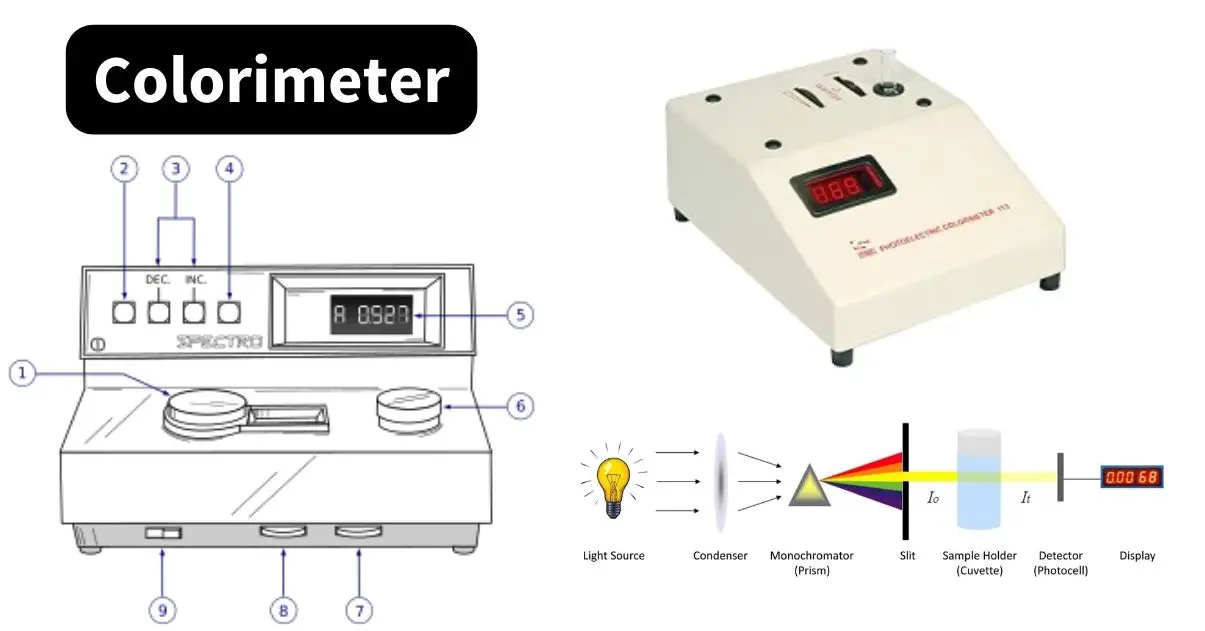
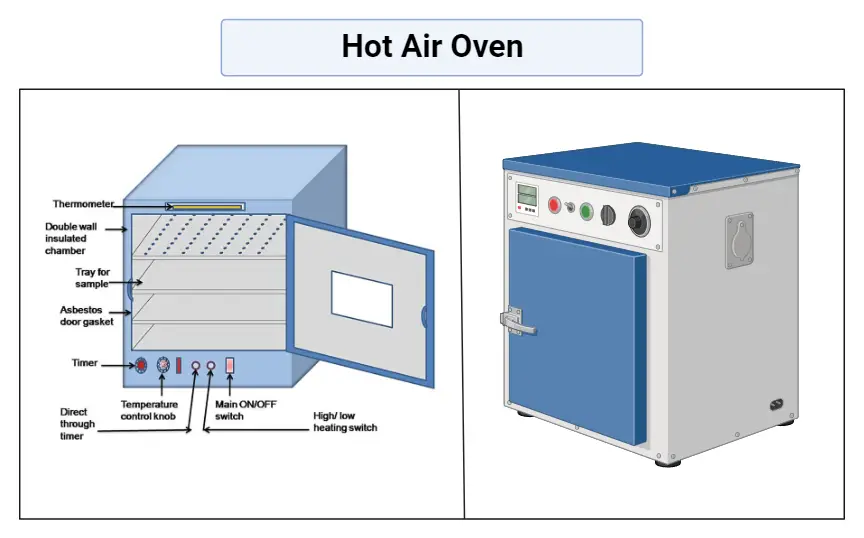
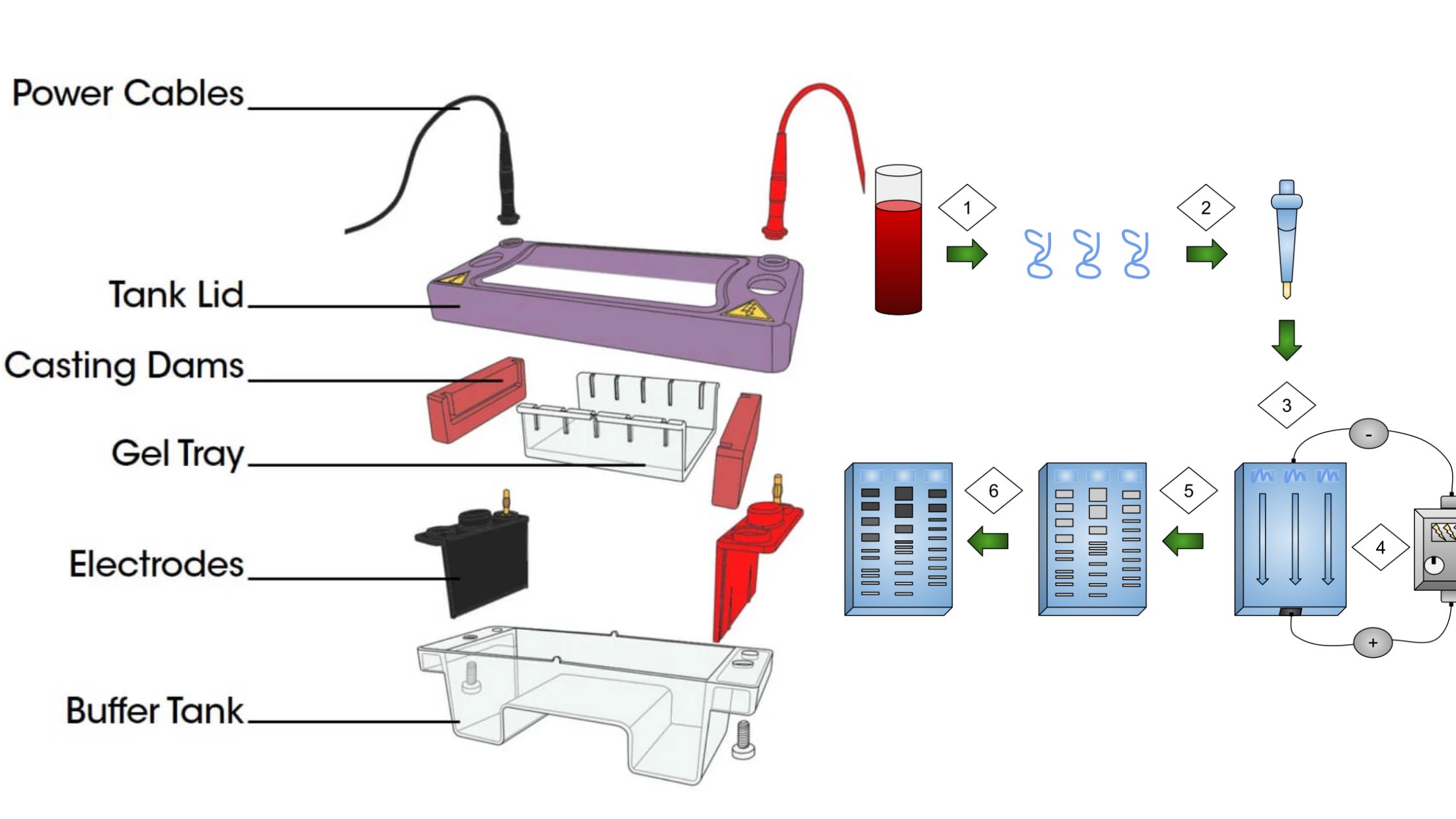
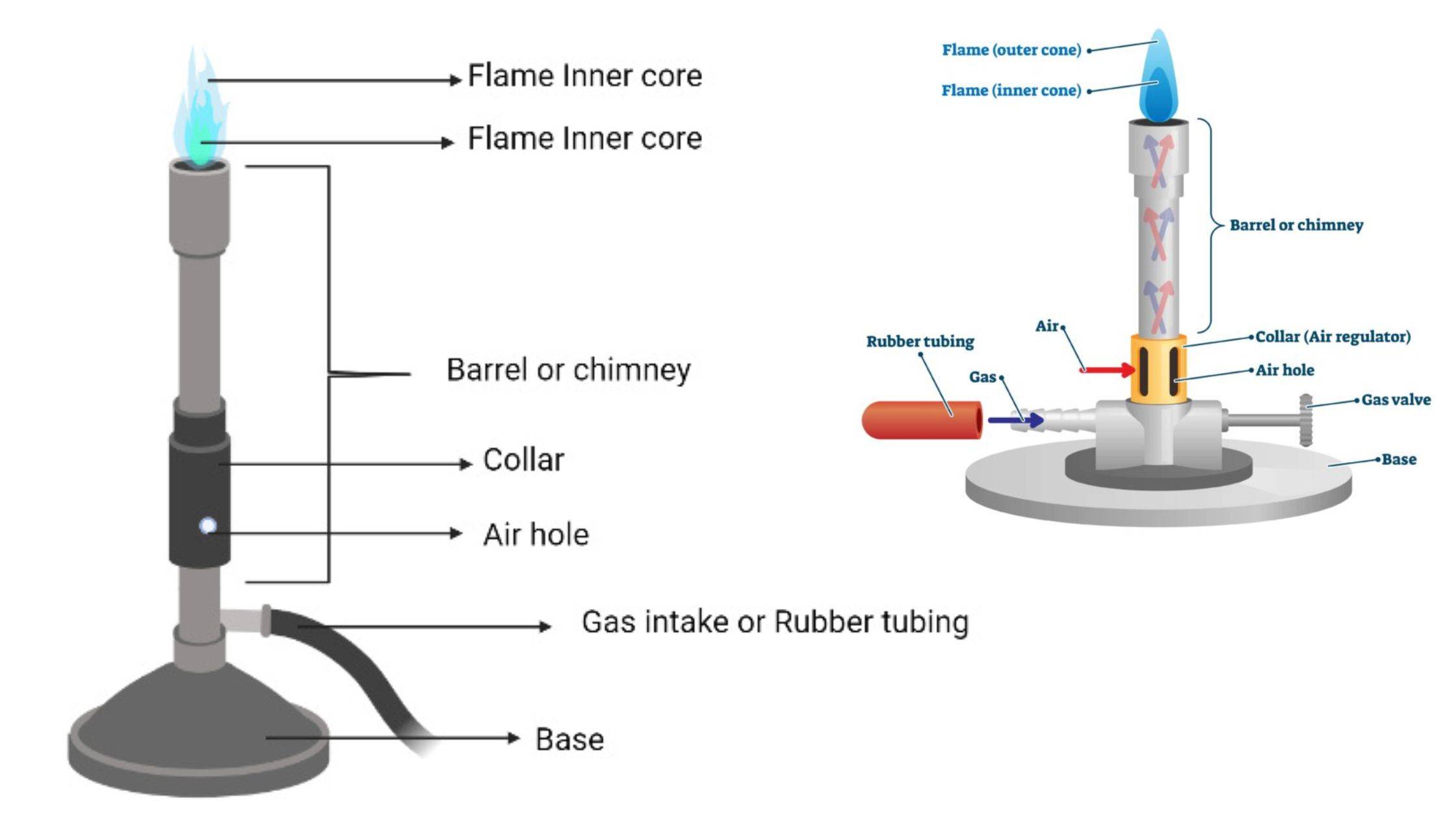
