Cellulose Acetate Electrophoresis (CAE) is a method of separating charged molecules, including proteins, based on their charge and size. It uses a cellulose acetate sheet as a medium for separation, and an electrical current to move the molecules through the medium. The charged molecules are separated based on their migration rate through the cellulose acetate medium, with smaller, more highly charged molecules moving faster than larger, less highly charged molecules.
CAE is an important method of protein separation and analysis, especially in the fields of biochemistry and molecular biology. It allows for the rapid and efficient separation of a wide range of protein molecules, including enzymes, structural proteins, and even DNA fragments.
The history of CAE can be traced back to the 1940s and 1950s, when researchers first started using cellulose acetate for protein separation. Over time, CAE has evolved into a sophisticated and widely used method of protein analysis, with numerous variations and refinements to the basic technique.
In conclusion, CAE is a powerful and versatile method for separating and analyzing proteins, providing valuable information about the molecular structure and function of these important biomolecules. It has a rich history of development and innovation, and continues to play an important role in the advancement of biochemistry and molecular biology.
Principle of Cellulose Acetate Electrophoresis
In cellulose acetate electrophoresis, a cellulose acetate membrane or stripe is employed as a support matrix to separate sample components. Similar to conventional zone electrophoresis, electrophoretic separation takes place in a homogenous buffer solution. The support matrix or medium is immersed in an electrophoresis running buffer with a specific pH value, and electrophoretic separation is carried out for a specific period of time.
Based on the size of the pores between the molecules of the support matrix, it can provide a molecular sieving effect for electrophoretic separation of the sample. Additionally, it can minimise convection currents caused by excessive heat during electrophoresis. When the separation process is complete, the sample components are separated into discrete bands or zones. Each band reflects sample elements that share similar or identical features.
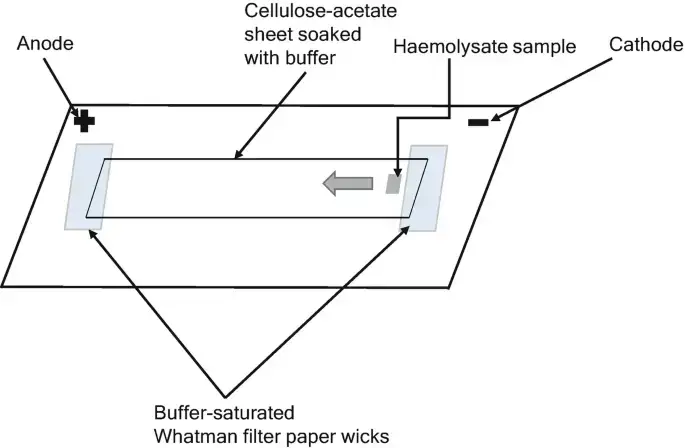
In cellulose acetate electrophoresis, cellulose acetate membranes in the shape of sheets or stripes are soaked with the running buffer. In an electrophoresis tank, each end of the buffer-saturated stripes overlaps with a filter paper wick that reaches into the buffer. About one-third or one-half of the length of the cellulose acetate membrane is covered with discrete samples of interest. An electric current is delivered to each end of the stripe, which functions as an anode and cathode, to initiate the separation. At the conclusion of electrophoresis, separated components might be stained or left unstained for visibility and subsequent quantitative analysis.
Electrophoresis on cellulose acetate can be used for quick screening by comparing the pattern of separation obtained from the sample of interest to a known standard. The method is suitable to numerous types of samples, including nucleic acids, proteins, polypeptides, dyes, and dye-polysaccharide combinations.
Materials Required for Cellulose Acetate Electrophoresis
- Cellulose acetate strip/membrane: The strip or membrane composed of cellulose acetate is a thermoplastic resin. By treating the cellulose paper with acetic anhydride, it is acetylated. It serves as a grid for support. In this instance, one side of the strip serves as the cathode and the other as the anode.
- Electrophoresis buffer: Electrophoresis buffer consists of Typically, a Tris-EDTA buffer (TEB) with a pH of 8.4 is used for blood analysis.
- Tank for electrophoresis: Utilized is a horizontal electrophoresis tank with a movable bridge.
