What is Precipitation Reaction?
- Precipitation reaction, also known as a precipitation antigen-antibody reaction, occurs when a soluble antigen interacts with its specific antibody under optimal conditions of temperature, pH, and the presence of an electrolyte. This reaction leads to the formation of an insoluble precipitate, resulting in the formation of an antigen-antibody complex.
- During a precipitation reaction, a lattice structure is established between the antigens and antibodies involved. In some cases, this lattice becomes visible as an insoluble precipitate. The antibodies responsible for aggregating the soluble antigens are referred to as precipitins.
- The formation of the antigen-antibody lattice and subsequent precipitation depends on the valency of both the antibody and the antigen. For the precipitation reaction to occur, the antibody must be bivalent, meaning it possesses two antigen-binding sites. Monovalent Fab fragments of antibodies are unable to form precipitates. On the other hand, the antigen must be bivalent or polyvalent, having at least two copies of the same epitope or different epitopes that can react with various antibodies present in polyclonal sera.
- To achieve effective lattice formation and precipitation, it is crucial that the concentrations of the antigen and antibody are appropriate relative to each other. If there is an excess of either the antigen or the antibody, efficient crosslinking and lattice formation may be hindered.
- In summary, a precipitation reaction is a specific type of antigen-antibody reaction where a soluble antigen and its corresponding antibody form an insoluble precipitate under specific conditions. This reaction involves the establishment of an antigen-antibody lattice, resulting in the visible formation of a precipitate.
Precipitation Reaction Definition
Precipitation reaction refers to a chemical reaction in which two soluble substances react to form an insoluble solid compound, known as a precipitate. This reaction occurs when two aqueous solutions containing ions combine, resulting in the formation of an insoluble product that settles out of the solution as a solid. The insoluble compound formed is typically a result of the combination of cations and anions present in the solution.
Precipitation Reactions Principle
- The principle of precipitation reactions is based on the interaction between soluble antigens and specific antibodies, which, under suitable conditions of temperature and pH, form insoluble complexes known as precipitates. These precipitates are formed due to the establishment of antigen-antibody lattices in the presence of appropriate electrolytes.
- When the precipitate remains suspended at the surface of the solution, it is referred to as a floccule, and the reaction is known as flocculation. In the process, soluble antigens and soluble antibodies combine to form an insoluble antigen-antibody complex:
- Antigen (soluble) + Antibody (soluble) → Ag-Ab complex (insoluble)
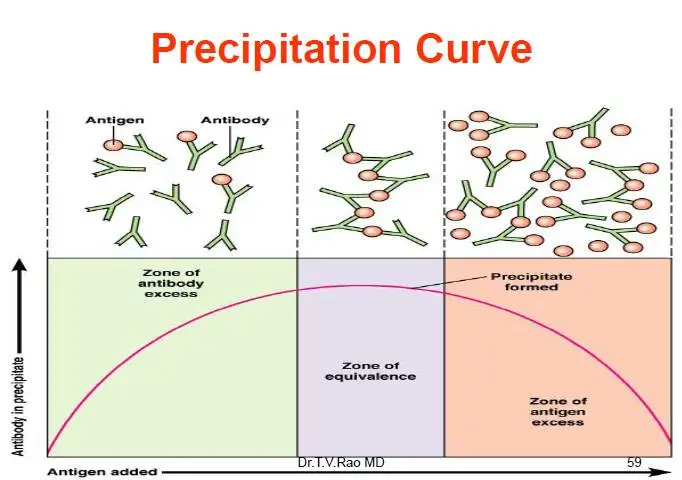
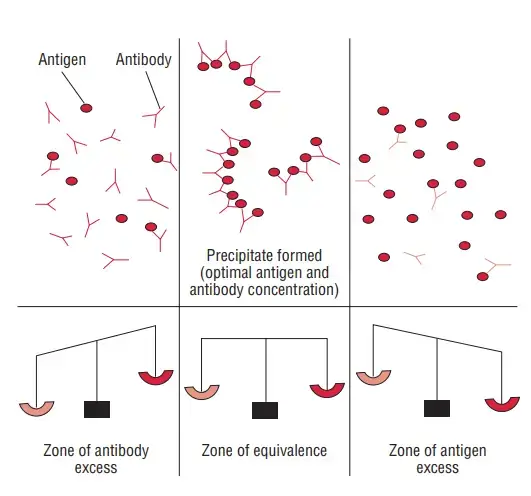
- For a precipitation reaction to occur, the ratio of antigens to antibodies must be equivalent. This point, where there are equal numbers of antigens and antibodies in the solution, is called the zone of equivalence.
- When the antigen concentration is lower than the antibody concentration, a phenomenon called the prozone occurs. In the prozone, the reaction is hindered due to inadequate lattice formation.
- Conversely, when the antigen concentration exceeds the antibody concentration, it results in the post zone phenomenon. In this case, the excess antigens interfere with proper lattice formation.
- The concept of lattice formation and the prozone/post zone phenomena was elucidated by Marrack in 1934, providing a better understanding of the principles underlying precipitation reactions.
Antigen (soluble) + Antibody (soluble) → Ag-Ab complex (insoluble)
What is Precipitation?
Precipitation, also known as immunoprecipitation, refers to the non-covalent interaction between soluble antigens and soluble antibodies, resulting in the formation of an insoluble precipitate. This process can occur both in vivo (within living organisms) and in vitro (in laboratory settings).
In this reaction, the antibodies involved are referred to as precipitins. It is important to note that precipitation does not occur with monovalent Fab fragments of antibodies. Only bivalent antibodies have the ability to participate in the precipitation reaction.
