To function properly, cells must be kept turgid. To function properly, a well-watered plant will grow and produce fruits and flowers. A plant that is not watered for several days will wilt and eventually die. This happens because water leaves the cell and causes the cell to lose its turgidity.
Based on the concentration of solute in the solution, water moves into or out the cell. Osmosis refers to the process of determining water’s net movement through a semipermeable membrane, from a low solute content region to a high one.
Tonicity refers to the effect of the osmotic pressure gradient. It is the difference in the water potential between two solutions separated by a partially porous cell membrane. Tonicity is determined by the concentration of selective membrane impermeable solvents across a cell membrane. This determines the direction and extent to which osmotic flux flows. This term is used to describe the shrinking or swelling of cells when they are immersed in an external solution.
Tonicity can only be affected by solutes that are unable to cross the membrane. Only these substances exert an effective osmotic force. Tonicity is not affected by solvents that can freely cross the membrane. They will always equilibrate at equal concentrations without any net solvent movement. It can also affect imbibition.
One solution can have one of three types of tonicity: hypertonic or hypotonic. Salt water is an example of a hypotonic solution.
What is tonicity? Tonicity refers to the ability of an extracellular solution to move water inside or outside a cell through the process called osmosis. It is also known as the solution’s osmolarity, which measures how much solute has been dissolved in a given amount of solution.
- Tonicity refers to the difference in concentration between a solution and another. Concentration refers to the concentration of solutes that are dissolved in a solution.
- Hypertonic is a solution that contains more solutes (or less water) than the other.
- Hypotonic solutions have a lower concentration and more water than other solutions.
- The same amount of solutes is found in isotonic solutions.
- Biology says that water’s movement across semipermeable membranes is determined by the relative tonicity of the environment to the cell.
- Water moves to balance the concentration gradients of solutes. It can move from high to low concentrations of solutes.
- Water’s polar nature is responsible for its ability to dissolve solutes.
- Temporary hydrogen bonds are formed between water molecules and other solutes due to the different electrical poles of each watermolecule. This allows water to be distributed and moved into new areas.
- Water’s tonicity is enhanced by adding solutes, but it is impossible to label the solution using one specific term of tonicity without comparing it to another.
- Similar results can be achieved with two different solutes. They require different amounts of water to dissolve. One solute, such as silicon, can take many water molecules to dissolve, while another could be completely dissolved in high concentrations.
- The osmolarity is the total concentration in all dissolved substances and can be used to describe the overall tonicity water.
Osmotic Pressure
Osmotic pressure is the pressure created by water diffusion across a membrane. The equivalent of the osmotic force is when the pressure in the water-flowing compartment is increased to stop water movement. This pressure is commonly referred to as hydrostatic (or ‘water-stopping pressure’).
This pressure is measured in tonicity. If there is no tendency for water or osmotic pressure to move across the membrane, it means that the concentrations of both solutes are equal. These solutions will be considered isotonic in relation to each other. The concentrations of solutes are usually higher on one side of the membrane than on the other.
Osmolarity
Osmolarity refers to the total amount of solutes present in a solution. Solution with a low level of osmolarity will have fewer solute particle per liter. However, solution with high osmolarity will have more solute particles for every liter. Water will flow from one side of a solution with a lower osmolarity than the other when it is separated by a membrane that is permeable to water but not to solute.
To describe the relative osmolarities of solutions, three terms are used: hypoosmotic (hyperosmotic), isoosmotic (isoosmotic), and hyperosmotic (hyperosmotic). If you compare two solutions with different osmolarities for example, the hyperosmotic solution is the one to choose. The hypoosmotic solution is the one with a lower osmolarity. Two solutions with the same osmolarity are called isoosmotic.
Osmosis vs Diffusion
Diffusion refers to the movement of particles from one region with a higher concentration to another. If you add sugar to water, it will diffuse through the water until the sugar concentration is constant throughout. A second example of diffusion is the spread of fragrances in a room.
As with diffusion, osmosis causes particles to look for the same concentration in the solution as they do during diffusion. The particles might be too large for semipermeable membranes to allow them to pass through. This allows water to move across the membrane. A semipermeable membrane can be used to separate sugar solutions from pure water. The pressure on the water side will allow the sugar solution to dilute. Does this mean that all the water will flow into your sugar solution? It is possible that the fluid exerts pressure on the membrane and equalizes the pressure.
For example, if you place a cell in water, water will flow into it, which causes it to swell. Is all the water going to the cell? No. No.
