What is Liquid Chromatography?
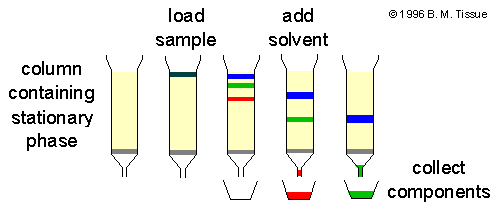
- Liquid chromatography (LC) is a technique for separating components in a mixture based on their interactions with a stationary phase while being transported by a liquid mobile phase. This approach can be used in a column or on a plane.
- The sample and the mobile phase are passed through a column or plane holding a stationary phase in LC. The stationary phase can be a solid, ionic-group-containing resin, liquid on an inert solid support, or porous inert particles. Depending on characteristics such as adsorption, size, partitioning, or ion exchange, the different solutes in the sample interact with the stationary phase to varying degrees.
- As the components move across the column or plane, they split due to differences in interaction. Because it provides for simple visualisation, liquid chromatography is particularly useful in separating coloured mixtures. However, even non-colored combinations can be visualised using numerous ways, such as ultraviolet light irradiation.
- Liquid chromatography is commonly employed in complicated mixtures to separate proteins, nucleic acids, and tiny compounds. It can be used for both analytical and preparative purposes. Analytical liquid chromatography is commonly employed for detection or quantification, and high-performance liquid chromatography (HPLC) instruments are frequently used.
- When opposed to conventional liquid chromatography, HPLC devices use a pump to drive the mobile phase through the system, resulting in improved resolution and faster analytical times. Conventional LC, on the other hand, is widely used in preparative-scale work to purify and isolate certain components of a mixture. Disposable columns are frequently used once and then discarded in ultratrace separations.
- Liquid chromatography is a versatile and powerful technology with several applications in chemistry, biology, pharmaceuticals, environmental analysis, and the food and beverage industries. It helps researchers to separate and analyse complicated mixtures, revealing important information about the composition and properties of various substances.
Types of Liquid Chromatography
Liquid chromatography can be classified into four main types based on the components involved in the chromatographic process:
- Reversed-Phase Chromatography: Reversed-phase chromatography utilizes a non-polar stationary phase and a polar mobile phase. Hydrophilic molecules present in the polar mobile phase are transported to the column and eluted, while hydrophobic molecules interact with and are retained by the non-polar stationary phase. Reversed-phase chromatography often employs organic solvents, aqueous buffers, or mixtures of both to elute compounds from the column. The stationary phase particles are typically covalently bonded with alkyl chains. Reverse-phase chromatography can also separate charged analytes through a process called ion pairing.
- Normal Phase Chromatography: Normal phase chromatography involves a polar stationary phase and a non-polar mobile phase. Commonly used stationary phases include silica and organic compounds with cyano and amino groups. Polar solvents such as chloroform, isopropanol, and ethyl acetate are mixed with non-polar solvents like hexane or heptane as the mobile phase. Normal phase chromatography is often employed for separating chiral compounds, cis-trans isomers, geometric isomers, and water-sensitive compounds. Less polar compounds are eluted first in this type of chromatography, while more polar compounds elute later.
- Ion Exchange Chromatography: Ion exchange chromatography employs an ionic stationary phase and an aqueous buffer as the mobile phase. This type of chromatography separates ions and polar compounds based on their affinity for the ion exchanger. Ion exchange chromatography can be further categorized into cation exchange chromatography and anion exchange chromatography. Cation exchange chromatography retains positively charged cations, while anion exchange chromatography retains negatively charged anions. It is commonly used to separate organic and inorganic ions from aqueous solutions. Ion exchange chromatography has high matrix tolerance and predictable elution patterns but is limited to ionizable compounds.
- Size Exclusion Chromatography (SEC) or Gel Filtration Chromatography: Size exclusion chromatography separates molecules based on their size and the pore size of the stationary phase. It is also referred to as gel filtration chromatography. SEC is particularly useful for separating industrial polymers and proteins. Larger molecules elute first in this technique, while smaller molecules are eluted later. In some cases, separation based on molecular mass can also be achieved using SEC.
These different types of liquid chromatography offer versatility in separating and analyzing diverse compounds, catering to various research and industrial applications. Researchers select the appropriate type of liquid chromatography based on the specific properties of the sample and the desired separation objectives.