On the other hand, the antigens involved in precipitation need to be bivalent or polyvalent. This means that they must possess multiple copies of the same epitope or have different epitopes that can interact with various antibodies. These antigens, which can bind with multiple antibodies, are sometimes referred to as precipitinogens.
During precipitation, the interaction between the multivalent antigens and the multiple cross-linked antibodies leads to the formation of a lattice structure. This lattice resembles a net-like arrangement and is crucial for the insoluble precipitate to form.
Overall, precipitation is a specific type of antigen-antibody interaction where soluble antigens and antibodies come together to form an insoluble precipitate. This process requires the antigens to be bivalent or polyvalent and the antibodies to be bivalent for effective lattice formation and precipitation to occur.
What is Prozone phenomenon?
- The prozone phenomenon refers to a situation in an antigen-antibody reaction where there is an excess of antibodies compared to the antigen. This imbalance prevents efficient lattice formation, resulting in the inhibition of precipitation. The prozone phenomenon occurs when there are too many antibodies present for the available antigens to bind effectively.
- In the prozone phenomenon, the excess antibodies bind to only a few antigens, and no cross-linkage occurs to form a lattice network. Consequently, the formation of an insoluble precipitate is hindered.
- On the other hand, the postzone phenomenon refers to the scenario where there is an excess of antigens compared to the antibodies. In this case, small antigen aggregates are surrounded by an excess of antigens, preventing the formation of a lattice network and inhibiting precipitation.
- To achieve detectable precipitation reactions, it is crucial to operate within the zone of equivalence, where the proportions of antigens and antibodies are balanced.
- When the precipitate remains suspended as floccules instead of sedimenting, the reaction is referred to as flocculation. Flocculation can occur when the conditions are not optimal for precipitation, and the resulting insoluble complexes form floccules that remain suspended in the solution.
- Precipitation reactions are based on the interaction between antibodies and antigens. They rely on soluble reactants coming together to form an insoluble product, the precipitate. These reactions are characterized by the formation of lattices or cross-links when antigens and antibodies are present in the appropriate proportions. Excess of either component hampers lattice formation and subsequent precipitation.
- Understanding the prozone and postzone phenomena is important in the interpretation of serological tests. False-negative reactions can occur if these phenomena are not taken into account. Diluting the antibody and retesting can correct false-negative results caused by the prozone phenomenon. In the postzone phenomenon, conducting the test at a later time when more antibodies have been produced may reveal their presence. A negative result in this case suggests that the specific antibody is unlikely to be present in the patient.
Features of Precipitation Reaction
Precipitation reactions exhibit several distinctive features:
- Soluble Antigen: In precipitation reactions, the antigen involved is soluble in the solution.
- Antigen-Antibody Interaction: The test relies on the interaction between the soluble antigen and its corresponding antibody. The reaction takes place in the presence of an electrolyte, at a specific pH and temperature, leading to the formation of a precipitate.
- Lattice Formation: The antigens and antibodies combine to form a lattice structure. In some cases, this lattice is visible as an insoluble precipitate.
- Precipitins: The antibodies responsible for aggregating soluble antigens are known as precipitins.
- Flocculation: When the precipitate remains suspended in the solution as floccules rather than settling at the bottom, it is referred to as flocculation.
- Valency Requirement: The formation of the antigen-antibody lattice depends on the valency of both the antigen and the antibody. The antibody must be bivalent, possessing two antigen-binding sites, while monovalent Fab fragments of antibodies do not have the ability to create a precipitate. The antigen must be bivalent or polyvalent, containing multiple copies of the same epitope or different epitopes that can interact with different antibodies present in polyclonal antisera.
These features collectively contribute to the characteristic behavior of precipitation reactions, allowing for the detection and analysis of antigen-antibody interactions.
Types of Precipitation Reaction
Precipitation reactions often fall into three categories: 1. Precipitation in solution 2. Agar precipitation 3. precipitation using an electric field in agar
1. Precipitation in solution
Precipitation in solution is illustrated by the ring and flocculation tests.
a. Ring test
- The ring test is a rapid and straightforward test used to detect the presence of antibodies in a sample. It is performed in a test tube and involves the sequential addition of the antibody solution and the antigen solution.
- The test begins by adding the sample containing the antibody solution into the test tube. This solution may be derived from a patient’s serum or other sources. Following this, the antigen solution is poured into the tube, combining with the antibody solution.
- If the antibody is present in the sample, it will interact with the corresponding antigen, leading to the formation of an insoluble precipitate. After a few hours, the test is considered positive if a distinct precipitate ring is observed in the middle of the test tube. The ring formation indicates the successful interaction between the antibody and the antigen, resulting in the formation of a visible precipitate.
- The ring test is valued for its simplicity and rapidity, making it suitable for initial screening purposes. However, it is important to note that the ring test provides a qualitative result, indicating the presence or absence of a precipitate. Further quantitative analysis may be required to determine the exact concentration or titer of the antibodies in the sample.
- Overall, the ring test offers a quick and convenient method to assess the presence of antibodies by observing the formation of a precipitate ring in a test tube.
Requirements for Ring Test
The ring test requires a few specific requirements to ensure accurate and reliable results:
- Test Tubes or Capillary Tubes: The test is typically performed in glass test tubes or capillary tubes. These tubes provide a controlled environment for the antigen and antibody solutions to interact.
- Serum Containing Reactant (Mainly Antibody): The sample used in the ring test should contain the reactant of interest, which is primarily the antibody. This serum can be obtained from a patient or another source.
- Corresponding Antigen Solution: The antigen solution that corresponds to the specific antibody being tested is required. The antigen is the target molecule that the antibody recognizes and binds to.
