What is Rickettsia?
- Rickettsia is a genus of small-sized intracellular bacteria that measure about 0.3 to 2 µm in size. Due to its small size, Rickettsia was initially mistaken for a virus. These bacteria reproduce through binary fission within the cytoplasm of eukaryotic cells and possess both DNA and RNA as their genetic material.
- Rickettsia bacteria are classified as gram-negative and are found in the intestinal tract of various arthropods such as ticks, fleas, mites, and chiggers. They are responsible for causing several diseases in humans, including Rocky Mountain spotted fever, rickettsialpox, epidemic typhus, and murine typhus.
- Within the Rickettsiaceae family, three genera are recognized: Rickettsia, Orientia, and Candidatus Cryptoprodotis. Rickettsia and Orientia are closely related to each other, while Candidatus Cryptoprodotis is primarily found in ticks and does not cause infections in humans.
- The genus Rickettsia comprises 25 known species, which are further divided into four groups. These bacteria are nonmotile, gram-negative, and highly pleomorphic, meaning they can take various forms, including cocci (0.1 μm in diameter), bacilli (1–4 μm long), or threads (up to about 10 μm long).
- The term “rickettsia” should not be confused with rickets, a deficiency disease caused by a lack of vitamin D. Instead, the bacterial genus Rickettsia was named after Howard Taylor Ricketts, in recognition of his groundbreaking research on tick-borne spotted fever.
- While “Rickettsia” technically refers to a single genus, the informal term “rickettsia” (plural “rickettsias”) is often used to refer to any members of the order Rickettsiales. These bacteria are obligate intracellular pathogens, relying on entry, growth, and replication within the cytoplasm of living eukaryotic host cells, typically endothelial cells. As a result, Rickettsia species cannot be cultured in artificial nutrient media but must be grown in tissue or embryo cultures. Chicken embryos are commonly used for this purpose, following a method developed by Ernest William Goodpasture and his colleagues in the 1930s.
- Numerous strains or species of Rickettsia are discovered and described each year. While some Rickettsia species are pathogenic to humans and animals, many are non-pathogenic to vertebrates, including humans, and exclusively infect arthropods such as aphids or whiteflies. These non-pathogenic Rickettsia bacteria often have a symbiotic relationship with their arthropod hosts. However, in medical literature, they are sometimes mistaken for pathogenic Rickettsia, highlighting the anthropocentric bias in the field.
- Pathogenic Rickettsia species are transmitted by various arthropods, including chiggers, ticks, fleas, and lice, and are responsible for both human and plant diseases. Some notable diseases caused by pathogenic Rickettsia include typhus, rickettsialpox, boutonneuse fever, African tick-bite fever, Rocky Mountain spotted fever, Flinders Island spotted fever, and Queensland tick typhus (Australian tick typhus). Fortunately, the majority of pathogenic Rickettsia bacteria are susceptible to antibiotics from the tetracycline group.
Features of Rickettsia
- Gram-negative: Rickettsia bacteria are classified as gram-negative, indicating that they have a distinct cell wall structure.
- Non-spore forming: Unlike some bacteria, Rickettsia does not form spores as a means of survival.
- Non-motile: Rickettsia bacteria do not possess flagella or other structures that enable motility.
- Pleomorphic: Rickettsia displays pleomorphism, which means it can take on different forms. It can appear as cocci (spherical shapes) with a diameter of approximately 0.1 µm, bacilli (rod-shaped) ranging from 1 to 4 µm in length, or thread-like structures up to 10 µm in length.
- Circular genome: The genetic material of Rickettsia consists of a circular genome, which ranges in size from 1 to 2.1 megabases (Mb).
- Replicates by binary fission: Rickettsia bacteria multiply through a process called binary fission. They divide into two identical daughter cells.
- Microcapsular protein layer: Rickettsia possesses a microcapsular protein layer above its outer membrane. This layer contributes to the bacteria’s interaction with host cells and may have a role in evading the immune system.
- Cannot survive in an artificial nutrient environment: Rickettsia species are obligate intracellular bacteria, meaning they require a living host cell to grow and replicate. They cannot survive and grow in an artificial nutrient medium.
- Can grow in tissue cells or chicken embryo culture: Rickettsia can be cultured and grown in tissue cells or chicken embryos. This method, developed in the early 1930s, allows researchers to study and propagate these bacteria for research purposes. Chicken embryos are commonly used in the culture of Rickettsia.