Small ions and molecules can cross semipermeable membranes, so that solutes (Na+, Cl+) behave as if simple diffusion was occurring.
Types of Tonicity in Solutions
Solutions can be classified based on their relative osmolarity to extracellular fluid.
1. Hypotonic Solutions
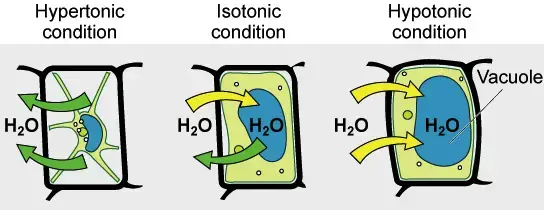
Hypotonic solutions have a lower concentration than other solutions. Hypotonic is a solution that is not contained in a cell. It has a lower concentration than the cytosol. Water diffuses into cells due to osmotic tension, making them appear bloated or turgid. If the gradient is sufficiently large, cells that do not have a cell wall, such as animal cells can be subject to excessive water uptake, which can cause cytolysis or cell rupturing. Plant cells in hypotonic solutions have a central vacuole that takes in extra water and pushes against the cell wall. It pushes back due to the rigidity in the cell wall, which prevents the cell from burst. This is known as turgor pressure.
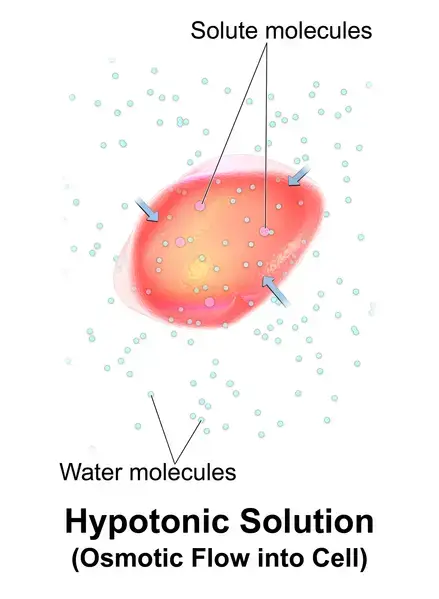
Example: A cell placed in hypotonic solution (example: tapwater) will experience a net flow in the cell’s extracellular environment. This causes the cell to expand or increase in volume.
2. Hypertonic Solutions
Hypertonic solutions have a higher concentration of non-permeating solvents than other solutions. The tonicity of a solution is usually determined by its solute concentration relative the other solution. A solution that is not inside a cell can be called hypertonic if it contains more solutes than the cell’s cytosol. In order to balance the solute concentrations, water will flow out of a hypertonic cell when it is submerged in the solution. In contrast, the cytosol can be classified as hypotonic, which is opposite to the outer solution.
Plant cells in hypertonic solutions experience a flexible cell membrane that pulls away from the rigid wall of the cell, but is still attached to the wall at points known as plasmodesmata. Plasmolysis is a condition where the plasmodesmata become constricted and cells take on the appearance a pincushion. Plant cells cannot be described as isotonic or hypotonic because of the significant pressure exerted on them by their cell walls.
Hypertonicity can be circumvented by some organisms. Saltwater, for example, is hypertonic for fish that live in it. The fish require a large area in their gills to be in direct contact with seawater for gas exchanging. They lose water osmotically from their gill cells. The fish respond by drinking large quantities of saltwater and excreting excess salt. Osmoregulation is the name of this process.
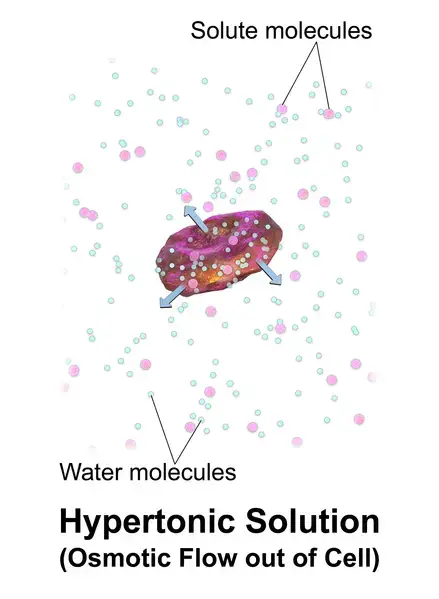
Example: A cell placed in hypertonic solutions (example: seawater) will experience a net flow outside of the cell. This causes the cell to shrink or lose volume.
