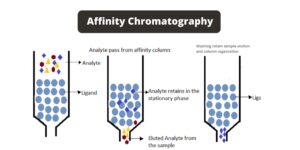
What is Hydrophobic Interaction Chromatography (HIC)?
- Hydrophobic Interaction Chromatography (HIC) is a protein purification technology that is used in both analytical and preparatory applications. It separates and purifies protein molecules using the principle of hydrophobicity, making it a preferable method over other chromatography techniques since it operates under less denaturing circumstances. This preserves the biological activity of the proteins during the purifying process.
- The hydrophobic interaction chromatography technology takes advantage of proteins’ hydrophobic characteristics, which result from the presence of hydrophobic amino acid residues in their structure. These hydrophobic interactions are important for protein folding and stability. A hydrophobic stationary phase or matrix, often comprised of alkyl chains or aromatic groups, is used in HIC, while the mobile phase is an aqueous solution with changing salt concentrations.
- The hydrophobic interactions between the proteins and the hydrophobic stationary phase occur during the chromatography process. Proteins with higher hydrophobicity interact more firmly with the stationary phase and are thus kept for a longer period of time, whereas proteins with lower hydrophobicity pass through more quickly. The strength of the hydrophobic contacts can be controlled by altering the salt concentration in the mobile phase, allowing for additional protein separation based on hydrophobicity.
- One of the most significant advantages of HIC is its ability to purify proteins while retaining biological activity. In comparison to other chromatography techniques, such as ion exchange chromatography or size exclusion chromatography, which can involve harsher denaturing conditions, the conditions utilised in HIC are softer. The HIC environment is less denaturing, reducing the likelihood of protein unfolding or aggregation and guaranteeing that purified proteins preserve their native structure and functioning.
- HIC can also be used to successfully remove contaminants or product aggregate species from aqueous solutions. It takes use of the hydrophobic differences between aggregates and target molecules. Because aggregates are more hydrophobic, they interact more strongly with the stationary phase, allowing target molecules to pass through more easily. Because of this differential interaction, contaminants or clumps in the protein solution can be removed selectively.
- HIC is frequently used in conjunction with other chromatographic techniques to achieve a high degree of purification in practise. It can be combined with ion exchange chromatography to create a multi-step purification procedure in which HIC is used to separate proteins based on their hydrophobicity and ion exchange chromatography is used to separate proteins based on their charge characteristics. HIC can also be used in conjunction with gel filtration chromatography to further separate proteins based on their size or molecular weight.
- Finally, hydrophobic interaction chromatography (HIC) is a good approach for protein purification because it preserves protein biological activity while providing a less denaturing environment. HIC facilitates the separation and purification of proteins based on their hydrophobicity by utilising their hydrophobic qualities, making it a valuable tool in the field of bioseparations and protein purification.
Principle of Hydrophobic Interaction Chromatography (HIC)
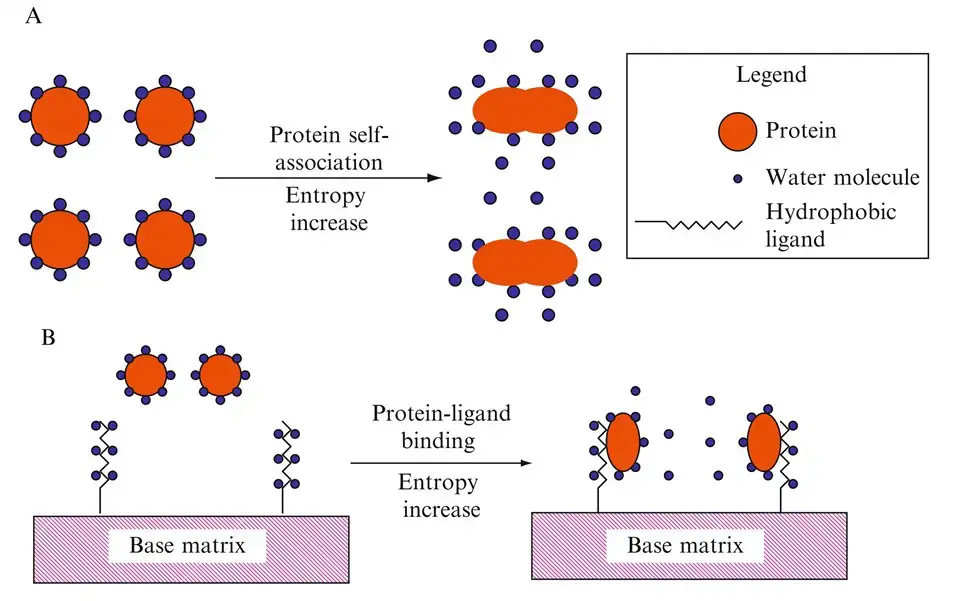
The principle of Hydrophobic Interaction Chromatography (HIC) is based on the interactions between the hydrophobic regions of sample protein molecules and a hydrophobic stationary phase or matrix. This principle allows for the separation and purification of proteins based on their hydrophobicity.