- Chemicals such as Glycerol: Glycerol is often used in the ring test to prevent the intermixing of the antigen and antibody solutions. By adding glycerol, a density gradient can be created, ensuring that the solutions remain separate during the test.
The use of glycerol in the ring test is beneficial because it helps maintain a clear separation between the antigen and antibody solutions. This separation allows for the formation of a distinct precipitate ring in the middle of the tube, confirming the positive result.
It is important to note that specific protocols may vary depending on the particular assay or test being conducted. Therefore, it is crucial to follow the recommended procedures and requirements provided by the test manufacturer or laboratory protocols to ensure accurate and reliable results in the ring test.
Ring Test Result Interpretation
- In the ring test, the interpretation of the results is based on the observation of a precipitate ring that forms between the antigen and antibody solutions after a certain period, typically around four hours.
- If a visible precipitate ring is observed in the middle of the test tube after the specified incubation time, the test is considered positive. The presence of the ring indicates that the antigen and antibody have successfully interacted and formed an insoluble precipitate at the interface of the two solutions.
- The positive result suggests that the sample contains the specific antibody that recognizes and binds to the corresponding antigen used in the test. This finding indicates the presence of the desired antibody in the tested sample.
- On the other hand, if no precipitate ring or only a faint ring is observed after the designated incubation time, the test is interpreted as negative. A negative result suggests the absence or insufficient concentration of the specific antibody in the tested sample.
- It is important to note that the interpretation of the ring test results should be done in consideration of the specific protocols and guidelines provided by the test manufacturer or laboratory performing the assay. The timing, observation criteria, and any additional factors for result interpretation may vary depending on the specific test being conducted.
Ring Test Applications
The ring test finds application in various areas, including the Lancefield technique for grouping Streptococcus spp. and the detection of anthrax using Ascoli’s test. Let’s explore these applications further:
- Lancefield Technique for Grouping Streptococcus spp.: The Lancefield technique is a method used to classify different species and strains of Streptococcus bacteria based on their specific antigens. In this technique, the ring test is employed to determine the presence or absence of specific Lancefield antigens. By combining the suspected Streptococcus sample with Lancefield-specific antisera in a test tube, the formation of a precipitate ring confirms the presence of the targeted antigen and aids in the classification of Streptococcus species.
- Ascoli’s Test for the Detection of Anthrax: Ascoli’s test, also known as the Anthrax Precipitation Test, is a diagnostic method used to detect the presence of antibodies against the causative agent of anthrax, Bacillus anthracis. In this test, the ring test principle is applied. The suspected serum sample from an individual is mixed with a known anthrax antigen solution. If antibodies against the anthrax antigen are present in the serum, they will bind with the antigen, resulting in the formation of a precipitate ring. The presence of the precipitate ring confirms the presence of antibodies against Bacillus anthracis and indicates a positive test result for anthrax infection.
b. Slide Test
The slide test is a diagnostic technique performed on glass slides, such as cavity slides, with various applications. Let’s explore the details of the slide test, including its requirements, result interpretation, and applications:
Slide Test Procedure:
- Preparation: A cavity slide is used, which has a shallow well or cavity in the center. The serum sample from the suspected patient is placed in the cavity of the slide.
- Mixing: A known antigen or antibody solution, specific to the target being tested, is added to the serum sample in the cavity. The mixture is thoroughly shaken or mixed to ensure proper interaction between the sample and the antigen or antibody.
- Observation: After a certain incubation period, the slide is examined under suitable conditions. In the case of a positive test, the presence of floccules, which are small clumps or aggregates, can be observed in the suspension on the slide.
Slide Test Requirements: The slide test requires the following components:
- Glass Slides (Cavity Slides): These are specially designed slides with a central cavity or well for holding the test samples.
- Serum Sample: A suspected patient’s serum sample is needed for testing. The serum contains the relevant antibodies or antigens being investigated.
- Known Antigen or Antibody Solutions: Specific antigen or antibody solutions, which are already characterized and known, are used for mixing with the serum sample to initiate the reaction.
Slide Test Result Interpretation:
In the slide test, a positive result is indicated by the presence of floccules in the suspension on the slide after proper mixing and incubation. The formation of floccules confirms the interaction between the antibodies or antigens in the sample and the specific antigen or antibody solution added to the slide.
Slide Test Applications
The slide test finds significant application in the detection of syphilis antigens using the VDRL (Venereal Disease Research Laboratory) test. The VDRL test is a slide test used to diagnose syphilis by detecting specific antibodies present in the patient’s serum. The floccules formation on the slide confirms the presence of syphilis antigens, indicating a positive test result.
The slide test can also be adapted for other diagnostic purposes, where the interaction between known antigens or antibodies and patient samples is assessed visually on a glass slide. This test format offers simplicity, ease of interpretation, and suitability for qualitative assessment of antigen-antibody interactions.
Overall, the slide test, such as the VDRL test, is a valuable diagnostic tool for detecting specific antigens or antibodies in patient samples, enabling the identification and diagnosis of various infectious diseases and other conditions.
b. Tube Test
The tube test is a flocculation test used to observe the formation of floccules when antigens or antibodies solutions are mixed in a tube. It is also applicable for both qualitative and quantitative diagnosis of toxins. Let’s delve into the details of the tube test, including its requirements, result interpretation, and applications:
Tube Test Procedure:
- Preparation: A test tube is used for performing the tube test. The antigens or antibodies solution, along with specific known antibodies or antigens, is added to the tube.
- Mixing: The contents in the test tube are thoroughly mixed to ensure proper interaction between the antigens or antibodies and their corresponding specific antibodies or antigens.
- Observation: After a suitable incubation period, the test tube is examined for the presence of floccules. Floccules are formed when the antigen-antibody complexes come together and precipitate out of the solution.