Other Varieties of Liquid Chromatography
Liquid chromatography encompasses a variety of techniques beyond the commonly known methods. Here are some other varieties of liquid chromatography:
- Partition Chromatography: In partition chromatography, both the stationary phase and the mobile phase are liquids. The stationary phase is an immiscible liquid that is different from the mobile phase. The separation occurs based on the differential partitioning of analytes between the two liquid phases.
- Liquid-Solid Chromatography: Liquid-solid chromatography is similar to partition chromatography, but the stationary phase is a bonded rigid silica or silica-based component inside the column. It can also be alumina in some cases. Analytes that have an affinity for the stationary phase will be adsorbed onto it, resulting in longer retention times, while those that do not will pass through the column with shorter retention times. This method includes both normal and reverse phases.
- Ion Exchange or Ion Chromatography: Ion exchange chromatography is specifically used to separate and determine ions. Columns with low ion exchange capacity are employed, and the separation is based on the equilibrium of ion exchange between the ions in solution and the oppositely charged ions fixed to the stationary phase. The stationary phase contains either positively or negatively charged functional groups, such as sulfonate (-SO3-) or a quaternary amine (-N(CH3)3+), serving as cation and anion exchangers, respectively.
- Size Exclusion Chromatography: Size exclusion chromatography separates molecules based on their size. The stationary phase consists of small particles of silica or polymer, forming uniform pores. Smaller molecules get trapped in the pores and elute from the column at a faster rate, while larger molecules pass through more quickly. There is no specific interaction between the analyte and the stationary phase in this method.
- Affinity Chromatography: Affinity chromatography involves binding a reagent, known as a ligand, to the analyte molecules in a sample. The stationary phase is typically composed of agrose or porous glass beads that immobilize the bonded molecule. Only the analyte molecules with a specific affinity for the ligand are retained in the column, while the unbound analyte passes through in the mobile phase. Elution conditions, such as pH or ionic strength, can be manipulated to change the binding and release of the analyte. Affinity chromatography is commonly used in biochemistry for protein purification.
- Chiral Chromatography: Chiral chromatography is employed to separate a racemic mixture into its enantiomeric parts. A chiral additive can be added to the mobile phase, or a stationary phase with chiral properties can be used. The most popular option is a chiral stationary phase, which is capable of recognizing the chirality of the analyte, leading to attractive forces and formation of inclusion complexes between the stationary phase and the analyte molecules.
These variations of liquid chromatography expand the capabilities of the technique, allowing for specialized separations and applications in diverse fields such as pharmaceuticals, biochemistry, and analytical chemistry.
Principle of Liquid Chromatography
- The principle of liquid chromatography is based on the affinity of molecules for the mobile phase. Components that have a higher affinity for the mobile phase will move more rapidly through the stationary phase and elute from the column faster. On the other hand, components with a lower degree of interaction with the mobile phase will move more slowly and elute from the column later.
- When two molecules with different polarities are present in a mixture and the mobile phase has a distinct polarity, these molecules will move at different speeds through the stationary phase. In column liquid chromatography, as the liquid mobile phase passes through the column, the components in the mobile phase interact with the solid stationary phase. This interaction is based on various physicochemical properties such as molecular size, charge, hydrophobicity, or specific binding interactions.
- The composition of the mobile phase is typically adjusted during a separation run to change the strengths of the interactions between the compounds of interest and the stationary and mobile phases. This alters the phase partitioning of each compound and determines the order in which they elute from the column.
- The eluate, or column effluent, is collected in fractions while monitoring the concentrations of the eluted compounds over time to generate a chromatogram, which is an elution curve. The mode of detection depends on the analyte being detected. For protein separations, a UV detector or spectrophotometer is commonly used to monitor protein elution by measuring light absorption at 280 nm. The resulting chromatogram is then analyzed to quantify the proteins in the eluate.
- The chromatogram shows distinct peaks, with each peak representing a resolved component. The area under the curve of each peak corresponds to the amount of that compound eluted from the column. It is important to note that a single peak may contain multiple protein species, so further analysis, such as gel electrophoresis, may be required.
- A typical chromatogram begins with an initial broad peak of weakly interacting proteins that elute quickly. The column is then washed until this first flowthrough peak is completely eluted. Proteins that interact more strongly with the resin are eluted by changing the composition of the elution buffer. The specific composition of the elution buffer depends on the properties of the molecules being separated and the chromatography media used.
- The elution can be achieved using a linear gradient or stepwise isocratic elution. By adjusting parameters such as the elution gradient, flow rate, column length, and resin particle size, the resolution and separation ability of a given resin can be optimized to yield well-separated, sharp peaks in the chromatogram.