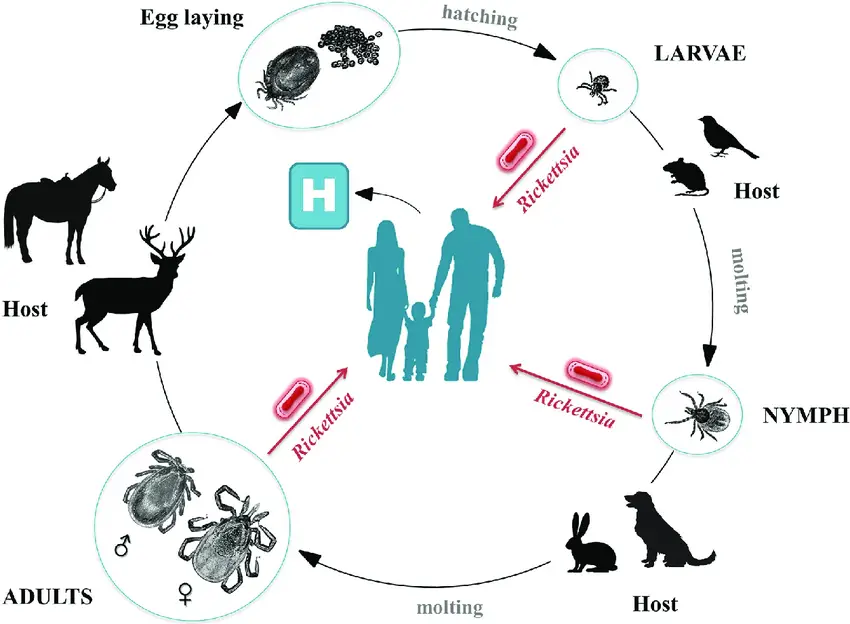
Transmission of Rickettsia
- Rickettsia bacteria are primarily transmitted to humans through direct contact with infected arthropod vectors during their feeding process. The arthropod vectors can include fleas, lice, mites, or ticks. These vectors become infected with Rickettsia bacteria by feeding on infected hosts, such as rodents or other mammals. When the infected arthropod vector subsequently feeds on a human, it can transmit the bacteria into the person’s bloodstream.
- Transmission of Rickettsia can also occur when a person unintentionally introduces the bacteria into their body. This can happen if a person scratches their skin and contaminates the arthropod bite wound or breaks in the skin with infectious fluids or feces from the arthropod vector. Similarly, crushing the arthropod vector at the bite site can also result in the inoculation of Rickettsia bacteria.
- In addition to these modes of transmission, certain rickettsial pathogens can infect humans through inhalation of bacteria or inoculation of the conjunctiva with infectious material. These routes of transmission are less common but can still lead to infection in specific cases.
- It is worth mentioning that some rickettsial pathogens, such as Anaplasma and Ehrlichia species, can be transmitted through transfusion of infected blood products or by organ transplantation. Although less common, these modes of transmission highlight the importance of appropriate screening and safety measures in blood banks and organ transplant procedures.
- Overall, the transmission of Rickettsia involves direct contact with infected arthropod vectors during their feeding process. Additional modes of transmission include inoculation of the bacteria through scratching, crushing the arthropod vector, inhalation, or inoculation of infectious material into the conjunctiva. Awareness of these transmission routes is crucial for implementing preventive measures and reducing the risk of rickettsial infections.
Diagnosis of Rickettsia
Diagnosing rickettsial diseases can be challenging, and it often relies on a combination of clinical recognition, epidemiological context, and laboratory testing. Here are some key points regarding the diagnosis of Rickettsia infections:
- Clinical Recognition and Epidemiologic Context: Timely presumptive diagnosis is typically based on clinical observation, taking into account the characteristic signs and symptoms associated with rickettsial diseases, as well as the patient’s exposure history to arthropod vectors. Experienced clinicians play a crucial role in recognizing the clinical features of these infections.
- Serologic Testing: Serologic tests are commonly used to confirm the diagnosis of rickettsial infections. Testing involves comparing acute-phase and convalescent-phase serum samples to detect a significant rise in antibody titers, typically a fourfold or greater increase, using indirect immunofluorescence antibody assays (IFA). It is important to note that cross-reactivity of antigens can occur, leading to antibodies that react in group-targeted serologic tests, indicating exposure at the group level.