Tube Test Requirements:
The tube test requires the following components:
- Test Tube: A standard glass or plastic tube is used for performing the test.
- Toxins and Antitoxins: For the quantitative and qualitative diagnosis of toxins, the respective toxins and antitoxins are used as the reactants in the test.
- Serum Sample: In the case of antigen or antibody detection, a serum sample containing the suspected antigen or antibody is required. Specific known antibodies or antigens are added to the tube for the reaction.
Tube Test Result Interpretation:
The tube test result is interpreted by observing the formation of floccules in the test tube. If floccules are formed, it indicates a positive test result. The presence of floccules confirms the interaction between the antigens and antibodies, leading to the precipitation and formation of visible aggregates.
Tube Test Applications:
The tube test was historically used for the detection of syphilis before the development of the VDRL (Venereal Disease Research Laboratory) test. The tube test for syphilis detection is known as the Kahn test. It involves the use of specific known antibodies for the detection of syphilis antigens. The formation of floccules in the test tube confirms the presence of syphilis antigens, indicating a positive test result.
Furthermore, the tube test is employed for the qualitative and quantitative diagnosis of toxins. By mixing toxins and antitoxins in the test tube, the formation of floccules indicates the presence of toxins, allowing for the detection and measurement of toxin levels.
In summary, the tube test is a flocculation test used for the qualitative and quantitative detection of antigens, antibodies, and toxins. It has applications in the diagnosis of syphilis and the assessment of toxin presence and levels.
2. Precipitation in agar
- Precipitation reaction in agar, also known as immunodiffusion, is a technique performed using agar, agarose gel, or polyacrylamide gel as the medium. Agarose gel is commonly preferred for this purpose. The use of gels provides a medium for the diffusion of reactants through the pores, allowing for the formation of clear, observable bands in the precipitation.
- Compared to precipitation reactions in a liquid medium, performing the reaction in agar offers several advantages. One advantage is the formation of distinct bands that can be easily observed and preserved for a longer period, facilitating further analysis and use. These bands allow for the differentiation of individual antigens within a mixture of antigens, providing valuable information about the specific components present in the sample.
- The process of precipitation in agar involves the diffusion of antigens and antibodies through the gel medium. As the reactants diffuse, they encounter each other, leading to the formation of antigen-antibody complexes and subsequent precipitation. The interaction between the reactants results in the formation of visible bands in the agar gel, which can be analyzed and interpreted.
- Precipitation reactions in agar are widely used in various applications, including immunological research, diagnostic testing, and antibody identification. The technique allows for the detection and characterization of specific antigens and antibodies in biological samples, helping to diagnose diseases, determine immune responses, and identify target molecules.
- In summary, precipitation reactions in agar provide a valuable tool for studying antigen-antibody interactions. The use of agar or agarose gel as the medium allows for the formation of observable bands, making it advantageous for analyzing and differentiating individual antigens within a mixture. This technique is widely employed in immunodiffusion assays, research, and diagnostic applications.
Types of immunodiffusion reactions
Immunodiffusion reactions are characterised according to (a) the number of diffusing reactants and (b) the direction of diffusion:
a. Single diffusion in one dimension (Oudin Procedure)
- Single diffusion in a single dimension, also known as Oudin’s technique, is a method of performing precipitation reactions where only one of the reactants, typically the antigens, is allowed to diffuse in a single direction towards their corresponding antibody.
- In this technique, a test tube is used as the reaction vessel. The medium for the reaction, usually agarose gel, is prepared and mixed with the antibody. The mixture is then heated to approximately 60 degrees Celsius and allowed to cool for a while to solidify the gel. Once the gel has cooled, the antigen solution or sample is added to the test tube. The antigens diffuse in a single direction, downwards into the gel, and interact with the antibody to form a line or ring of precipitate.
- The requirements for performing single diffusion in a single dimension include a test tube, agarose gel as the medium, a known antibody solution, and the solution or sample containing the antigens.
- The interpretation of the test results involves observing the formation of precipitate after a certain period. As the antigens diffuse downwards, they interact with the antibody in the gel, resulting in the formation of a line or ring of precipitate. The presence of a precipitate confirms the test as positive. Additionally, the appearance of multiple rings or bands indicates the presence of different types of antigens in the solution, allowing for the differentiation and identification of specific antigens.
- Single diffusion in a single dimension is a valuable technique in immunological research and diagnostic applications. It allows for the detection and characterization of specific antigens in samples, providing important information for disease diagnosis, antibody identification, and immune response analysis.
- In summary, single diffusion in a single dimension, also known as Oudin’s technique, involves the diffusion of antigens in a single direction towards their respective antibodies in a gel medium. The formation of a line or ring of precipitate confirms the presence of antigens and allows for the identification of specific types of antigens. This technique is useful in various immunological applications and provides insights into antigen-antibody interactions.
b. Single diffusion in two dimensions (Radial immunodiffusion)
- Single diffusion in double dimension, also known as radial immunodiffusion, is a technique invented by Mancini for performing precipitation reactions in a gel medium.
- In this technique, agarose gel is mixed with the antibody solution and then poured onto a Petri plate or glass slide to form a solid gel medium. A well is created in the gel using a well borer or any suitable tool. The antigen solution or sample is then poured into the well.
- The requirements for performing single diffusion in double dimension include Petri plates or glass slides as the reaction surface, the antibody solution mixed with agarose gel, a well borer for creating the well, and the sample or solution containing the antigens.