- Overall, the principle of liquid chromatography relies on the differential affinity of molecules for the mobile and stationary phases, allowing for the separation and analysis of complex mixtures based on their physicochemical properties.
Liquid Chromatography Instrumentation

Liquid chromatography (LC) instrumentation typically comprises various components that work together to facilitate the separation and analysis of compounds. Here is an overview of the key components in a basic LC system:
- Solvent Inlet Filter: The solvent inlet filter is placed at the entrance of the LC system to remove any particulate matter or impurities present in the solvent before it enters the system. This helps maintain the integrity of the system and prevents clogging or damage to other components.
- Pump: The pump is responsible for delivering the mobile phase or solvent at a controlled flow rate. It ensures a consistent and precise flow of the solvent through the system, allowing for accurate separations. Pumps can be either isocratic (delivering a constant solvent composition) or gradient (allowing for changing solvent compositions over time).
- Inline Solvent Filter: Similar to the solvent inlet filter, the inline solvent filter helps remove any remaining impurities or contaminants that may be present in the solvent after passing through the pump. It ensures the purity of the solvent before it enters the injection valve and subsequently the separation column.
- Injection Valve: The injection valve is a crucial component that allows for the introduction of the sample into the system. It controls the sample injection and directs it onto the separation column. The valve can be manually or automatically operated, enabling precise and reproducible sample introduction.
- Precolumn Filter: The precolumn filter is positioned after the injection valve and serves to protect the separation column from any particles or debris that may be present in the sample. It helps maintain the longevity and efficiency of the column by preventing blockages or contamination.
- Column: The column is the heart of the liquid chromatography system where the separation of compounds takes place. It contains a stationary phase that interacts with the analytes of interest, allowing for their separation based on various physical and chemical properties. The column can be packed with different materials depending on the separation mode and target analytes.
- Detector: The detector is used to monitor the eluted compounds from the column. It detects and measures the concentration of the separated compounds, providing data that can be used for qualitative and quantitative analysis. Common types of detectors used in LC include UV-Vis absorbance detectors, fluorescence detectors, refractive index detectors, and mass spectrometers.
- Recorder: The recorder captures and records the signals generated by the detector, allowing for the visualization and analysis of the chromatographic peaks. It provides a graphical representation of the separation, enabling the identification and quantification of the separated compounds.
- Backpressure Regulator: The backpressure regulator controls the pressure within the system, helping to maintain a consistent flow rate and prevent fluctuations that could impact the separation. It ensures the optimal performance of the LC system and protects the column from excessive pressure.
- Waste Reservoir: The waste reservoir collects the waste or eluent that is not required for analysis. It allows for the safe disposal of the solvent and any residual sample or contaminants.
These components work in synergy to facilitate the separation, detection, and analysis of compounds in liquid chromatography. Advanced LC systems may incorporate additional features and modules to enhance performance, automation, and data processing capabilities.
Liquid Chromatography Protocol
The liquid chromatography protocol consists of six main steps:
- Column Equilibration: The chromatography column is equilibrated with a buffer that is compatible with the protein of interest and the chosen resin. Typically, 5-10 column volumes of equilibration buffer are passed through the column to ensure proper conditioning.
- Sample Loading: Once the column is equilibrated, the sample containing the proteins of interest is loaded onto the column. The sample is generally loaded in a buffer with the same composition as the equilibration buffer to maximize protein interaction with the stationary phase.
- Column Washing: After the proteins have been immobilized on the stationary phase, weakly or nonspecifically bound proteins are removed by washing the column with several column volumes of wash buffer. The wash buffer can be the same as the equilibration buffer or may contain specific components to disrupt weak interactions.
- Sample Elution: Following the washing step, proteins that strongly interact with the resin are eluted from the column by changing the composition of the buffer that passes over the resin. The elution buffer disrupts the interactions between the proteins and the stationary phase, allowing them to be released from the column.
- Final Column Washing: Once the protein of interest has been eluted, any remaining bound proteins are washed off the resin by increasing the strength of the elution buffer. This step ensures thorough removal of all proteins from the column.
- Column Regeneration: After the elution and final washing steps, the column is regenerated for future use. This can involve saturating the column with equilibration buffer or filling it with a storage buffer, depending on the specific requirements of the chromatography system.