- Power source: It is necessary to have a power supply that can offer consistent current. It can reach 400 volts.
- Filter paper wicks: 3mm Whatman filter paper wick with the same length as an electrophoretic tank’s width.
- For detection and quantification of the separated components, a spectrophotometer, zymogram paper, and blotting materials are required.
Cellulose Acetate as Electrophoretic Support Matrix
- Cellulose acetate is generated from the acetylation of pure cellulose-based filter sheets. Generally, the C-3 and C-6 locations of the glucose ring are acetylated.
- Cellulose acetate has bigger holes than common electrophoretic matrices like agarose and polyacrylic acid. The sieving effect that cellulose acetate has on the separate components is consequently minimal or nonexistent.
- In fact, it can be extrapolated that the mobility of the components in the sample during cellulose acetate electrophoresis is mostly determined by the component’s overall charge rather than its size.
- In comparison to gel or capillary electrophoresis, cellulose acetate electrophoresis has a limited ability to distinguish between extremely similarly sized components. This is referred to as its resolution.
- Similarly, cellulose acetate electrophoresis, unlike other matrices, is appropriate for the separation of peptides or proteins based on their isoelectric point (pI)—where the peptides or proteins have a net negative charge.
- Electrophoresis of proteins or peptides on cellulose acetate yields comparable results to isoelectric focusing (IEF), despite the absence of a pH gradient during cellulose acetate electrophoresis.
Application of Cellulose Acetate Electrophoresis
Therefore, it is sometimes called haemoglobin electrophoresis. Electrophoresis of cellulose acetate has the following applications:
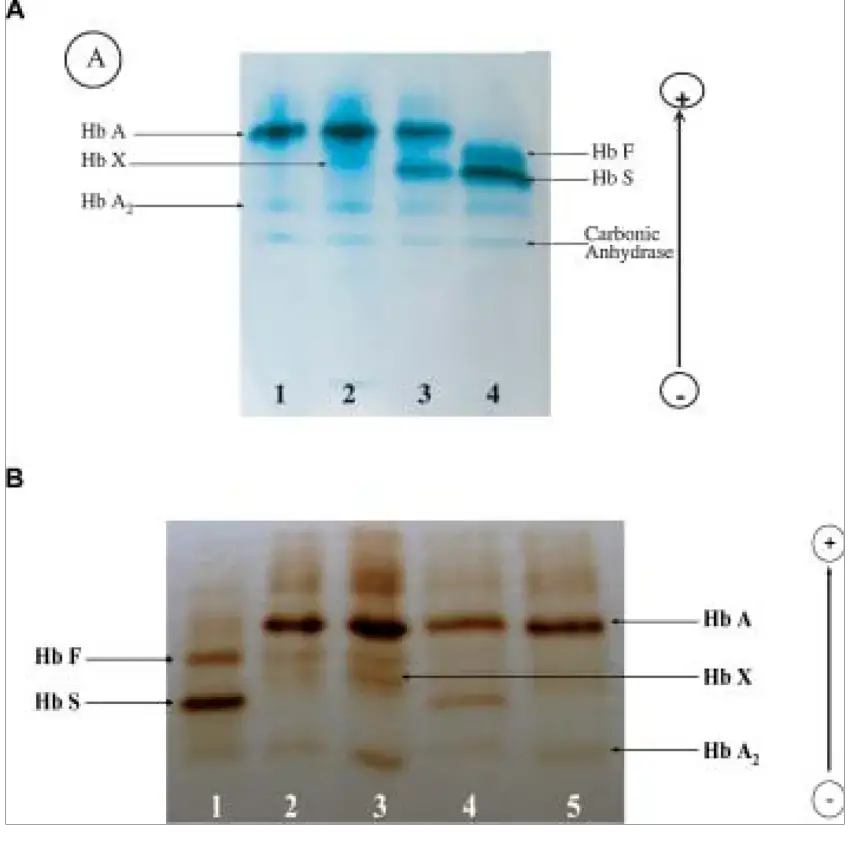
- Blood analysis: Cellulose acetate electrophoresis can be used to detect anomalies in haemoglobin, particularly in individuals with undiagnosed sickle cell anaemia. Similarly, serum proteins such as glycoproteins and albumin are evaluated with this method. It is used for beta thalassemia prenatal diagnosis.
