Animal models of autoimmune illnesses have provided invaluable insights into the mechanism of autoimmunity, human autoimmunity, and prospective therapies. Certain inbred animal breeds develop autoimmunity naturally, and autoimmunity can also be caused by certain experimental procedures.
Autoimmunity Can Develop Spontaneously in Animals
- Several spontaneously occurring autoimmune illnesses in animals share significant clinical and pathologic parallels with certain autoimmune diseases in humans.
- Certain inbred mouse strains have served as particularly useful models for shedding light on the immunologic abnormalities that contribute to the development of autoimmunity.
- New Zealand Black (NZB) mice and F1 hybrids of NZB and New Zealand White (NZW) mice suffer autoimmune illnesses that resemble systemic lupus erythematosus.
- Between 2 and 4 months of age, NZB mice spontaneously develop autoimmune hemolytic anaemia, at which point several auto-antibodies are detectable, including antibodies to erythrocytes, nuclear proteins, DNA, and T cells.
- 18 months after birth, F1 hybrid animals develop glomerulonephritis due to immune-complex deposits in the kidney and die prematurely.
- As in humans with SLE, autoimmunity is more prevalent in female (NZB NZW)F1 hybrids. A mouse strain known as MRL/lpr/lpr develops an accelerated and severe form of systemic autoimmune illness resembling systemic lupus erythematosus.
- These mice are homozygous for the faulty fas gene gene lpr, which has been identified as the lpr gene. The protein encoded by the fas gene belongs to the TNF family of cysteine-rich membrane receptors.
- When the normal Fas protein interacts with its ligand, it transmits a signal that induces apoptosis in Fas-containing cells. This pathway may be responsible for the killing of target cells by certain CTLs.
- Fas is also important for the death of hyperactivated CD4+ cells in the periphery. Normally, when mature peripheral T cells become activated, they are induced to express both Fas antigen and Fas ligand. Fas-bearing cells are driven to die when they come into touch with a surrounding activated cell expressing Fas ligand.
- Fas ligand may potentially interact with Fas from the same cell, resulting in cellular death. In the absence of Fas, mature peripheral T cells do not die, and these activated cells continue to proliferate and generate cytokines, causing lymph nodes and spleen to become excessively swollen.
- Humans exhibit fas expression defects comparable to those described in the lpr mice, which can have serious implications. Nonetheless, there is no correlation between fas expression and SLE in humans, indicating that the lpr mouse may not be an accurate model for SLE.
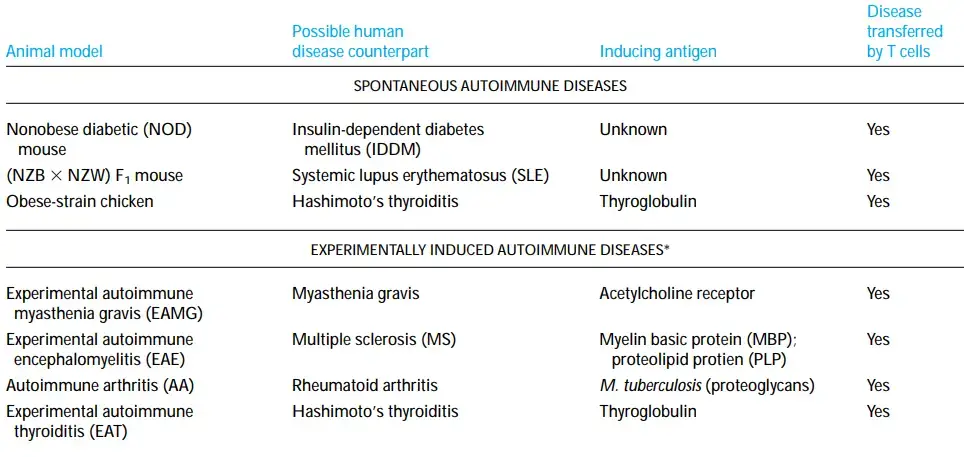
- The nonobese diabetic (NOD) mouse, which spontaneously develops a form of diabetes similar to human insulin-dependent diabetes mellitus, is another useful animal model (IDDM).
