and can hold different volumes, such as 100, 250 or 500 ml liquids, 1000 ml liquids, or 2000 ml.
- Flasks can be either flat or round-bottomed.
- Sometimes, the flasks can also be graduated to indicate the volume of liquid.
- Some modifications can be made to Erlenmeyer flasks according to customer requirements. For example, a beak is made near the neck to connect to rubber tubing or other equipment. These flasks can be called side-arm flasks.
- During sterilization, a cotton plug is placed in the mouth the flask.
- Before microbiological use, it must be sterilized.
6. Volumetric flasks
- It is used for preparing solution of exact strength.
- The upper portion is narrow and cylindrical, and marked with a point. This marks denotes the water level that must be maintained at this point.
- The lower half of the body is round and voluminous.
- Its flat base allows it to be placed properly on the surface.
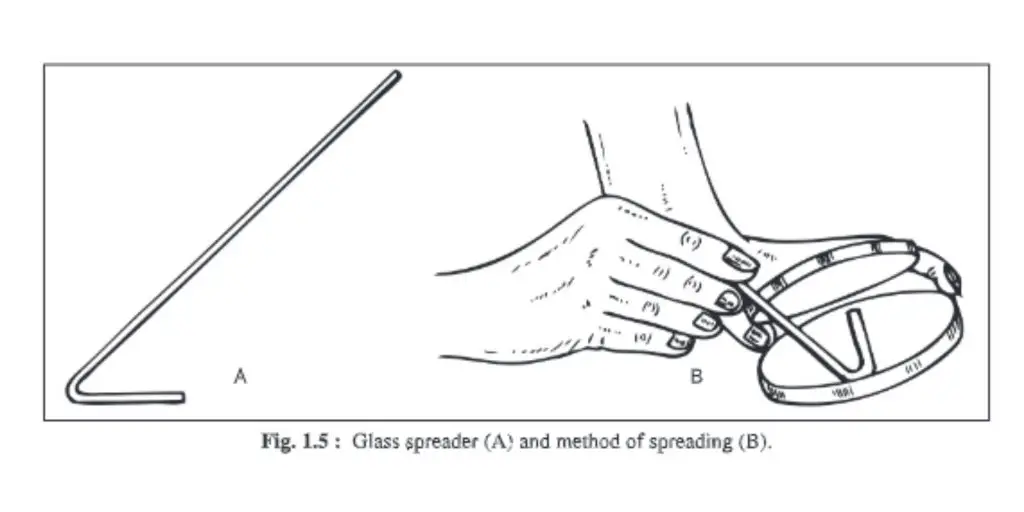
7. Glass spreader
- You can make a glass spreader by benting a piece of glass rod into an L-shaped shape.
- It is used for spreading microorganisms evenly on agar surfaces in liquid medium.
- The long arm is held in your hand, while the small arm is flame-sterilized and placed on agar.
- It is brought forth and returned so that liquid microorganisms can be separated and evenly distributed on the entire surface of the agar.
8. Haemocytometer
- A device that measures blood cells is called a haemocytometer.
- This can also be used to count other cells, such as bacteria and spores.
- The haemocytometer is made up of large squares. Each square is 1 x 1x 0.1mm = 0.1mm3 volume. It has an area of 1×1 mm = 1mm2. The chamber depth is 0.1mm. A cubie millimeter, or 0.0001 cm, or 1 ml, is equal to 10-4ml. Hence. To calculate the bacterial count per ml, multiply the large square by 104.
- Each large square has 25 medium-sized squares. They are 0.2 mm long, 0.2 inches wide and 0.1 inch deep. The volume is 0.04 mm3 with an area of 0.04mm and area of 0.04mm. This is equal to 1/25 of large squares or 1/25 0.1mm3.
- Each square of medium size is divided into 16 smaller squares with 0.0025mm2 area volumes of 0.000255 mm3.