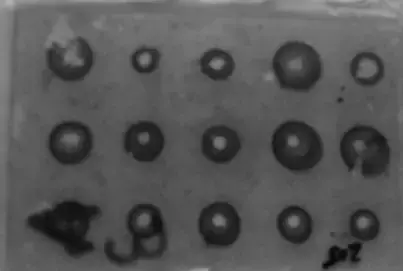
- The interpretation of the test results involves observing the formation of a precipitate ring around the well. As the antigen diffuses through the well in all directions within the gel, it interacts with the antibody, leading to the formation of a visible ring of precipitate. The presence of a precipitate ring confirms the test as positive, and this characteristic ring formation is why the technique is called radial immunodiffusion.
- The diameter of the precipitate ring can provide qualitative and quantitative information. If the concentration of antigens in the sample is higher, it will result in a ring of larger diameter, while a lower antigen concentration will produce a smaller ring. This feature of the technique allows for both qualitative analysis, indicating the presence or absence of the antigen, and quantitative estimation, providing a measure of antigen concentration.
- Single diffusion in double dimension has various applications in immunology. It can be used for the estimation of immunoglobulins, such as IgG, IgA, and IgM, in biological samples. It is also employed in the detection of specific proteins, such as transferrin, in serum. Additionally, this technique can be utilized for diagnosing and monitoring viral infections, such as influenza, by detecting specific viral antigens in patient samples.
- In summary, single diffusion in double dimension, or radial immunodiffusion, is a technique in which agarose gel mixed with antibody solution is used to create a gel medium. The antigen solution is poured into a well in the gel, and the formation of a precipitate ring around the well confirms the presence of the antigen. This technique allows for both qualitative and quantitative analysis of antigens and finds applications in the estimation of immunoglobulins, protein detection, and viral infection diagnosis.
c. Double diffusion in one dimension (Oakley Fulthorpe procedure)
- Double diffusion in a single dimension, also known as the Oakley-Fulthrope technique, is a precipitation reaction technique that allows the interaction between antigens and antibodies to occur in a gel medium.
- To perform this technique, the antibody is first mixed with agar or agarose gel and placed in a test tube. Then, another layer of plain agar is added on top of the antibody-agar mixture. Finally, the antigen solution is poured over the plain agar layer.
- The requirements for conducting double diffusion in a single dimension include test tubes, agar or agarose gel, buffering chemicals to maintain the appropriate pH, and the antibody and antigen solutions.
- Interpretation of the test results involves observing the formation of a precipitate band in the plain agar layer. When the concentrations of the antigen and antibody are equivalent, a visible band of precipitate forms in the plain agar layer, indicating a positive test result.
- The double diffusion in a single dimension technique provides valuable information about the interaction between antigens and antibodies. By observing the position and characteristics of the precipitate band, insights can be gained about the specificity and affinity of the antigen-antibody reaction.
- This technique has various applications in immunology and diagnostics. It can be used to study antigen-antibody interactions, determine the presence of specific antigens or antibodies in a sample, and analyze the specificity and cross-reactivity of antibodies. It is also employed in serological tests for the diagnosis of infectious diseases and in research laboratories for various immunological investigations.
- In summary, double diffusion in a single dimension, or the Oakley-Fulthrope technique, is a precipitation reaction technique performed in a gel medium. The interaction between antigens and antibodies takes place in the plain agar layer between two layers of antibody-agar mixture. The formation of a precipitate band confirms a positive test result. This technique is used for studying antigen-antibody interactions, detecting specific antigens or antibodies, and analyzing antibody specificity in various immunological applications and diagnostic tests.
d. Double diffusion in two dimensions (Ouchterlony procedure)
- Double diffusion in two dimensions, also known as the Ouchterlony procedure, is a technique used to study the interaction between antigens and antibodies in a gel medium.
- To perform this technique, agar or agarose gel is poured onto a Petri plate, and a central well is created. Multiple wells are made around the central well. The antibody solution is placed in the central well, while the antigen solutions are added to the surrounding wells. The plate is then incubated in a moist chamber for one or two days.
- In double diffusion in two dimensions, both antigens and antibodies diffuse in two directions, horizontally and vertically, within the gel medium. As the antigens and antibodies diffuse towards each other, they form precipitate lines.
- The requirements for conducting double diffusion in two dimensions include Petri plates or glass slides, agar or agarose gel, a well borer to create the wells, antigen and antibody solutions, and a moist chamber to maintain the appropriate conditions for incubation.
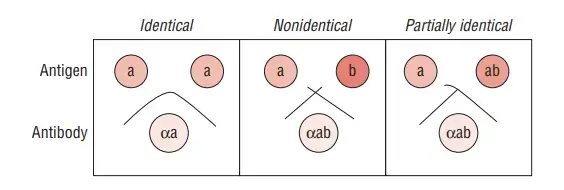
- The interpretation of the test results involves observing the formation of precipitate lines. At the point of equivalence, where the concentrations of antigens and antibodies are optimal, distinct precipitate lines are observed. The pattern of the precipitate lines provides information about the antigen-antibody interaction.
- If the wells contain similar antigens, the precipitate lines merge or fuse together. If the wells contain different antigens, the precipitate lines do not merge but instead cross each other. In the case of partially similar antigens, spur formation can be observed, where the precipitate lines extend outward from the individual wells.
- Double diffusion in two dimensions has various applications in immunology and diagnostics. It is used for the detection of specific infections, such as smallpox and Corynebacterium diphtheriae. It is also employed in the identification of fungal infections and the study of antigen-antibody interactions in research and clinical settings.
- In summary, double diffusion in two dimensions, or the Ouchterlony procedure, is a technique that allows the study of antigen-antibody interactions in a gel medium. Precipitate lines are formed as antigens and antibodies diffuse towards each other in both horizontal and vertical directions. The pattern of the precipitate lines provides information about the interaction specificity. This technique finds applications in the detection of infections and the study of antigen-antibody reactions in various fields of immunology and diagnostics.