It is important to note that size exclusion chromatography (SEC) does not require specific buffer changes, as it relies on the differential exclusion of molecules based on size. In SEC, there are no true wash and elution steps since separation occurs solely based on the size of the molecules.
By following this liquid chromatography protocol, researchers can separate and purify proteins based on their interactions with the stationary and mobile phases, enabling the analysis and isolation of specific compounds from complex mixtures.
Rf Value
The Rf value, which stands for Retention factor, is a characteristic identification value used in chromatography. It is calculated as the ratio of the distance traveled by the analyte to the distance traveled by the solvent front. The Rf value is specific to each compound and can be used to analyze and identify different substances.
The formula for calculating the Rf value is:
Rf = Distance traveled by analyte / Distance traveled by solvent
In practice, the Rf value is determined by measuring the distance a particular compound travels on a chromatographic plate or column compared to the distance traveled by the solvent front. The distance traveled by the analyte is usually measured from the point of application to the center of the spot or peak, while the distance traveled by the solvent front is measured from the point of application to the leading edge of the solvent front.
The Rf value is a dimensionless quantity and typically ranges between 0 and 1. It is influenced by various factors such as the chemical nature of the analyte, the composition of the mobile phase, and the characteristics of the stationary phase. These factors determine the extent of interaction between the analyte and the stationary phase, which affects the analyte’s mobility and ultimately its Rf value.
The Rf value is useful in chromatographic analysis because it provides a means to compare and identify different compounds. Each compound will have a unique Rf value under specific chromatographic conditions. By comparing the Rf value of an unknown compound with the Rf values of known reference compounds, it is possible to tentatively identify the unknown substance.

For example, if substances A, B, and C are being analyzed, their Rf values would be calculated as follows:
Rf value of A = Distance traveled by substance A / Distance traveled by solvent (X)
Rf value of B = Distance traveled by substance B / Distance traveled by solvent (X)
Rf value of C = Distance traveled by substance C / Distance traveled by solvent (X)
By comparing these Rf values with those of known compounds, it becomes easier to identify and differentiate between different substances in a mixture.
Disadvantages of Liquid Chromatography
While liquid chromatography has many advantages, it also comes with certain disadvantages that should be considered. Here are some of the disadvantages of liquid chromatography:
- Limited Visibility of Separation: Liquid chromatography relies on the visualization of separation bands or peaks to assess the efficacy of the separation. If the components in the sample solution are not visible, it can be challenging to determine the success of the separation. While certain visualization methods like UV irradiation or colored compounds can aid in detection, the inability to observe all components visually can make the evaluation of the separation more difficult.
- Manual Operation: Traditional liquid chromatography methods, such as column chromatography, often require manual operation by the scientist. This includes controlling the flow of the mobile phase, detecting each separation band, and collecting individual components. The manual nature of the process introduces a higher potential for experimental error and requires more time and effort compared to automated techniques like high-performance liquid chromatography (HPLC).
- Potential for Experimental Errors: Due to the manual handling and operation involved in liquid chromatography, there is an increased risk of experimental errors. Inaccurate measurement of mobile phase flow rates, poor column packing, or inconsistent sample application can all affect the quality and reproducibility of the separation. These errors can lead to reduced resolution, lower separation efficiency, and compromised results.
- Cost and Equipment Requirements: While liquid chromatography can be less expensive compared to other separation methods like HPLC or gas chromatography (GC), it still requires specific equipment and consumables. For flash chromatography, the use of sophisticated air pumps and vacuum pumps can increase the overall cost. Additionally, the need for proper column materials and specialized detectors can add to the expenses.
- Sample Size Limitations: Liquid chromatography techniques may have limitations when it comes to handling small sample volumes. Column chromatography, in particular, may require larger sample volumes to achieve satisfactory separation. This can be a disadvantage when working with limited or precious samples.
It is important to consider these disadvantages while selecting a chromatographic method, weighing them against the specific requirements of the analysis, the available resources, and the desired outcomes. Advances in technology and the introduction of automated systems have addressed some of these limitations, offering more precise and efficient liquid chromatography methods.
Advantages of Liquid Chromatography
Liquid chromatography offers several advantages as a separatory technique for the separation of mixtures:
- Versatility: Liquid chromatography is a versatile technique that can separate a wide range of compounds, including organic and inorganic compounds, biomolecules, pharmaceuticals, environmental pollutants, and more. It can be applied to various sample types, such as liquids, gases, and solids, making it a valuable tool in many fields of research and analysis.