- PCR Assays and Immunohistochemical Analyses: Molecular diagnostic techniques, such as polymerase chain reaction (PCR) assays and immunohistochemical analyses, can provide valuable information. PCR can be performed on various sample types, such as swabs or biopsies from eschars (if present), biopsy specimens of rash lesions, or whole blood samples. Eschar samples are particularly useful for species-specific diagnosis. However, the timing and quality of specimens can influence the accuracy of these tests.
- Anaplasmosis and Ehrlichiosis Diagnosis: For suspected anaplasmosis or ehrlichiosis, PCR of a whole blood sample is the preferred diagnostic test. Additionally, examination of a buffy coat, a layer of white blood cells separated from whole blood, can provide presumptive evidence of infection by identifying characteristic inclusion bodies called intraleukocytic morulae within leukocytes.
- Notifiable Diseases and Commercial Laboratory Testing: Spotted fever rickettsiosis, anaplasmosis, and ehrlichiosis are nationally notifiable diseases in the United States. Commercial laboratories offer testing for rickettsial diseases, including scrub typhus, anaplasmosis, and ehrlichiosis. However, some species-targeted serologic tests may only be available through the Centers for Disease Control and Prevention’s (CDC) Rickettsial Zoonoses Branch.
Epidemiology of Rickettsia
- Epidemiology of Rickettsia refers to the study of the distribution, transmission, and patterns of infection of the bacteria belonging to the Rickettsia genus. Rickettsia is a group of intracellular bacteria that are globally distributed throughout tropical, subtropical, and temperate regions, largely influenced by the presence of small blood-sucking arthropods.
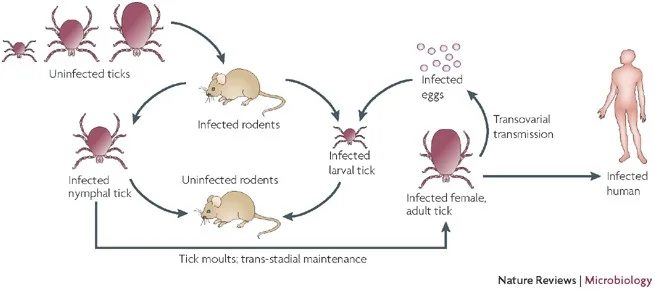
- Rats serve as the primary reservoir for Rickettsia typhi, which is responsible for causing murine typhus. The bacteria are transmitted to mammals through the bite of the rat flea, acting as a vector. These fleas become infected with R. typhi by feeding on the blood of infected rats. When the infected flea subsequently bites a human or another mammal, it can transmit the bacteria, leading to murine typhus.
- Ticks are another important hematophagous arthropod vector involved in the transmission of rickettsioses. They transmit tropical rickettsioses by biting humans and injecting bacteria into the bloodstream through their saliva. The transmission process requires approximately six hours of attachment and feeding on the host’s skin surface before the bacteria can be transmitted effectively.
- Tick-borne rickettsioses and spotted fever groups are transmitted by ticks through two primary mechanisms: transtadial transmission (from one stage of the tick’s life cycle to another) and transovarial transmission (transfer of bacteria from adult female ticks to their eggs). This means that ticks can pass the infection from one generation to the next, allowing the bacteria to persist in the tick population.
- Apart from direct tick bites, the infection can also be spread through other means. Scratching, itching, or rubbing the skin contaminated with infectious tick feces can introduce the bacteria into the body. Furthermore, rickettsioses can be considered emerging zoonotic diseases during international travel. Ticks can be transported by birds, Northern American flying squirrels, and their ectoparasites, facilitating the spread of these bacteria to new regions.
- Typhus rickettsiae, which cause diseases such as murine typhus, can also undergo an alternative zoonotic cycle. This means that in addition to the primary cycle involving rats and fleas, other animals and arthropods can be involved in the transmission, amplification, or maintenance of the bacteria.
- The occurrence of human spotted fever rickettsioses tends to be highest during the summer months when there is increased outdoor activity and greater exposure between humans and ticks. It is worth noting that certain age groups are considered high-risk populations for specific rickettsial diseases. Children between the ages of 5 and 9 years old and older adults aged 40 to 64 years old are particularly vulnerable to Rocky Mountain spotted fever.