- Analysis of proteins: During cellulose acetate electrophoresis, different amino acids present in proteins are separated based on charge under the influence of an electric current for protein analysis. This study aids forensic, molecular, and clinical laboratories. Cellulose acetate membrane electrophoresis (CAME) is a time-honored technique for evaluating protein panels.
- Polypeptide dyes analysis: Polypeptide dyes are utilised in the textile industry and in molecular biology for labelling purposes. Electrophoresis on cellulose acetate assists in analysing the nature and constituents of polypeptide dyes.
- Analysis of nucleic acid: Cellulose acetate electrophoresis is also useful for examining RNA and DNA components during nucleic acid analysis.
Advantages of Cellulose Acetate Electrophoresis
Although cellulose acetate electrophoresis is a historic technique, it is not extensively employed. There are numerous benefits. Cellulose acetate electrophoresis is superior to other methods of separation due to its simple setup, shorter running time, straightforward detection, and precise zone creation. Below are descriptions of the benefits:
- Easy setup: In cellulose acetate electrophoresis, the apparatus setup is incredibly straightforward. It does not require complex equipment, such as establishing gels in gel electrophoresis or maintaining capillaries in capillary electrophoresis.
- Timely detection: There are numerous methods for identifying separated zones in cellulose acetate electrophoresis, such as the extremely convenient zymogram paper, which allows for timely detection. Similarly, employing blotting techniques and a spectrophotometer is quite simple.
- Reduced in duration: Less than two hours are necessary to complete the electrophoresis. In comparison to gel electrophoresis and capillary electrophoresis, the setup time for agarose electrophoresis is greater than two hours, and the electrophoresis itself takes approximately one hour to complete. Therefore, it requires less time than other techniques.
- No tailing effect: Other methods of protein separation can leave a tailing effect after separation, but this method does not. After separation, the electrophoresis of cellulose acetate does not leave such effects.
- Superior zone formation: the zone generated following separation in cellulose acetate electrophoresis is more evident than in paper electrophoresis. The resolution and clarity of the bands are enhanced.
Limitation of Cellulose Acetate Electrophoresis
The cellulose acetate electrophoresis is highly favourable, however it does have some drawbacks, which are addressed in the following section.
- Only appropriate in an alkaline environment: Electrophoresis of cellulose acetate can only be performed when the buffer is alkaline.
- Probability of electroosmosis: During electrophoresis of cellulose acetate, the sulfonic and carboxylic acid residues can generate electroosmosis.
- Difficulty in quantification: The procedure is difficult to quantify and requires intensive blotting techniques to quantify the amount of components following separation.
FAQ on Cellulose Acetate Electrophoresis
What is cellulose acetate electrophoresis?
Cellulose acetate electrophoresis is a laboratory technique used to separate and analyze different charged species, such as proteins or nucleic acids, based on their charge and size.
How does cellulose acetate electrophoresis work?
Cellulose acetate electrophoresis works by applying an electrical field to a sample of charged species suspended in a gel. The gel matrix, made of cellulose acetate, provides a porous environment in which the charged species can move in response to the electrical field. Smaller species move faster than larger species, leading to separation of the sample based on size and charge.
What are the advantages of cellulose acetate electrophoresis?
Cellulose acetate electrophoresis has several advantages, including simplicity, low cost, ease of use, and the ability to separate a wide range of species. It is also highly versatile, allowing for the separation of a variety of charged species, including proteins and nucleic acids.
What are the limitations of cellulose acetate electrophoresis?
The limitations of cellulose acetate electrophoresis include low resolution compared to other electrophoresis techniques, such as polyacrylamide gel electrophoresis, and the tendency of some species to aggregate, making accurate separation difficult.
What is the procedure for cellulose acetate electrophoresis?
The procedure for cellulose acetate electrophoresis typically involves preparing the sample, casting the gel, loading the sample into the gel, and running the electrophoresis. The separated species are then visualized using staining or other detection methods.