- Selectivity: Liquid chromatography allows for excellent selectivity in separating complex mixtures. Different types of liquid chromatography, such as reversed-phase, normal phase, ion exchange, and affinity chromatography, offer specific interactions with the analytes, enabling the separation based on different properties like polarity, charge, size, and specific binding interactions.
- Sensitivity: Liquid chromatography can achieve high sensitivity in detecting and quantifying analytes. It is often coupled with sensitive detection techniques such as ultraviolet-visible (UV-Vis) spectroscopy, fluorescence spectroscopy, mass spectrometry, or electrochemical detection, enhancing the ability to detect and measure trace amounts of compounds in complex samples.
- Resolution: Liquid chromatography provides good resolution for the separation of closely related compounds. By optimizing various parameters such as column type, mobile phase composition, and flow rate, it is possible to achieve efficient separation and resolve even complex mixtures into distinct peaks or bands.
- Sample Compatibility: Liquid chromatography is compatible with a wide range of sample matrices, including aqueous and non-aqueous samples. It can handle a variety of sample sizes, from microliters to large volumes, making it suitable for both analytical and preparative purposes.
- Automation: While it is mentioned in the content that liquid chromatography can be performed manually, it is important to note that modern liquid chromatography systems often incorporate automation, allowing for precise control of parameters, automated sample injection, and data acquisition. Automation enhances reproducibility, reduces human error, and increases the overall efficiency of the separation process.
- Availability and Cost-effectiveness: Liquid chromatography equipment and consumables, such as columns and solvents, are widely available in the market. Additionally, glassware commonly used in liquid chromatography is readily available and relatively inexpensive compared to other analytical techniques, making it a cost-effective option for separation needs.
These advantages make liquid chromatography a popular and widely used technique in various fields, including pharmaceutical analysis, environmental monitoring, food and beverage analysis, forensic analysis, and many more.
Applications of Liquid Chromatography
Liquid chromatography, a versatile analytical technique, finds numerous applications across various industries and fields. Here are some key applications of liquid chromatography:
- Ink Sample Testing: Liquid chromatography is extensively employed in forensic science and document examination to analyze ink samples. By separating and identifying the different components present in the ink, it helps determine the origin, age, and composition of the ink, aiding in the investigation of fraudulent documents or forged signatures.
- Environmental Analysis and Cleanliness Testing: Liquid chromatography plays a crucial role in environmental analysis. It is used to detect and quantify pollutants, such as pesticides, heavy metals, and organic contaminants, in soil, water, and air samples. Additionally, it is employed in cleanliness testing to assess the levels of residue or impurities on surfaces, ensuring compliance with safety and quality standards.
- Food Analysis and Quality Control: In the food industry, liquid chromatography is employed for analyzing various components and contaminants in food products. It enables the identification and quantification of vitamins, amino acids, sugars, food additives, and pesticide residues, ensuring food safety and quality control. It is also utilized to detect food fraud, such as adulteration or mislabeling of food products.
- Pharmaceutical and Chemical Industries: Liquid chromatography is extensively utilized in the pharmaceutical and chemical industries for drug analysis, quality control, and research purposes. It plays a vital role in drug development, assisting in the identification, quantification, and purification of active pharmaceutical ingredients (APIs). It is also employed for impurity profiling, stability testing, and the analysis of complex mixtures in chemical research.
- Forensic Science and Hospitals: Liquid chromatography is employed in forensic toxicology to analyze biological samples, such as blood or urine, for the detection and quantification of drugs, toxins, or poisons. It helps in determining drug overdose, drug abuse, or exposure to toxic substances. In hospitals, liquid chromatography is used in clinical laboratories for analyzing patient samples to diagnose diseases, monitor drug levels, and assess therapeutic interventions.
These are just a few examples of the broad range of applications of liquid chromatography. Its versatility, high separation efficiency, and sensitivity make it an invaluable tool in various scientific and industrial fields, aiding in research, quality control, and the development of safer and more effective products.
Liquid Chromatography Considerations
When designing protein purification workflows using liquid chromatography, several considerations need to be taken into account to achieve optimal results. The following factors play a crucial role in the design and implementation of a successful chromatographic separation:
- Resolution: Resolution refers to the ability to separate different components in a chromatogram. It is important to achieve clear separation between peaks to isolate the molecules of interest effectively. Factors such as the properties of the resin, buffer composition, flow rate, and sample volume influence the resolution. Different chromatographic techniques may result in varied resolutions for specific compounds.