3. Isotonic Solutions
If a solution’s effective osmole is equal to that of another solution, it is considered isotonic. Biology says that the solutions to either side of a cell’s membrane are isotonic when the concentrations of solutes in the outside and inside of the cell equal each other. The cell does not shrink or swell in this situation because there is no concentration gradient that would cause large amounts of water to diffuse across its membrane. The plasma membrane allows water molecules to freely diffuse in both directions. Because the rate of water diffusion in each direction is equal, there will be no water loss or gain.
Hypotonic solutions can be iso-osmolar if the solution is able penetrate the cell membrane. Hypotonic solutions can cause red blood cells to lysis by being hypotonic with iso-osmolar levels of urea. This is because urea enters the cell through a gradient in its concentration, then water. Normal saline’s osmolity, 9g NaCl dissolved into water to a volume of 1 liter, is close to that of NaCl found in blood (about 295 mOsm/L). Normal saline is nearly isotonic to plasma. Unlike urea, sodium and chloride ions cannot freely pass through plasma membranes.
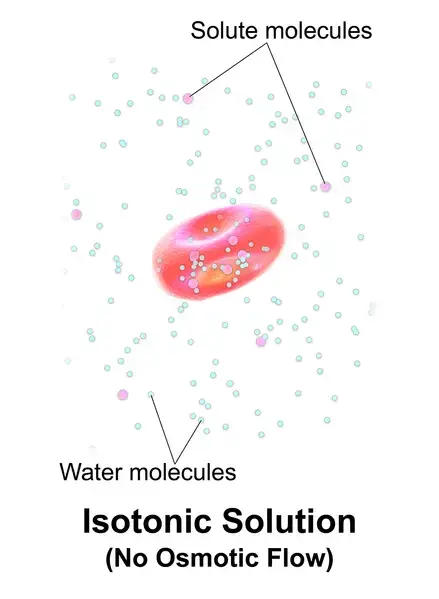
Example: A cell placed in hypertonic solutions (example: seawater) will experience a net flow outside of the cell. This causes the cell to shrink or lose volume.
Tonicity in living systems
A hypertonic solution will cause water to leave a cell and cause it to shrink. An isotonic environment has no net water movement so the cell’s size does not change. Hypotonic environments will cause water to enter cells and cause them to swell.
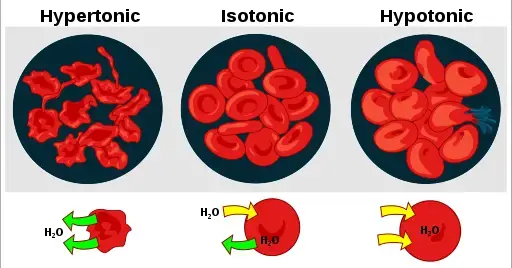
In the case of a red blood cell, isotonic conditions are ideal, and your body has homeostatic (stability-maintaining) systems to ensure these conditions stay constant. A hypotonic solution will cause a red blood cell to bloat and explode. Conversely, a hypertonic environment will cause it to shrivel and make its contents more concentrated and dense.
Hypotonic extracellular solutions are ideal for plant cells. The cell will not burst or lyse because the plasma membrane can only expand up to the limits of its rigid cell wall. The cytoplasm of plants is a bit hypertonic, so water can only enter cells until it reaches its internal pressure, which is called the Turgor pressure.
This balance is vital for the plant’s health. The extracellular fluid can become hypertonic or isotonic, which will cause water to escape the plants’ cells. You may have seen this as wilting. Hypertonic conditions can cause the cell membrane to detach from its wall and constrict the cells, a condition known as plasmolysis (left panel).
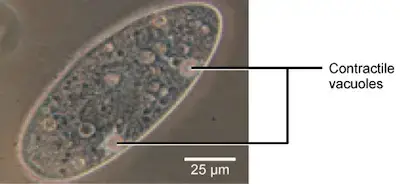
Tonicity is a problem for all living creatures, especially those without rigid cell walls or who live in hypo- or hypertonic environments. Paramecia (pictured below) and amoebas are two examples of protists who lack cell walls. They may have contractile vacuoles, which are specialized structures. Contractile vacuoles collect excess water from cells and pump it out. This prevents the cell from lysing from taking in water from its hypotonic environment.