What are the applications of cellulose acetate electrophoresis?
Cellulose acetate electrophoresis is widely used in molecular biology and biochemistry for a variety of applications, including protein analysis, nucleic acid analysis, and the detection of genetic disorders.
Can cellulose acetate electrophoresis be used to separate DNA?
Yes, cellulose acetate electrophoresis can be used to separate DNA based on size, with smaller fragments moving faster through the gel matrix than larger fragments.
How does the pH of the running buffer affect cellulose acetate electrophoresis?
The pH of the running buffer can affect cellulose acetate electrophoresis because it influences the charge on the species being separated. In general, a neutral pH is best for optimal separation, as this provides a neutral environment that does not interfere with the migration of the species through the gel.
What is the role of the voltage in cellulose acetate electrophoresis?
The voltage applied during cellulose acetate electrophoresis plays a critical role in the separation of the species. The voltage creates an electrical field that drives the movement of the charged species through the gel matrix, leading to separation based on size and charge.
How is the resolution of cellulose acetate electrophoresis determined?
The resolution of cellulose acetate electrophoresis is determined by the separation of closely related species. A higher resolution gel will lead to a clearer separation of species that are similar in size and charge, while a lower resolution gel will result in less distinct separation of these species.
References
- Forget, B. G., & Bunn, H. F. (2013). Classification of the Disorders of Hemoglobin. Cold Spring Harbor Perspectives in Medicine, 3(2), a011684–a011684. https://doi.org/10.1101/cshperspect.a011684
- Jorgenson, J. W. (1986). Electrophoresis. Analytical Chemistry, 58(7), 743A-760A. https://doi.org/10.1021/ac00298a001
- Kohn, J. (1969). Separation of haemoglobins on cellulose acetate. Journal of Clinical Pathology, 22(1), 109–111. https://doi.org/10.1136/jcp.22.1.109
- Kohn, J. (1962). Cellulose Acetate Electrophoresis. Proceedings of the Association of Clinical Biochemists, 2(1), 19–20. https://doi.org/10.1177/036985646200200114
- Kohn, J. (1957). An Immuno-electrophoretic Technique. Nature, 180(4593), 986–987. https://doi.org/10.1038/180986a0
- Marengo-Rowe, A. J. (1965). Rapid electrophoresis and quantitation of haemoglobins on cellulose acetate. Journal of Clinical Pathology, 18(6), 790–792. https://doi.org/10.1136/jcp.18.6.790
- Mashkour, M., Afra, E., Resalati, H., & Mashkour, M. (2015). Moderate surface acetylation of nanofibrillated cellulose for the improvement of paper strength and barrier properties. RSC Advances, 5(74), 60179–60187. https://doi.org/10.1039/C5RA08161K
- Nowotny, A. (1979). Immunoelectrophoresis. In Basic Exercises in Immunochemistry (pp. 235–237). https://doi.org/10.1007/978-3-642-67356-6_72
- Righetti, P. G., & Gelfi, C. (2001). 14. Electrophoresis. In Helmut Guenzler & A. Williams (Eds.), Handbook of Analytical Techniques (pp. 346–347). WILEY-VCH Verlag GmbH.
- Rocco, R. M. (2005). Joachim Kohn (1912–1987) and the Origin of Cellulose Acetate Electrophoresis. Clinical Chemistry, 51(10), 1896–1901. https://doi.org/10.1373/clinchem.2005.056572
- Walker, J. M. (2010). 10 Electrophoretic techniques. In K. Wilson & J. M. Walker (Eds.), Principles and Techniques of Biochemistry and Molecular Biology (7th ed.). Cambridge: Cambridge University Press.
- Westermeier, R., Gronau, S., Becket, P., Buelles, J., Schickle, H., & Theßeling, G. (2005). Electrophoresis in Practice: A Guide to Methods and Applications of DNA and Protein Separations (4th, revised ed.). Wiley-VCH Verlag.
- Wild, B. J., & Bain, B. J. (2004). Detection and quantitation of normal and variant hemoglobins: an analytical review. Annals of Clinical Biochemistry, 41(5), 355–369. https://doi.org/10.1258/0004563041731600