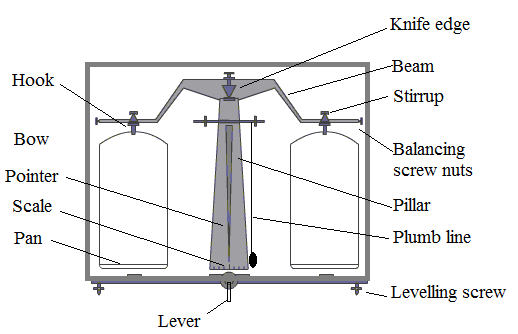
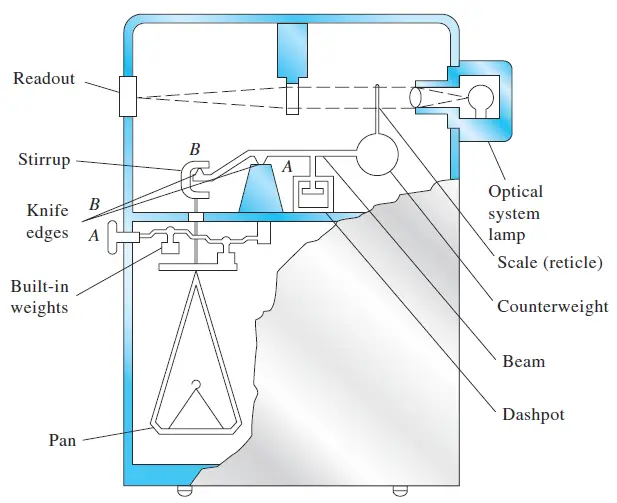
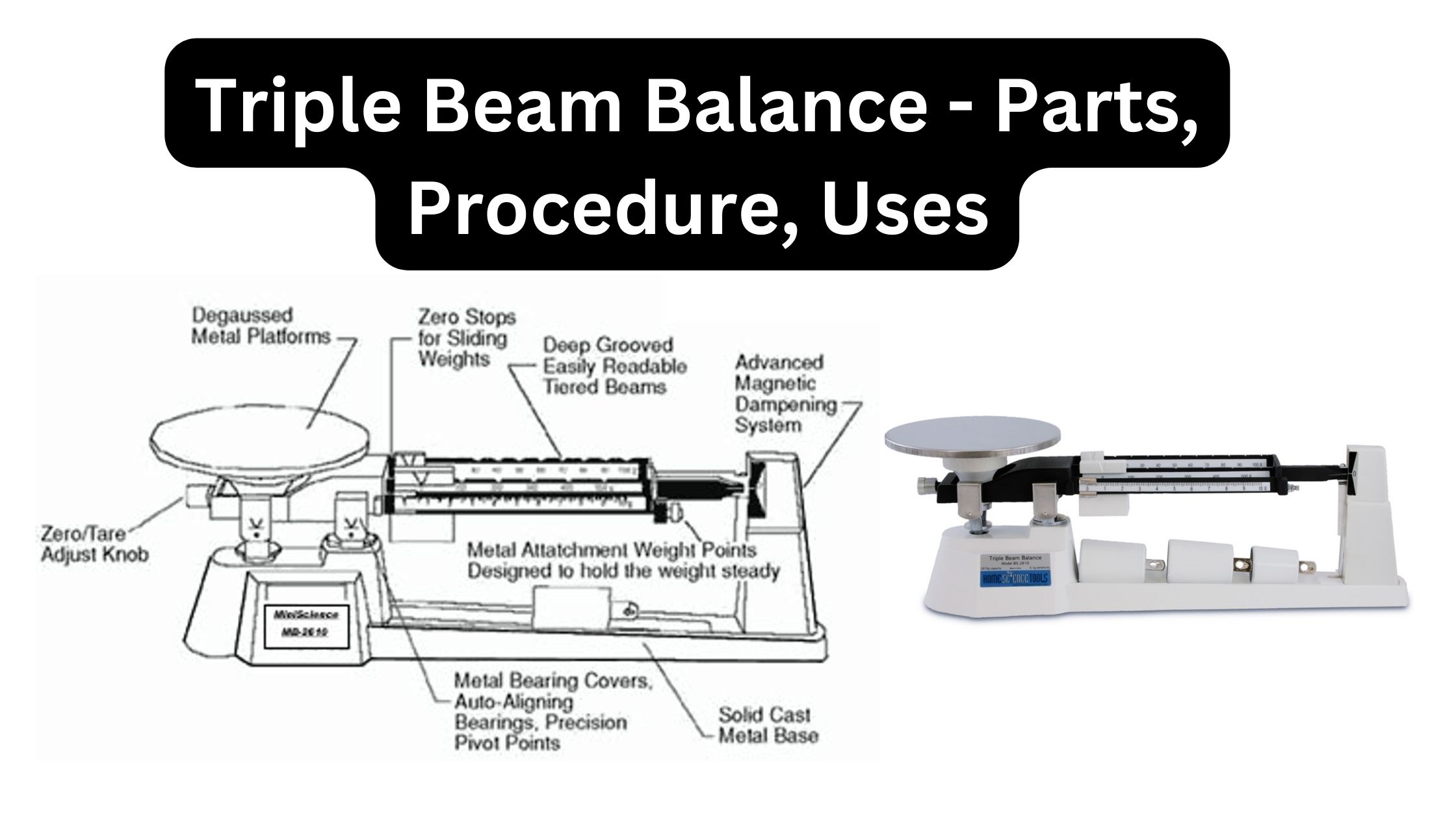