In HIC, the sample protein molecules are introduced into a column containing a high-salt buffer. The presence of the salt in the buffer plays a crucial role in promoting the binding of the proteins to the hydrophobic stationary phase. The salt reduces the solvation of the sample molecules, exposing their hydrophobic regions. As a result, the hydrophobic regions of the proteins interact with the hydrophobic media, leading to their adsorption onto the stationary phase.
The amount of salt required to promote binding is inversely proportional to the hydrophobicity of the protein molecules. Proteins with higher hydrophobicity will have more exposed hydrophobic regions, requiring less salt for binding. Conversely, proteins with lower hydrophobicity will need a higher salt concentration to promote their interaction with the hydrophobic media. This property allows for the separation of proteins based on their relative hydrophobicity.
To elute the bound protein molecules from the HIC column, a decreasing salt gradient is typically employed. By reducing the salt concentration in a gradient manner, the strength of the hydrophobic interactions between the proteins and the stationary phase is gradually weakened. Consequently, the proteins are released from the column in the order of increasing hydrophobicity.
In addition to the salt gradient, mild organic modifiers or detergents can be added to the elution buffer to facilitate sample elution. These modifiers or detergents disrupt the hydrophobic interactions between the proteins and the stationary phase, further aiding in the release of the proteins from the column.
The principle of protein adsorption to HIC media is complementary to other chromatography techniques such as ion exchange chromatography and size exclusion chromatography. While ion exchange chromatography separates proteins based on their charge properties and size exclusion chromatography separates them based on their size or molecular weight, HIC separates proteins based on their hydrophobicity.
In summary, the principle of Hydrophobic Interaction Chromatography involves the use of a high-salt buffer to reduce solvation and expose the hydrophobic regions of protein molecules. The hydrophobic interactions between the exposed hydrophobic regions and the hydrophobic stationary phase lead to the adsorption of the proteins. By employing a decreasing salt gradient and potentially incorporating mild organic modifiers or detergents, the bound proteins can be effectively eluted from the column in the order of increasing hydrophobicity.
Protocol of Hydrophobic Interaction Chromatography (HIC)
The protocol for Hydrophobic Interaction Chromatography (HIC) involves several key steps to effectively separate and purify proteins based on their hydrophobicity. Here is a general outline of the HIC protocol:
- Column Preparation: HIC media, consisting of alkyl or aryl ligands coupled to an inert, porous matrix, are packed into a chromatography column as a packed bed arrangement. The matrix provides the hydrophobic surface for protein binding. Prior to sample application, the column is equilibrated with an appropriate buffer to fill the pores and spaces between particles in the matrix.
- Sample Application: The protein sample, typically dissolved in a moderately high salt buffer, is applied to the HIC column. The buffer selected usually contains 1-2M ammonium sulfate or 3M sodium chloride. These salts promote the key interaction between the protein sample and the hydrophobic medium while minimizing the interaction with other less hydrophobic proteins or impurities.
- Washing: After sample application, the column is washed with the high salt buffer to remove non-bound proteins and impurities. This step helps in reducing background noise and achieving better separation.
- Elution: The salt concentration in the elution buffer is gradually lowered to initiate the elution of proteins. This is typically done by employing a salt gradient, starting from a higher salt concentration and gradually decreasing it. The manipulation of salt gradients allows for the differential elution of proteins based on their hydrophobicity. Proteins with lower hydrophobicity, or those less strongly bound to the hydrophobic stationary phase, will be eluted first.
- Final Wash: After the desired proteins have been eluted, a final wash step is performed to remove any tightly-bound proteins that may still be attached to the column. This wash is typically conducted using a salt-free buffer or a buffer with a significantly lower salt concentration. Additionally, additives can be included in the buffer to promote the desorption of bound proteins. These additives may include water-miscible alcohols, chaotropic salt solutions (which “salt-in” proteins), or detergents.
- Harsher Conditions (if required): In some cases, if there are proteins that are strongly bound to the column and cannot be effectively eluted using the standard protocol, harsher conditions may be necessary. These conditions can include the use of 0.5-1.0M sodium hydroxide, 70% ethanol, or 30% isopropanol to remove all bound proteins. However, it is important to note that these harsher conditions may also denature the proteins to some extent.
By following this protocol, Hydrophobic Interaction Chromatography (HIC) allows for the effective separation and purification of proteins based on their hydrophobicity, while preserving their biological activity. The specific details of the protocol may vary depending on the specific HIC media and system being used.