- Similar to the human disease, NOD mouse disease begins with lymphocyte infiltration of the pancreatic islets. In addition, similar to IDDM, there is a high correlation between specific MHC alleles and the development of diabetes in these mice.
- Experiments indicate that T cells from diabetic mice can transmit the disease to non-diabetic recipients. When the immune systems of normal mice are killed by lethal dosages of x-rays and subsequently rebuilt using bone-marrow cells from NOD mice, the reconstituted mice acquire diabetes.
- In contrast, the NOD mice do not acquire diabetes when their immune systems are damaged by x-irradiation and then replaced with normal bone marrow cells.
- Multiple studies have indicated that CD4+ T cells play a crucial role in the NOD mice, and new data implicates the TH1 fraction in disease progression.
- Several additional spontaneous autoimmune illnesses in animals have acted as models for comparable human diseases.
- Obese-strain chickens have humoral and cell-mediated response to thyroglobulin similar to that observed in Hashimoto’s thyroiditis.
Autoimmunity Can Be Induced Experimentally in Animals
- Some animals are amenable to experimental induction of autoimmune dysfunctions resembling human autoimmune disorders.
- In 1973, when rabbits were inoculated with pure acetylcholine receptors from electric eels, one of the first of these animal models was identified by chance.
- The animals quickly developed a muscle weakening comparable to that of myasthenia gravis. This experimental autoimmune myasthenia gravis (EAMG) was demonstrated to occur when antibodies to the acetylcholine receptor prevented acetylcholine from stimulating muscle synapses.
- Within a year, it was discovered that auto-antibodies to the cholinergic receptor were the cause of myasthenia gravis in humans, proving the utility of this animal model.
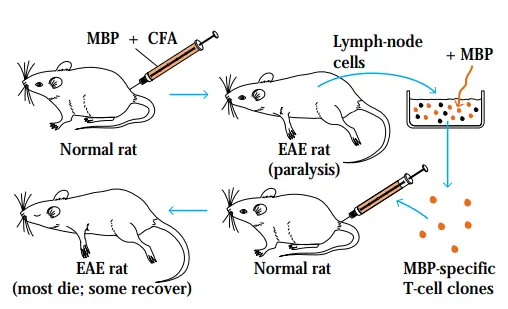
- Experimental autoimmune encephalomyelitis (EAE) is an additional animal model that has significantly advanced our knowledge of autoimmunity. This is one of the most thoroughly researched models of autoimmune illness.
- EAE is exclusively mediated by T cells and can be generated in a number of species through immunisation with myelin basic protein (MBP) or proteolipid protein (PLP) in Freund’s complete adjuvant.
- Within two to three weeks, the animals acquire cellular infiltration of the central nervous system’s myelin sheaths, culminating in demyelination and paralysis.
- Some animals acquire a chronic form of the disease that resembles chronic relapsing-remitting multiple sclerosis in people.
- Those who recover are resistant to illness development following a repeat MBP and adjuvant injection.
- The EAE mouse model provides a technique for testing MS therapies. Because MBP- or PLP-specific T-cell clones are identified in the periphery, for instance, it is thought that these clones avoided negative selection in the thymus.
- Recent animal investigations demonstrate that MBP taken orally may induce autotolerance in these antigen-specific peripheral T-cell clones.
- These studies have paved the road for MS patient clinical trials. Several animals can be induced with experimental autoimmune thyroiditis (EAT) by immunising with thyroglobulin in full Freund’s adjuvant.
- The development of both humoral antibodies and TH1 cells directed against thyroglobulin leads to thyroid inflammation. EAT seems to most closely resemble Hashimoto’s thyroiditis. In contrast to EAE and EAT, which are both induced by self-antigen immunisation, autoimmune arthritis (AA) is created by immunising rats with Mycobacterium tuberculosis in complete Freund’s adjuvant.
- These animals develop arthritis with similar characteristics to human rheumatoid arthritis.