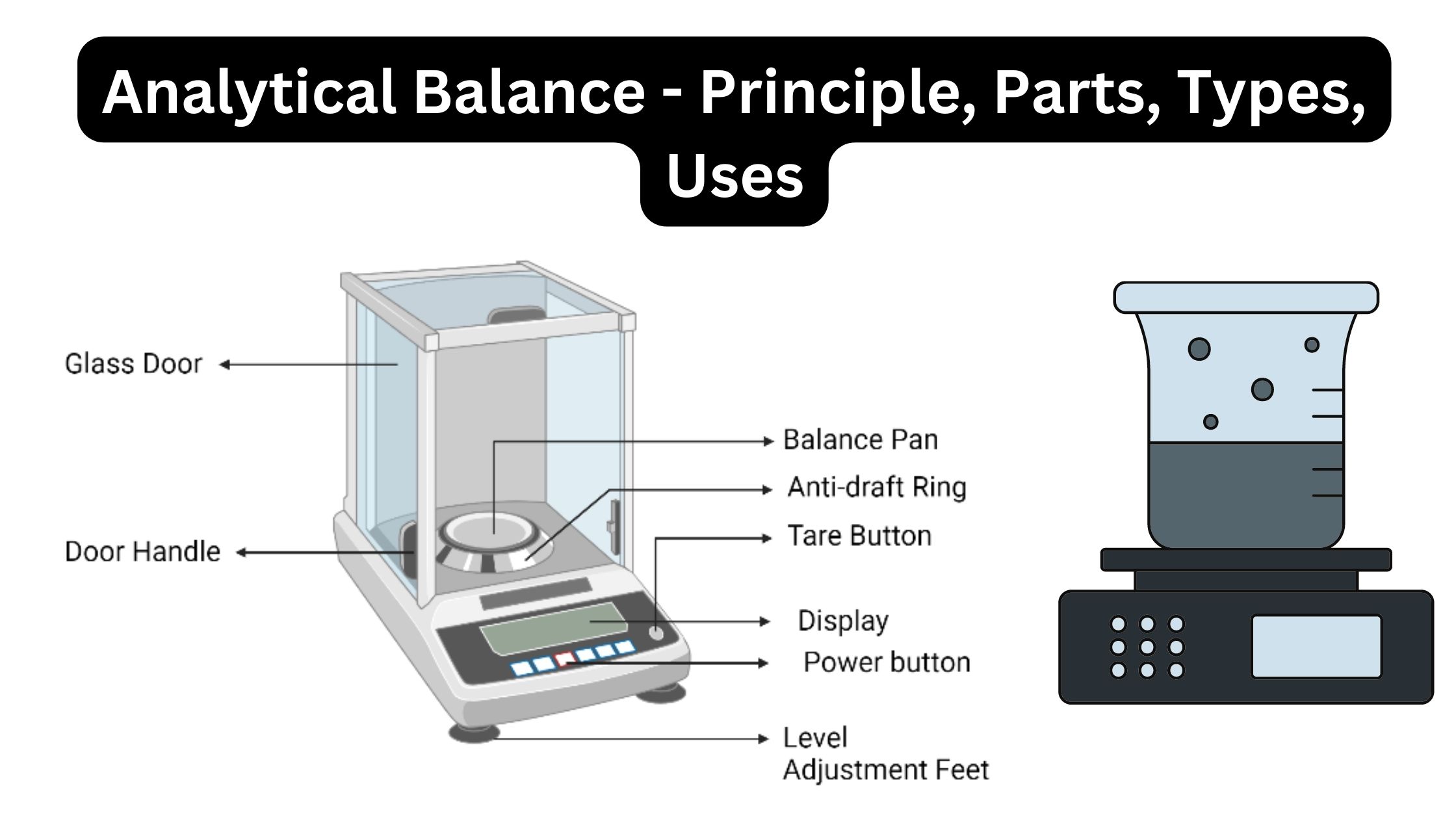
Colorimeter – Definition, Principle, Parts, Procedure, Applications
A colorimeter is a scientific instrument used to measure the absorbance of light by a colored solution to determine the concentration of solutes. Based on the Beer-Lambert law, the principle of colorimeter explains how light intensity changes as it passes through a solution. The instrument mainly consists of essential parts of colorimeter like a light … Read more