- Studies analyzing the GeoSentinel database have indicated that approximately 3.1% of travelers are affected by rickettsioses. This highlights the importance of considering these diseases when evaluating individuals with febrile illnesses who have recently traveled to regions where rickettsial infections are endemic.
- It is believed that global warming may contribute to the increase in rickettsial infections and the northward expansion of tick species. As climatic conditions change, it can create more favorable environments for ticks to thrive and expand their geographical range, potentially exposing more individuals to rickettsial infections.
- In conclusion, understanding the epidemiology of Rickettsia is crucial for developing effective prevention and control strategies. Factors such as the presence of competent vectors, reservoir hosts, and environmental conditions play significant roles in the distribution and transmission of rickettsioses. Ongoing surveillance, public health education, and appropriate vector control measures are essential for mitigating the impact of these diseases on human health.
Pathogenesis of Rickettsioses
- The pathogenesis mechanism of rickettsioses involves a series of steps that occur after the bacteria invade the host’s body through various routes of infection. The primary modes of transmission include bites from infected ticks or mites, ingestion of infected louse or flea feces through the fecal-oral route, and inhalation of the organisms or their feces in certain occupational settings.
- Once inside the host, Rickettsia bacteria invade the vascular endothelium, the layer of cells that line the blood vessels. They enter the endothelial cells, multiply within the cytoplasm, and subsequently spread through the bloodstream to different parts of the body. The bacteria target and infect both the vascular endothelium and smooth muscle cells. This invasion triggers an immune response from the host, leading to an increase in immune-effector responses.
- The disseminated infection of Rickettsia leads to increased permeability of vascular cells, resulting in the accumulation of fluids in the interstitial surroundings. This fluid accumulation can lead to a decrease in blood volume, potentially causing hypovolemic shock, a condition characterized by low blood volume and inadequate tissue perfusion. In severe cases, this can result in life-threatening complications and even death.
- Apart from the vascular endothelium, Rickettsia can affect other vital organs in the body. The brain, liver, lungs, heart, and adrenal glands are particularly susceptible to infection, leading to various diseases such as meningoencephalitis (inflammation of the brain and meninges) and interstitial pneumonitis (inflammation of the lung tissue).
- In some cases, infection can occur through inhalation of the bacteria or their feces, predominantly observed among laboratory workers with poor hygienic practices. This route of infection poses a risk of respiratory involvement and can lead to respiratory symptoms and complications.
- When the kidneys are infected, the decrease in fluid perfusion can cause acute renal failure. The reduced blood flow to the kidneys hampers their normal function, resulting in the inability to effectively filter waste products from the blood.
- The vascular leakage of plasma into the alveolar spaces of the lungs can disrupt normal gas exchange, leading to hypoxemia (low oxygen levels in the blood). This can contribute to respiratory distress and further complications.
- The host’s cellular immunity plays a crucial role in inhibiting intracellular rickettsiae. Inducible nitric oxide production by endothelial cells, activated by cytokines such as gamma interferon and tumor necrosis factor, is an important mechanism for killing the bacteria within these cells.
- Natural killer cells also contribute to the immune response against rickettsial diseases. They produce immune responses that help eliminate the bacteria from infected cells.
- Additionally, the humoral immune response, mediated by antibodies, plays a role in preventing reinfection. Antibodies are produced against the outer membrane proteins of Rickettsia, aiding in the recognition and clearance of the bacteria.
- In summary, the pathogenesis mechanism of rickettsioses involves the invasion of vascular endothelium, dissemination of the bacteria through the bloodstream, and subsequent infection of various organs. The immune response triggered by the host aims to control and eliminate the bacteria, with cellular and humoral immunity playing important roles in combating the infection. Understanding the pathogenesis mechanisms can provide insights into the development of diagnostic, preventive, and therapeutic strategies against rickettsial diseases.
Signs and Symptoms of rickettsial diseases
The signs and symptoms of rickettsial diseases can vary depending on the specific type of infection. However, common features include fever, headache, and malaise, along with the presence of a widespread rash. Here are some specific signs and symptoms associated with different types of rickettsial diseases:
- Rocky Mountain Spotted Fever:
- Gradual or abrupt onset, typically occurring 2-8 days after a tick bite
- Fever, headache, confusion, muscle aches, and gastrointestinal symptoms
- Rash develops around day 2-3, starting with small red blotches on the wrists and ankles that eventually become widespread and may sometimes blister
- It is important to note that around 20% of cases do not develop a rash, known as spotless Rocky Mountain spotted fever.