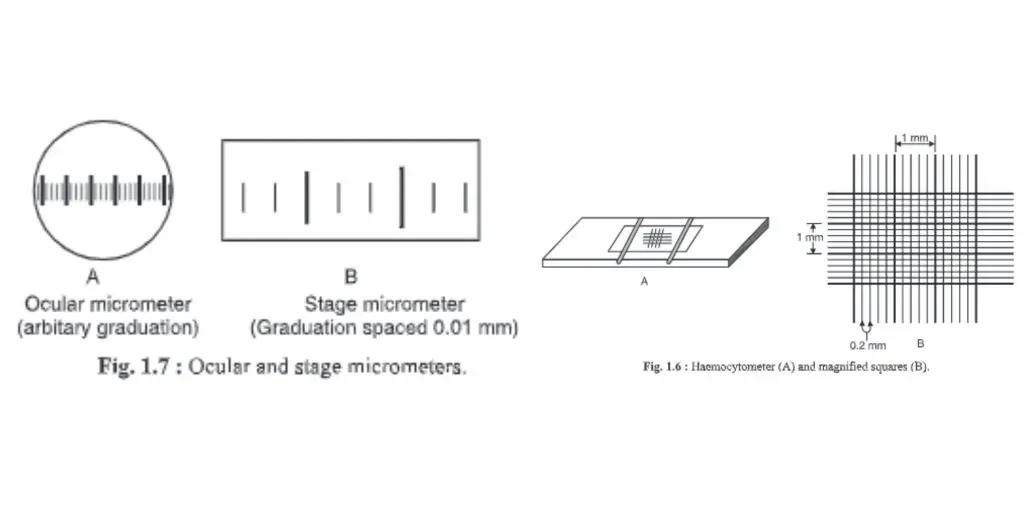
9. Ocular micrometer
- The ocular microscope is a round disc that has been divided into small pieces, i.e. Divisions marked O-100.
- It is found inside the eyepiece of a microscope.
- The objective of the microscope determines the distance.
- A stage micrometer can be used to determine the distance.
- It can be used to determine the exact dimension of any microorganism, cell, or microscopic material.
10. Stage micrometer
- The stage micrometer is a glass slides that are graduated in 1mm. The scale is only 1 mm (1 000 mm).
- One mm is then divided into 100 small divisions and 10 large divisions. A large division is split into 10 smaller divisions (10×10 = 100 divisions).
- These divisions are equally placed. The minimum count is 0.01mm, or 1000/100 = 10. This means that every smaller division of a stage micrometer equals 10 mm.
- It is used for calibrating the ocular microscope.
11. Cleaning of glassware
It is important to ensure that glassware used in microbiology laboratories is clean and neat. The glassware should be chemically cleaned after it has been received and before any work begins. Dirty glassware must be cleaned by first removing grease using a rag, or by being soaked in benzene, chloroform, and then by overnight soaking in chromic acids. Concentrated nitric acid or sulphuric acids can be used if the former is not affected. The cleaner is then rinsed in tap water, followed by distilled water. Some glassware can contain spores of fungus or bacteria that came with the packaging materials. Glassware made from factory may also contain alkali. Therefore, 2-3% of HC1 must be applied for 24 hours to neutralize the alkali.
After glassware is used, the medium must be removed. Then, the former can be treated with a 3% commercially-available lysol solution and washed with boiling water. It can then be dried and stored in an oven for between 140-180degC for 3-8 hours. You can also clean the used slides, pipettes, covers slips, Petri dishes, etc. as detailed above.
As a cleaning agent for glassware, chromic acid is used extensively. It is a mixture sodium dichro-mate and concentrated sulfuric acid. It has powerful oxidizing and solvent properties.
Preparation of chromic acid
- First Method: Take 5 grams of sodium/potassium dimer (K2Cr2O7, Na2Cr2O7) in your hand and mix it in 5 ml of distilled (250ml) water. Slowly add 100ml concentrated H2SO4 and stir it continuously. Allow the mixture to cool down to 40°C. Then, store it in a dry glass bottle.
- Second Method: Add 1g of Na2Cr2O7/K2Cr2O7 to 100ml concentrated H2SO4 and stir. Mix the mixture several times to prevent cake formation. Let it cool at 40°C. Store in a dry, clean bottle.