Factors that Affect Hydrophobic Interaction Chromatography (HIC)
Several factors play a crucial role in influencing the performance and outcomes of Hydrophobic Interaction Chromatography (HIC). These factors should be carefully considered and optimized for successful protein separation and purification. Here are some key factors that can affect HIC:
- Ligand: The choice of ligand used in HIC determines the adsorption behavior of proteins. For example, straight-chain alkyl ligands exhibit hydrophobic interactions, while aryl ligands can show both aromatic and hydrophobic interactions. The specific ligand selected will impact the selectivity and binding characteristics of the HIC system.
- Matrix: The matrix refers to the support material that carries the ligand. Hydrophilic carbohydrates such as cross-linked agarose and synthetic copolymer materials are commonly used as matrix materials. Different matrices can exhibit variations in selectivity, even with the same ligand. Therefore, the choice of matrix can influence the separation efficiency and binding characteristics of the HIC system.
- Degree of Substitution: The degree of substitution refers to the density of ligand molecules attached to the matrix. The binding capacity of a protein is generally directly proportional to the degree of substitution. However, higher levels of ligand substitution can increase the strength of the interaction between the ligand and the protein, making it more challenging to elute the proteins during the purification process.
- Temperature: Temperature can affect the affinity of hydrophobic interactions in HIC. Higher temperatures can enhance the strength of these interactions. Additionally, temperature can influence the structure and solubility of proteins. However, temperature is not commonly utilized to modulate elution in HIC and is typically controlled to maintain stability and prevent protein denaturation.
- pH: The pH of the mobile phases used in HIC typically falls within the neutral range of 5-7. The effect of pH on protein-medium interactions can vary depending on the specific protein. In general, as pH increases, the hydrophobic interaction between the medium and the protein tends to decrease due to increased protein charge. While pH can affect the degree of protein binding, it is not commonly employed to elute solute molecules in HIC.
- Salt Concentration: The addition of salt to the buffer and sample is important for promoting ligand-protein binding in HIC. However, high salt concentrations can also lead to protein precipitation. Sodium, ammonium, or potassium sulfates are effective in promoting interactions between the ligand and the protein but can also increase the risk of precipitation. It is crucial to optimize the salt concentration to achieve the desired binding and elution properties while minimizing protein precipitation.
By carefully considering and optimizing these factors, researchers can tailor HIC conditions to achieve efficient and effective protein separation and purification. It is important to note that the optimal conditions may vary depending on the specific proteins and HIC system being used, and experimental optimization is often necessary to achieve the best results.
Uses of Hydrophobic interaction chromatography
Hydrophobic Interaction Chromatography (HIC) has a wide range of uses in the separation and purification of various biomolecules, primarily proteins, as well as other organic compounds. Here are some key applications of HIC:
- Protein Purification: HIC is particularly valuable for the purification of proteins that possess hydrophobic regions or groups. It allows for the separation of proteins based on their relative hydrophobicity, making it a powerful technique for protein purification. HIC is often preferred over other methods because it operates under milder, less denaturing conditions, thereby preserving the biological activity of the proteins to a greater extent.
- Separation of Hydrophilic and Hydrophobic Molecules: HIC enables the separation of hydrophilic and hydrophobic biological molecules from each other. By exploiting the differences in their hydrophobic properties, HIC can effectively separate compounds based on their affinity for the hydrophobic stationary phase. This makes it useful for separating and purifying a wide range of biomolecules, including proteins, peptides, nucleic acids, carbohydrates, and lipids.
- Removal of Impurities and Aggregate Species: HIC can be utilized to remove impurities and aggregate species from protein solutions. By exploiting the hydrophobic interactions between the impurities or aggregates and the hydrophobic stationary phase, HIC selectively retains these species while allowing the target proteins to pass through. This purification step is often employed in conjunction with other chromatographic techniques, such as ion exchange or gel filtration chromatography, to achieve a high level of purity.
- Sample Pre-concentration: HIC can also be used for sample pre-concentration, where dilute samples with low target molecule concentrations are concentrated onto the HIC column. By selectively binding the target molecules through hydrophobic interactions, HIC helps to concentrate and enrich the desired compounds, enabling further downstream analysis or characterization.
- Separation of Organic Compounds: In addition to proteins, HIC can be applied to the separation of other organic compounds that possess hydrophobic groups. This includes small molecules, peptides, pharmaceuticals, natural products, and synthetic organic compounds. HIC’s ability to exploit hydrophobic interactions makes it a valuable tool for separating and purifying a diverse range of hydrophobic compounds.