- Rickettsialpox:
- Irregular fluctuating fever lasting less than 1 week
- Headache, chills, muscle aches, runny nose, sore throat, nausea, vomiting, and abdominal pain
- A red raised spot develops at the site of the mite bite, later forming a dry scab known as an eschar
- The rash is distributed on the face, neck, trunk, and extremities and can be easily mistaken for the rash of varicella (chickenpox).
- Boutonneuse Fever:
- Fever, headache, malaise, and muscle aches
- Rash appears on days 3-5 of illness, spreading from the extremities to the trunk, neck, face, palms, and soles within 36 hours
- The rash is spotty and blotchy and may persist for 2-3 weeks
- In about half of the cases, a dry scab known as a tache noire (black spot) may develop.
- Louse-Borne Typhus:
- Abrupt onset occurring 1-2 weeks after a louse bite
- Fever and intractable headache
- Rash appears on days 4-7 of illness, spreading from the trunk to the extremities (usually not affecting the face, palms, and soles)
- The rash starts as splotchy and develops into raised red spots
- Recurrence of the disease, known as Brill-Zinsser disease, is usually milder.
- Murine Typhus:
- Similar to louse-borne typhus but tends to have a milder and shorter course
- Flea bite does not typically result in the formation of an eschar
- Fever, headache, and other nonspecific symptoms may be present.
- Scrub Typhus:
- Generalized swelling of the lymph nodes is common
- Fever and headache
- Rash appears 1-3 weeks after a mite bite and presents as a dry scab-like lesion
- The rash is usually limited to the trunk and has a short duration.
Complications from rickettsial diseases
While complications from rickettsial diseases are relatively uncommon when diagnosed and treated promptly, certain complications can arise in some cases. Here are some potential complications associated with rickettsial diseases:
- Bronchopneumonia: In some severe cases, rickettsial infections can lead to the development of bronchopneumonia, which is characterized by inflammation and infection of the bronchial tubes and the lungs.
- Congestive heart failure: Rickettsial infections may cause myocarditis, which is inflammation of the heart muscle. In severe cases, this inflammation can lead to congestive heart failure, a condition where the heart is unable to pump blood efficiently.
- Multi-organ failure: In rare instances of severe rickettsial infections, multi-organ failure can occur. This condition involves the failure of multiple organs, such as the heart, lungs, liver, kidneys, or other vital organs.
- Deafness: Some rickettsial infections, such as certain forms of spotted fever, may rarely result in hearing loss or deafness as a complication.
- Disseminated intravascular coagulopathy (DIC): DIC is a condition characterized by abnormal blood clotting and bleeding. In severe cases of rickettsial infections, DIC can occur as a complication.
- Myocarditis: Inflammation of the heart muscle, known as myocarditis, can lead to various cardiac complications and may be observed in some cases of rickettsial diseases.
- Endocarditis: Endocarditis refers to inflammation of the lining of the heart, typically affecting the heart valves. Although rare, rickettsial infections can lead to endocarditis as a potential complication.
- Glomerulonephritis: Certain rickettsial infections can result in glomerulonephritis, which is inflammation of the kidney’s glomeruli. This can lead to kidney dysfunction and potentially require medical intervention.
Diagnosis of Rickettsioses
The diagnosis of rickettsioses involves various laboratory methods and tests. Here are some key aspects of the diagnostic process:
- Clinical observation plays an important role in the initial diagnosis of rickettsioses. The characteristic clinical features, such as flu-like symptoms and the development of a rash, can provide important clues for the diagnosis.
- Immunohistochemical detection is used by taking skin biopsies of skin lesions to detect antigens specific to Rickettsia. This method can help confirm the presence of the bacteria in the affected tissues.
- Serological tests are commonly employed to confirm the diagnosis of rickettsioses. These tests detect specific antibodies against Rickettsia in serum samples. It’s important to note that antibodies might not be detectable during the initial stage of infection, so repeat testing may be necessary to capture the immune response.
- Culturing Rickettsia bacteria in artificial laboratory settings can be challenging. They are typically isolated in viable eukaryotic host cells, such as embryonated eggs, tissue cultures, antibiotic-free cell cultures, or susceptible laboratory animals. Common cell lines used for the maintenance of Rickettsia include HeLa, Hep2, Detriot-6, and mouse fibroblasts. Isolation of the bacteria often involves cultivation in developing 5 to 6 days old chick embryos. Laboratory animals like Guinea pigs and mice can be used to isolate Rickettsia species from animal specimens.