Use of Double diffusion
Double diffusion in two dimensions has been utilised for:
- Antibody demonstration in smallpox serodiagnosis
- Antigen identification for fungi
- Detection of antibodies to nuclear antigens that can be extracted.
- The technique for demonstrating the toxigenicity of Corynebacterium diphtheriae is the Elek precipitation test in gel.
Advantages of Immunodiffusion
Immunodiffusion reactions are advantageous in the following ways:
- The precipitation line is apparent as a band in this test, which can also be dyed for preservation.
- The test can detect identity, cross-reaction, and nonidentity between distinct antigens in a combination of responding substances.
3. Precipitation in agar with an electric field
Immunoelectrophoresis
- Immunoelectrophoresis is a technique that combines immunodiffusion with electrophoresis to separate and detect specific antibodies and antigens. It involves the use of agar gel, an electric field, and the principles of diffusion and electrophoresis to generate precipitate lines and identify various antigens in serum samples.
- The application of immunoelectrophoresis is particularly useful in the detection of antibodies, including abnormal proteins such as myeloma proteins, that may be present in human serum.
- To perform immunoelectrophoresis, a drop of antigen is placed in an agar well on a glass slide. The agar gel containing the antigen is then subjected to an electric current. Under the influence of the electric field, the antigens within the gel migrate based on their charge and size during electrophoresis.
- After the electrophoresis step, a trough is cut into the agar gel and filled with the corresponding antibody. As the antigen and antibody diffuse towards each other, a series of precipitation lines are formed. The movement of the antigens in the electric field and their interaction with the antibodies result in the formation of distinct precipitate lines.
- One of the primary advantages of immunoelectrophoresis is its ability to identify multiple antigens in serum samples. By utilizing both diffusion and electrophoresis, this technique allows for the separation and visualization of various antigens based on their charge and size.
- Immunoelectrophoresis is particularly valuable in the detection of normal and abnormal proteins in human serum. Abnormal proteins, such as myeloma proteins, can be identified using this technique, providing essential information for diagnosis and monitoring of certain conditions.
- In summary, immunoelectrophoresis is a technique that combines immunodiffusion with electrophoresis to separate and detect specific antibodies and antigens. By utilizing agar gel, an electric field, and the principles of diffusion and electrophoresis, immunoelectrophoresis enables the identification of multiple antigens in serum samples. This approach is widely used in the detection of abnormal proteins, including myeloma proteins, in human serum.
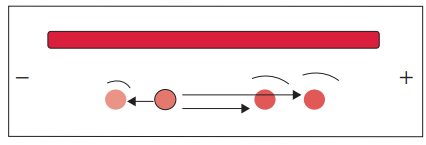
Counter-current immunoelectrophoresis
- Counter current electrophoresis is a technique used for the detection of specific antigens, such as those found on the surface of the Hepatitis B virus or amoebas. It is based on the principle that antigens migrate towards the anode (positive electrode) while antibodies migrate towards the cathode (negative electrode) when an electric field is applied.
- To perform counter current electrophoresis, agarose gel is applied to a glass slide and two wells are created. One well contains the antigen sample, while the other well contains the corresponding antibody. The gel with the wells is then subjected to an electric current.
- Under the influence of the electric field, the antigens within the gel migrate towards the anode, while the antibodies migrate towards the cathode. This movement is facilitated by the flow of charged particles through the agarose gel.
- Within approximately 30 to 60 minutes, a precipitate line becomes visible at the interface between the migrating antigens and antibodies. This precipitate line indicates the interaction and formation of antigen-antibody complexes.
- Counter current electrophoresis offers several advantages, including its relatively rapid results and simplicity of the technique. By utilizing the principles of electrophoresis and the specific migration of antigens and antibodies, this method allows for the efficient detection of target antigens in a relatively short time.
- The application of counter current electrophoresis is particularly useful in the detection of specific antigens, such as those found on the surface of amoebas or the Hepatitis B virus. This technique provides valuable information for diagnostic purposes and the identification of infectious agents.
- In summary, counter current electrophoresis is a technique that utilizes agarose gel, an electric field, and the migration of antigens and antibodies towards opposite electrodes. By applying an electric field, the movement of antigens and antibodies is facilitated, leading to the formation of a visible precipitate line at the interface. This method is commonly used for the detection of antigens on the surface of various pathogens and has applications in medical diagnosis and research.

Rocket electrophoresis
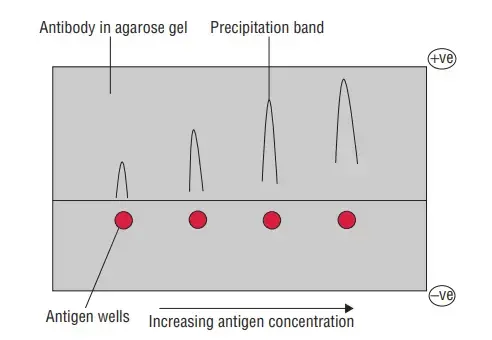
- Rocket electrophoresis is an analytical technique that is an extension of radioimmunodiffusion. It is used for the estimation and quantification of specific antigens, such as immunoglobulins and anti-streptolysin O proteins. This method is a modification of Laurell’s radial immunodiffusion technique.
- To perform rocket electrophoresis, agarose gel is mixed with antibodies at a specific pH that prevents their movement or diffusion. Wells are created in the gel, and the antigens of interest are placed in these wells. The gel is then subjected to an electric field.
- Under the influence of the electric field, only the antigens in the wells diffuse through the agarose gel. As the antigens migrate, they form precipitate bands or lines. These bands appear in the shape of cones or rockets, giving the technique its name.