Overall, Hydrophobic Interaction Chromatography (HIC) is a versatile technique with widespread applications in the separation and purification of proteins and other hydrophobic compounds. Its ability to preserve biological activity, separate hydrophilic and hydrophobic molecules, and remove impurities makes it a valuable tool in various fields, including biotechnology, pharmaceuticals, biochemistry, and research laboratories.
Example of Hydrophobic interaction chromatography
An example of the application of Hydrophobic Interaction Chromatography (HIC) is the separation of plant proteins from crude extracts. Plant tissues contain a diverse range of proteins with different hydrophobic characteristics, making HIC an effective technique for their separation and purification. Here’s an overview of how HIC can be used in this context:
- Preparation of Crude Extract: Plant tissues are collected and processed to obtain a crude extract containing a mixture of proteins. The extraction process can involve grinding the plant material, homogenization, and subsequent solubilization of proteins using appropriate buffers.
- Column Equilibration: A HIC column is selected based on the specific requirements of the experiment, considering factors such as ligand type, matrix material, and column dimensions. The column is then equilibrated with a buffer containing a moderately high salt concentration. This buffer choice is important as it promotes the hydrophobic interactions between the plant proteins and the HIC stationary phase.
- Sample Loading: The crude extract is applied to the equilibrated HIC column. The hydrophobic interactions between the proteins in the crude extract and the hydrophobic stationary phase of the column result in the selective adsorption of proteins with hydrophobic regions or groups. The non-hydrophobic proteins are not retained and pass through the column.
- Washing: After the sample loading, the column is washed to remove non-bound proteins and impurities. This step helps to reduce background noise and further purify the target proteins.
- Elution: The bound plant proteins are subsequently eluted from the HIC column. This can be achieved by decreasing the salt concentration in a step-wise or gradient manner. The elution conditions are optimized to selectively elute the plant proteins based on their hydrophobicity. Proteins with lower hydrophobicity will be eluted first, while those with higher hydrophobicity will require a lower salt concentration or more hydrophobic elution conditions to be released from the column.
- Analysis and Further Processing: The eluted fractions containing the separated plant proteins are collected and analyzed using various techniques such as SDS-PAGE, Western blotting, mass spectrometry, or enzymatic assays to determine their purity and identity. Depending on the downstream applications, the purified plant proteins can be further processed, characterized, or utilized for various research or industrial purposes.
By employing Hydrophobic Interaction Chromatography (HIC) in the separation of plant proteins from crude extracts, researchers can effectively isolate and purify specific proteins based on their hydrophobic characteristics. This enables further analysis, characterization, and utilization of these proteins in various fields, including plant biology, biotechnology, food science, and pharmaceutical research.
FAQ
What is Hydrophobic Interaction Chromatography (HIC)?
HIC is a chromatographic technique used for the separation and purification of biomolecules, particularly proteins, based on their hydrophobic properties.
Which types of biomolecules can be separated using HIC?
HIC is primarily used for the separation and purification of proteins. However, it can also be applied to the separation of other biomolecules and organic compounds with hydrophobic groups.
What factors affect the separation in HIC?
Factors such as the type of ligand, ligand density, degree of substitution, temperature, pH, and salt concentration can affect the separation efficiency in HIC.
What is the typical salt concentration used in HIC?
HIC is typically performed using high-salt buffers, such as 1-2 M ammonium sulfate or 3 M sodium chloride, to promote the hydrophobic interactions between the proteins and the matrix.
How are proteins eluted in HIC?
Proteins are eluted from the HIC column by decreasing the salt concentration, either using a decreasing salt gradient or by incorporating mild organic modifiers or detergents in the elution buffer.
What are the advantages of using HIC?
HIC allows for the separation of proteins in their native state without denaturation. It can be used for the isolation of protein complexes and the study of protein folding and unfolding.
What is the principle behind HIC?
The principle of HIC is based on the exposure of hydrophobic regions in proteins when the solvation of the proteins is reduced by high salt concentrations. These exposed hydrophobic regions interact with the hydrophobic ligands in the chromatography matrix.
What is the binding capacity of HIC media?
The binding capacity of HIC media refers to the maximum amount of protein that can be bound to the matrix. It is influenced by factors such as the type of ligand, ligand density, and the degree of substitution.
How does HIC work?
HIC utilizes the reversible interaction between hydrophobic ligands in the chromatography matrix and hydrophobic regions of proteins. Proteins are adsorbed onto the hydrophobic ligands in a high-salt buffer and can be eluted using a decreasing salt gradient.
Can HIC be combined with other chromatographic techniques?
Yes, HIC can be used in combination with other chromatographic techniques, such as ion exchange chromatography or size exclusion chromatography, to achieve higher purification levels and resolve complex mixtures of biomolecules.