- In the diagnosis of Rocky Mountain spotted fever, a direct fluorescent antibody (DFA) test can be used. This method involves using fluorescently labeled antibodies that specifically bind to Rickettsia antigens, allowing for rapid and specific detection.
- Newly developed serological tests, such as the indirect fluorescent antibody (IFA) test and enzyme-linked immunosorbent assay (ELISA), are increasingly used for the early diagnosis of rickettsial diseases. These tests detect specific antibodies in patient serum samples, aiding in the identification of recent or ongoing infections.
- It’s important to consider that the choice of diagnostic tests may vary depending on the specific clinical presentation, the stage of infection, and the availability of laboratory resources. Combination testing using different methods and repeated testing may be necessary to improve diagnostic accuracy.
In summary, the diagnosis of rickettsioses involves a combination of clinical observation, immunohistochemical detection, serological tests, and in some cases, culturing of Rickettsia bacteria. Advancements in serological techniques have facilitated early detection, aiding in prompt and appropriate treatment. Diagnostic methods continue to evolve, allowing for more accurate and timely identification of rickettsial infections.
Treatment of Rickettsioses
The treatment of rickettsioses involves the administration of appropriate antibiotics to target the underlying bacterial infection. Here are some important considerations regarding the treatment of rickettsioses:
- Presumptive Treatment: Due to the potential severity of rickettsial infections and the need for prompt treatment, a presumptive approach is often adopted. This means that if there is a high clinical suspicion of rickettsial infection based on symptoms, epidemiological factors, and geographic location, antibiotics are initiated without waiting for confirmatory laboratory tests.
- Antibiotic Choices: The primary antibiotics used in the treatment of rickettsioses include doxycycline, tetracycline, chloramphenicol, and fluoroquinolones. Among these, doxycycline is the most commonly recommended and effective choice. It is a broad-spectrum antibiotic that has excellent activity against Rickettsia bacteria. Doxycycline should be administered as early as possible after the diagnosis is suspected or confirmed.
- Alternative Antibiotics: In some situations, such as in pregnant women or individuals with specific contraindications to doxycycline, alternative antibiotics may be considered. Chloramphenicol is an example of an alternative antibiotic that can be used in these cases. However, it is important to note that chloramphenicol is generally considered less effective than doxycycline and is associated with a higher risk of adverse effects.
- Duration of Treatment: The duration of antibiotic treatment for rickettsioses varies depending on the specific type of infection, severity of the illness, and the response to treatment. In general, treatment is continued for a minimum of 7 to 14 days, and in severe cases or complications, it may need to be extended.
- Supportive Care: In addition to antibiotic treatment, supportive care is essential for managing rickettsial infections. This may include measures such as rest, adequate hydration, and management of specific symptoms or complications. In severe cases, hospitalization may be required for close monitoring and supportive interventions.
It is important to consult with a healthcare professional for a proper diagnosis and to determine the most appropriate antibiotic treatment regimen based on the specific clinical presentation, local guidelines, and individual patient factors. Early initiation of antibiotic therapy is crucial to prevent complications and improve outcomes in rickettsioses.
Prevention and Control of Rickettsioses
Prevention and control strategies play a crucial role in reducing the risk of rickettsioses. Here are some important measures to consider:
- Personal Protection: To minimize the risk of infection, individuals should use protective clothing, such as long sleeves, long pants, and closed shoes, when in areas where ticks, lice, mites, and fleas are prevalent. Applying insect repellent creams or sprays on exposed skin can also help prevent bites from these vectors. It is important to follow the manufacturer’s instructions when using insect repellents.
- Tick Avoidance: Activities such as bushwalking, camping, or spending time in areas with high vegetation should be approached with caution. These environments may have a higher population of ticks and other vectors. Avoid direct human contact with vegetation and brush, as well as grassy and wooded areas where ticks are commonly found. Checking the body for attached ticks and promptly removing them can significantly reduce the chance of transmission.
- Vector Control: Implementing measures to control the population of vectors, such as ticks, lice, mites, and fleas, can help reduce the transmission of rickettsial infections. This may involve environmental management, such as reducing the presence of tick habitats or implementing insecticide treatments in infested areas.