Calculating Osmotic and Hydrostatic Pressure
Osmosis is the process where water flows across a membrane to react to different concentrations of solutes. It creates a pressure across the membrane that’s called osmotic tension. Hydrostatic pressure is the pressure that stops water from flowing through a membrane. Osmotic pressure can be defined as the hydrostatic pressure needed to stop it. This membrane can be either an artifical bilayer of lipids, a plasma membrane, or a layer made up of cells.
This is how you can approximate the osmotic pressure of a dilute solution P:
P = RT (C1 + C2 + .. + Cn)
where R is the gas constant (0.082 liter-atmosphere/degree-mole), T is the absolute temperature, and C1 … Cn are the molar concentrations of all solutes (ions and molecules).
Similar to the above, the osmotic tension across a membrane that separates two solutions is:
P = RT (ΔC)
DC is the difference between the solute concentrations of the two solutions. If the membrane is permeable only to water, but not to other solutes (the proportionality factor RT) then the osmotic pressure across the membrane will be proportional to the solute concentration difference.
Examples of Tonicity in Living Systems
In Animals
Red blood cells are required to live in an isotonic environment in order to maintain homeostasis. Hypotonic solutions will cause red blood cells to inflate and burst. Contrarily, if the cells are placed in a hypertonic environment, they will shrink and become densely concentrated. It can even lead to cell death.
In Plants
The hypotonic environment provides the ideal conditions for cells to function. The cell membrane (plasma) must expand to the required level in order to function properly without being lysed. Plant cytoplasm is usually slightly hypertonic to extracellular environment. This allows water to enter cells until internal turgor pressure stops further infiltration.
If a plant isn’t watered, its extracellular fluid becomes too acidic, which causes water to escape from the plant cells. This causes wilting by causing a decrease in turgor pressure. The cell membrane can become detached from the cell wall in a hypertonic environment. This causes the cell to contract, which is known as plasmolysis.
Paramecium, an organism that lacks a cell wall and amoebae have specialized structures called contractile vessels which maintain the cell’s osmotic pressure. It pumps out excess water from cells to protect them from lysis.
What is a Hypotonic Drink?
Hypotonic drinks typically have less than 4g sugar (carbohydrates), per 100ml, and low osmotic pressure. This can be used to quench thirst. Hypotonic drinks offer athletes very little energy with a range of sugars. Hypotonic sports drinks are more effective than water for absorbing energy quickly. It is ideal for leisure sports and shorter, more strenuous work.
What is a Hypertonic drink?
Hypertonic drinks typically have more than 8g sugar (carbohydrates), and a higher osmotic pressure that bodily fluids. It is primarily intended to provide energy. It does not provide a thirst-ending result. Hypertonic drinks can be consumed more slowly than water. Ideal to be used thirty to an hour before sports/training/exertion and right away once sports/training/exertion. Hypertonic drinks also are helpful for athletes or staff United Nations agency realize that they have a small amount of additional energy throughout their training/competition/ toil. It is also ideal for long-term, less strenuous activities like driving, learning, and gaming.
What is a Isotonic Drink?
An isotonic beverage typically has between 4g-8g of sugar (carbohydrates), and has the same osmotic pressure and bodily fluids. Associate in Nursing isotonic drinks are as obsessed with the body as water. They are meant to satisfy thirst and provide energy for the body. They are ideal for endurance sports.
Osmosis and tonicity worksheet
References
- https://www.khanacademy.org/science/ap-biology/cell-structure-and-function/mechanisms-of-transport-tonicity-and-osmoregulation/a/osmosis
- https://en.wikipedia.org/wiki/Tonicity#:~:text=Tonicity%20is%20a%20measure%20of,a%20partially%20permeable%20cell%20membrane.
- https://biologydictionary.net/tonicity/
- https://www.biologyonline.com/dictionary/tonicity
- https://www.thoughtco.com/osmotic-pressure-and-tonicity-3975927
- https://www.thoughtco.com/hypertonic-definition-and-examples-605232
- http://www.vivo.colostate.edu/hbooks/pathphys/topics/osmosis.html
- https://www.wikidoc.org/index.php/Tonicity
- https://www.slideshare.net/EmmaAtman/biology-osmosis-and-tonicity-66134486
- https://ibiologia.com/tonicity/
- https://www.sciencefacts.net/tonicity.html
- https://www.thoughtco.com/osmotic-pressure-and-tonicity-3975927
- https://en.wiktionary.org/wiki/tonicity