- The height of the rocket-shaped precipitate band is directly proportional to the amount of antigen present in the sample. The measurement is taken from the well to the apex of the rocket. By comparing the height of the rocket to a standard curve or reference, the quantity of the antigen can be determined.
- Rocket electrophoresis is commonly used for the quantification of serum antigens. It provides a quantitative assessment of specific antigens in a sample, allowing for the measurement of their concentration or level. This information is valuable in various applications, including clinical diagnosis, research, and monitoring the progress of diseases or immune responses.
- In summary, rocket electrophoresis is an extension of radioimmunodiffusion that utilizes agarose gel mixed with antibodies to quantify specific antigens. The technique relies on the migration of antigens through the gel under the influence of an electric field, resulting in the formation of precipitate bands in the shape of cones or rockets. By measuring the height of these rockets, the amount of antigen in a sample can be determined. Rocket electrophoresis is widely employed in the estimation of immunoglobulins, anti-streptolysin O proteins, and other serum antigens for diagnostic and research purposes.
Two-dimensional immunoelectrophoresis
- Rocket electrophoresis is a subtype of two-dimensional immunoelectrophoresis. It is a double diffusion technique utilised for both qualitative and quantitative examination of sera for a wide variety of antigens.
- This test consists of two stages. In the first step, electrophoresis is used to separate antigens in solution. In the second stage, electrophoresis is performed perpendicular to the first stage in order to produce rocket-like precipitation.
- In this test, a small trough is cut into agar gel on a glass plate and then the antigen solution is added.
- Antigens migrate into the gel at a pace proportionate to their net electric charge once an electric current has been conducted through the gel.
- In the second stage, following electrophoresis, the gel fragment containing the separated antigens is placed on a second glass plate and antibody-laden agar is poured around it.
- A second electric potential is delivered perpendicular to the initial migratory direction.
- Antigens that have been pre-separated migrate at a pace proportionate to their net charge into a gel containing antibodies, where they precipitate with the antibodies to form precipitates.
- This approach is both qualitative and quantitative in that it recognises distinct antigens present in the serum solution and detects the amount of different antigens present in the solution, respectively.
Turbidimetry and nephelometry
- Both turbidimetry and nephelometry rely on the phenomenon of light scattering by precipitates in a solution in order to detect and quantify precipitation reactions in serum.
- Turbidimetry is the measurement of precipitate turbidity or cloudiness in a solution.
- In this procedure, a detector is positioned in direct line with the incident light to gather light that has passed through the solution.
- Thus, it quantifies the decrease in light intensity caused by reflection, absorption, or scattering. Light is scattered proportionally to the size, shape, and concentration of precipitates in solution.
- Nephelometry is an advancement of this technique since it measures the light scattered at a specific angle from the incident beam as it passes through a suspension containing antigen–antibody precipitate.
- The amount of light dispersed is an indicator of the solution’s concentration. A constant amount of antibody would result in an increase in antigen–antibody complexes as antigen concentration increases.
- Therefore, the connection between antigen concentrations, as shown by the production of antigen–antibody complexes, and light scattering is close to linear.
- Using a computer, this technique can estimate the exact antigen or antibody concentrations in the serum.
- In order to increase the sensitivity of this system, laser beams have been utilised as the incident light source.
Nephelometry is presently the preferred technique in many laboratories for measuring plasma proteins, such as IgG, IgM, and IgA, complement components, RA (rheumatoid arthritis) factor, ASLO (antistreptolysin O), etc.
Applications
Countercurrent immunoelectrophoresis has numerous applications:
- It is a quick and highly specific approach for detecting antigen and antibodies in serum, cerebrospinal fluid, and other body fluids for the diagnosis of a variety of infectious disorders, including bacterial, viral, fungal, and parasitic infections.
- It is typically used to detect Hepatitis B surface antigen (HBsAg), -fetoprotein, hydatid and amoebic antigens in the serum, and cryptococcal antigen in the cerebrospinal fluid.
Limitations of Precipitation Reaction
The precipitation reaction, while widely used in diagnostic immunology, has certain limitations that should be taken into consideration. These limitations include:
- Lower Sensitivity: Compared to other techniques such as agglutination, the sensitivity of precipitation reactions can be relatively lower. This means that the amount of antigen or antibody required for a detectable precipitate may be higher, leading to a potential decrease in sensitivity.
- Time-Consuming: Precipitation reactions can be more time-consuming compared to some other immunological techniques. The formation of visible precipitates often requires a certain incubation period, and the reaction may take hours or even days to complete.
- Requirement for Polyvalent Antigens: Precipitation reactions may not occur properly or may not occur at all in the absence of polyvalent antigens. Polyvalent antigens possess multiple antigenic determinants that can interact with multiple antibodies, facilitating the formation of visible precipitates. In the absence of polyvalent antigens, the precipitation reaction may not yield reliable results.
- Requirement for Equivalent Numbers of Antigens and Antibodies: Precipitation reactions require the presence of equivalent numbers of antigens and antibodies for optimal results. If there is an imbalance between the concentrations of antigens and antibodies, the formation of precipitates may be hindered or incomplete, leading to inaccurate or inconclusive results.
- Technical Expertise Required: Certain techniques involving precipitation reactions, such as precipitation in agar with electrophoresis, require expertise or trained professionals to perform accurately. These techniques may involve complex procedures, such as the preparation of agarose gels, application of electric fields, and interpretation of the results. Without proper knowledge and experience, the accuracy and reliability of the precipitation reaction may be compromised.