- Public Health Education: Raising awareness about rickettsioses, their modes of transmission, and preventive measures is crucial. Educating individuals, particularly those residing in or traveling to areas where rickettsial infections are endemic, can help promote personal protection practices and reduce the risk of exposure.
- Occupational Safety: Occupational groups at higher risk of exposure, such as laboratory workers handling Rickettsia bacteria or individuals working in environments with a high prevalence of vectors, should adhere to appropriate safety protocols. This includes using personal protective equipment, practicing good hygiene, and following proper handling and disposal procedures.
- Vaccination: Currently, there is no vaccine available for rickettsial infections. Therefore, prevention mainly relies on avoiding direct contact with the vectors and implementing personal protective measures.
- Surveillance and Early Detection: Active surveillance programs can help identify areas with a higher incidence of rickettsial infections and monitor disease trends. Early detection of cases allows for prompt treatment and implementation of control measures.
In summary, prevention and control of rickettsioses involve personal protective measures, avoidance of direct contact with vectors, environmental management, public health education, and surveillance. Until a vaccine becomes available, individuals should focus on reducing exposure to ticks, lice, mites, and fleas, which are responsible for transmitting these infections.
FAQ
What is Rickettsia infection?
Rickettsia infection refers to an infection caused by bacteria belonging to the genus Rickettsia. These bacteria are primarily transmitted to humans through arthropod vectors, such as ticks, fleas, lice, and mites.
How is Rickettsia infection diagnosed?
Diagnosis of Rickettsia infection typically involves a combination of clinical recognition, epidemiological context, and laboratory testing. Serological tests, PCR assays, and immunohistochemical analyses are commonly used to confirm the presence of the bacteria.
What is the treatment for Rickettsia infection?
The primary treatment for Rickettsia infection involves the administration of antibiotics, such as doxycycline, tetracycline, chloramphenicol, or fluoroquinolones. Prompt initiation of antibiotic therapy is important to prevent complications and improve outcomes.
Can Rickettsia infection be prevented?
Yes, Rickettsia infection can be prevented. Measures such as using protective clothing, applying insect repellents, and avoiding direct contact with vectors (ticks, fleas, lice, and mites) can reduce the risk of transmission. Additionally, environmental control measures and public health education play a role in prevention.
Are there vaccines available for Rickettsia infection?
Currently, there are no vaccines available for Rickettsia infection. Prevention mainly relies on avoiding contact with infected vectors and implementing personal protective measures.
What are the common symptoms of Rickettsia infection?
Common symptoms of Rickettsia infection include fever, headache, malaise (general feeling of unwellness), and a widespread rash. The specific symptoms may vary depending on the type of Rickettsia species involved.
How are Rickettsia infections transmitted?
Rickettsia infections are primarily transmitted through the bite of infected arthropod vectors. This can occur during the feeding process when the vectors, such as ticks or fleas, transmit the bacteria to humans. Transmission can also occur when infectious fluids or feces from the vectors contaminate the skin through scratching or crushing of the vector.
Are Rickettsia infections contagious from person to person?
No, Rickettsia infections are not considered contagious from person to person. The primary mode of transmission is through arthropod vectors, and human-to-human transmission is rare.
Can pets transmit Rickettsia infections?
Pets, such as dogs and cats, can become infected with certain Rickettsia species, but their role in transmitting the bacteria to humans is generally minimal. However, it is still important to protect pets from arthropod vectors to prevent their own infections.
Are Rickettsia infections common worldwide?
Rickettsia infections are found globally, but their prevalence varies depending on geographical factors, climate, and the presence of suitable arthropod vectors. Different species of Rickettsia may be more common in specific regions, leading to variations in the incidence of infection.
References
- Snowden J, Ladd M, King KC. Rickettsial Infection. [Updated 2023 Jan 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431127
- https://www.merckmanuals.com/home/infections/rickettsial-and-related-infections/overview-of-rickettsial-infections
- https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/rickettsial-diseases
- https://reference.medscape.com/article/968385-overview
- https://bestpractice.bmj.com/topics/en-gb/1604
- https://tripprep.com/library/rickettsial-infections/traveler-summary
- https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/conditions/infectious+diseases/rickettsial+infections/rickettsial+infections+-+including+symptoms+treatment+and+prevention