- Yield: In preparative chromatography, where the goal is to purify a protein for downstream applications, yield becomes a significant consideration. Yield is defined as the amount of the desired protein fraction recovered after purification. It is crucial to maximize the recovery of the protein of interest while maintaining sufficient purity.
- Sample Integrity: For certain applications, such as crystallography or enzymatic studies, maintaining sample integrity is vital. This involves preserving the native conformation and enzymatic activity of the protein during purification. Factors like buffer selection, the addition of protease inhibitors, and efficient purification techniques should be considered to ensure sample integrity throughout the process.
- Sample Purity: Sample purity is a key consideration to obtain a pure protein fraction. Merely observing a single peak in a chromatogram does not guarantee sample purity, especially when dealing with co-eluting compounds. It is necessary to assess sample purity using techniques like gel electrophoresis (SDS-PAGE) or other analytical methods to confirm the absence of contaminants.
It is important to note that sample purity, integrity, and yield often exhibit an inverse relationship. A purification workflow that provides high sample purity may require a longer separation time, potentially resulting in reduced yield or protein inactivation. Balancing these factors is crucial, taking into account the specific downstream applications and the level of purity and activity required.
In some cases, absolute sample purity is essential, such as in antibody production for diagnostic or therapeutic purposes. However, for certain enzymatic studies, functional purity may be sufficient, allowing for tolerable levels of contaminants that do not interfere with or enhance the activity of the protein of interest. Maximizing sample integrity and yield while meeting the purity requirements for the intended applications is a critical consideration in liquid chromatography-based protein purification workflows.
FAQ
What is liquid chromatography (LC)?
Liquid chromatography is a separation technique used to separate and analyze components of a sample based on their interactions with a stationary phase and a mobile phase. It involves passing a liquid sample through a column containing a stationary phase, where the different components separate based on their affinity for the stationary phase.
What are the different types of liquid chromatography?
There are several types of liquid chromatography, including high-performance liquid chromatography (HPLC), ion chromatography, size exclusion chromatography, affinity chromatography, and reversed-phase chromatography, among others. Each type utilizes different principles and stationary phases for specific applications.
What is the role of the mobile phase in liquid chromatography?
The mobile phase, typically a liquid solvent or a mixture of solvents, carries the sample through the column. It helps in the separation of components by interacting differently with the stationary phase and the sample components, leading to their differential elution.
What is the stationary phase in liquid chromatography?
The stationary phase is a material that is packed inside the column and interacts with the sample components to facilitate their separation. It can be a solid support with bonded functional groups or a liquid phase coated onto a solid support, depending on the type of liquid chromatography being employed.
How is the separation achieved in liquid chromatography?
The separation in liquid chromatography is achieved by exploiting the differences in chemical or physical properties of the sample components, such as polarity, size, charge, or affinity for the stationary phase. These differences cause the components to interact differently with the stationary phase, resulting in their separation as distinct peaks.
What are the typical detectors used in liquid chromatography?
Commonly used detectors in liquid chromatography include UV-Vis absorbance detectors, fluorescence detectors, refractive index detectors, and mass spectrometers. These detectors measure the physical or chemical properties of the eluted components and provide signals that can be used for qualitative or quantitative analysis.
How is liquid chromatography used in pharmaceutical industries?
Liquid chromatography is extensively used in the pharmaceutical industry for various purposes, including drug analysis, purity testing, impurity identification, quality control, and formulation development. It helps in ensuring the safety, efficacy, and quality of pharmaceutical products.
What are the advantages of liquid chromatography?
Liquid chromatography offers several advantages, such as high separation efficiency, versatility in separation modes, wide applicability to different sample types, and compatibility with various detectors. It allows for accurate quantification, sensitivity, and selectivity in compound analysis.
What are some common challenges in liquid chromatography?
Challenges in liquid chromatography can include method development and optimization, column selection, sample preparation, matrix interference, peak broadening, resolution limitations, and reproducibility issues. These challenges often require careful consideration and optimization to achieve reliable and accurate results.
How can liquid chromatography be coupled with other techniques?
Liquid chromatography can be coupled with various techniques to enhance its capabilities. For example, coupling LC with mass spectrometry (LC-MS) combines the separation power of liquid chromatography with the identification and structural information provided by mass spectrometry. Other common couplings include LC with UV-Vis spectrometry, fluorescence spectroscopy, or nuclear magnetic resonance (NMR) spectroscopy, among others, to gain additional information about the separated compounds.