Applications of Precipitation Reaction
The precipitation reaction has numerous applications in diagnostic immunology. It is commonly utilized in immunological diagnostics for various purposes. Some of the specific applications of precipitation reactions are:
- Detection of Syphilis: The precipitation reaction is widely used in the detection of syphilis in patients. Tests such as the VDRL (Venereal Disease Research Laboratory) test and the Kahn test employ precipitation reactions to identify the presence of syphilis antibodies in patient samples.
- Separation of Specific Proteins: Precipitation reactions can be employed to separate specific proteins from complex mixtures. By using specific antibodies that bind to the target proteins, the proteins of interest can be precipitated out of the mixture, allowing for their isolation and further analysis.
- Microbial Grouping: Precipitation reactions are used for the grouping and identification of different microbes based on the presence of specific antigens. For example, in the Lancefield technique, precipitation reactions are employed to group different strains of Streptococcus based on the specific antigens present on their cell surfaces.
- Toxin Standardization: Precipitation reactions are used for the standardization of toxins with their respective antitoxins. By combining the toxin and antitoxin, a precipitation reaction can be observed, and this reaction can be used to determine the potency or concentration of the toxin.
- Immunological Diagnostics: Precipitation reactions are widely used in various immunological diagnostic tests. These tests rely on the interaction between antigens and antibodies, leading to the formation of visible precipitates. These reactions can be used to detect the presence of specific antibodies or antigens in patient samples, aiding in the diagnosis of infectious diseases, autoimmune disorders, and other immunological conditions.
Precipitation Reaction vs Agglutination Reaction
| Precipitation Reaction | Agglutination Reaction |
|---|---|
| Binding of soluble antigens with soluble antibodies | Binding of insoluble antigens with soluble antibodies |
| Formation of precipitate or floccule | Formation of clumps |
| Takes more time to occur | Takes less time to occur |
| Lower sensitivity compared to agglutination | Higher sensitivity compared to precipitation |
| Involves larger size antigens | Involves smaller size antigens |
| Examples: Ring test, VDRL test | Examples: ABO Blood Grouping, Widal test, etc. |
FAQ
What is precipitation reaction?
Precipitation reaction refers to the formation of a visible insoluble complex (precipitate) when soluble antigens react with their corresponding soluble antibodies.
How does precipitation reaction differ from agglutination reaction?
In precipitation reaction, soluble antigens bind with soluble antibodies, forming a precipitate or floccule. In contrast, agglutination reaction involves the binding of insoluble antigens with soluble antibodies, resulting in the formation of clumps.
What are the applications of precipitation reaction?
Precipitation reaction has various applications in diagnostic immunology, including the detection of syphilis (VDRL test), grouping of microbes, separation of specific proteins, and standardization of toxins with antitoxins.
Is precipitation reaction time-consuming?
Yes, precipitation reaction can be more time-consuming compared to other techniques, as it may require a few hours or even days for the formation of visible precipitate.
What are the limitations of precipitation reaction?
Some limitations of precipitation reaction include its lower sensitivity compared to agglutination, the requirement of polyvalent antigens for proper reaction, the need for equivalent numbers of antigens and antibodies, and the expertise required for certain techniques like precipitation in agar with electrophoresis.
How is the sensitivity of precipitation reaction compared to agglutination reaction?
The sensitivity of precipitation reaction is generally lower than that of agglutination reaction. Agglutination reaction is known for its higher sensitivity in detecting smaller size antigens.
Which tests are examples of precipitation reactions?
Tests such as the Ring test and VDRL test (used for syphilis detection) are examples of precipitation reactions.
Can precipitation reaction be used for protein separation?
Yes, precipitation reaction can be employed for the separation of specific proteins. By precipitating proteins using their specific antibodies, they can be separated from the mixture.
What happens during precipitation reaction in terms of antigen-antibody interaction?
During precipitation reaction, soluble antigens and soluble antibodies come into contact, leading to the formation of an insoluble antigen-antibody complex. This complex then precipitates, becoming visible as a precipitate or floccule.
Are there any other techniques similar to precipitation reaction?
Yes, there are other related techniques, such as immunodiffusion, immunoelectrophoresis, and rocket electrophoresis, which combine aspects of precipitation reaction with other principles to enhance sensitivity and specificity in immunological diagnostics.
References
- Ouchterlony, Ö. T. G. (1998). Precipitation Reaction. Encyclopedia of Immunology, 1999–2002. doi:10.1006/rwei.1999.0506
- Deshmukh, Amol & Dighe, Pravin & Tiwari, Kundan & Garud, Supriya. (2020). PRECIPITATION REACTIONS IN IMMUNOLOGY. EUROPEAN JOURNAL OF PHARMACEUTICAL AND MEDICAL RESEARCH. 7. 214-216. http://ecoursesonline.iasri.res.in/mod/page/view.php?id=62004
- https://testbook.com/learn/chemistry-precipitation-reaction/
- https://www.aakash.ac.in/important-concepts/chemistry/precipitation-reaction
- https://mlinjawi.kau.edu.sa/Files/0001735/files/18847_precipitation%20reactions.pdf
- https://www.biosciencenotes.com/types-of-precipitation-reaction/
- https://www.brainkart.com/article/Precipitation-Reactions–Test,-Application_20189/
- https://uomosul.edu.iq/public/files/datafolder_2913/_20191231_051628_760.pdf
- https://www.onlinebiologynotes.com/precipitation-tests-and-types/
- https://microbenotes.com/introduction-to-precipitation-reaction/
- https://www.slideshare.net/doctorrao/antigen-and-antibody-reactionsprecipitation-methods
- https://www.slideshare.net/learnmicrobiology/precipitation-reaction-247588289
- https://microbiologynote.com/ring-precipitation-test/
- https://biologyreader.com/precipitation-reaction.html