What is Chromatography?
- Essential for scientific study, chromatography is used extensively in the separation, identification, and purification of mixture components. This method is fundamental in many different scientific disciplines and helps to evaluate complicated mixes both qualistically and quantitatively.
- Chromatography is fundamentally based on its many techniques, each designed to take use of unique properties like molecule size, binding affinities, and electrical charge among others. This adaptability makes chromatography a vital instrument in many different scientific fields, usually the only practical way to separate complicated mixtures into their component parts.
- Two fundamental components—the stationary phase and the mobile phase—are crucial to the chromatographic process. Usually a liquid or gas, the mobile phase moves the mixture across the stationary phase—usually a solid or viscous liquid immobilised on a solid support. Differential migration rates resulting from the interactions between the mixture components and the phases thereby facilitates the separation. Components with less affinity to the stationary phase migrate more quickly as the mobile phase moves, while those with more affection remain and thus separate the components of the mixture according on their inherent characteristics.
- All things considered, chromatography is a pillar method in scientific research as its wide application and capacity to discriminate depending on a spectrum of molecular properties support developments in many different fields of science.
Definition of Chromatography
Chromatography is a laboratory technique for the separation of a mixture by passing it in a solution or suspension or as a vapor (as in gas chromatography) through a medium in which the components move at different rates.
How does Chromatography work?
- Chromatography moves components of a mixture through two phases—a stationary phase and a mobile phase—based on the idea of separation of these components by differential migration. Whereas the stationary phase is a solid or liquid supported on a solid that remains motionless, the mobile phase consists of a fluid carrying the mixture to be separated on.
- Introduced into the system, the mixture moves over the surface of the stationary phase in the mobile phase. Variations in polarity, size, or affinity lead each component in the mixture to interact differently with the stationary phase, therefore separating them. While certain components interact less and move more rapidly with the mobile phase, others may attach tightly to the stationary phase and travel slowly.
- The components split from one another as they move at various speeds, allowing their unique identification and study. Chromatography is a flexible instrument for chemical analysis and purification as the particular conditions and materials used for the stationary and mobile phases may be changed to maximize the separation process for many types of combinations.
Applications Of Chromatography
Because chromatography can separate, identify, and measure components within a mixture, it is a flexible analytical method with many uses in many different domains. among the main uses are:
- Chromatography has great application in the pharmaceutical industry for medication purification, quality control, and component analysis. It guarantees the chemical purity of active medicinal components and facilitates the formulation chemical composition analysis.
- Environmental testing is used in environmental samples—including soil, air, and water—to find and measure contaminants. By means of trace level identification of pollutants including toxins, heavy metals, and pesticides, chromatography greatly facilitates environmental monitoring and cleaning projects.
- Food and beverage analysis uses chromatography to help find additives, preservatives, and poisons such aflatoxins or pesticides. By means of flavor compound, vitamin analysis, and nutritional component analysis, it guarantees the quality and authenticity of food items as well.
- Scientific research spanning the disciplines of chemistry, biology, and materials science depends critically on chromatography. Analyzing complicated combinations, researching reaction mechanisms, and developing novel materials or chemicals all find utility here.
- Chromatography is used in forensic science to examine narcotics, poisons, and explosive remnants among other elements discovered at crime sites. In the examination of criminal cases, it is essential as it helps to connect evidence to suspects.
- Chromatography is applied in the examination of biological samples like blood and urine for the presence of medicines, hormones, and other biomolecules in clinical and medical research. It backs biological research, therapeutic medication monitoring, and diagnostics.
- In the petrochemical and energy industry, chromatography is applied to examine crude oil and its fractions in order to ascertain their composition and track operations. Furthermore crucial in the evolution of biofuels and in the quality monitoring of petrol and energy products is this.
What are Mobile phase and stationary phase?
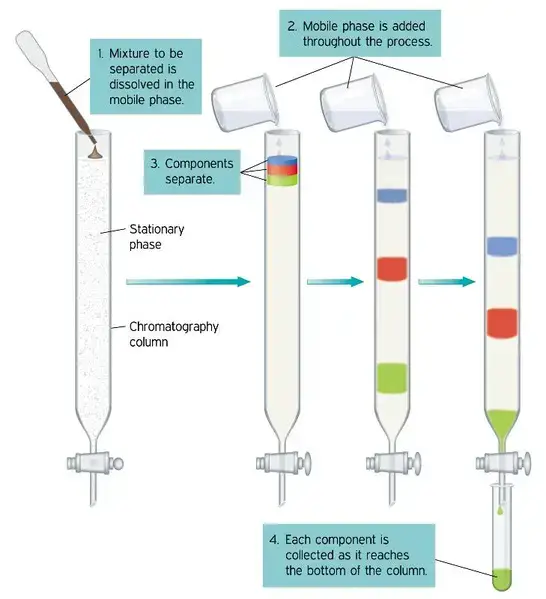
- Chromatography is the method of separating mixture components by use of the interaction between two separate phases: the stationary phase and the mobile phase. Comprehending the principles of chromatographic methods depends on an awareness of these two components.
- A fundamental part of the chromatographic process, the stationary phase is distinguished by its permanent place in the system. It can be made of either solid particles or a liquid layer covered over a solid support, like metal or glass. The selective adsorption or interaction of the components of the mixture finds a medium in this phase. The particular properties of the combination under analysis as well as the chromatographic technique used affect the stationary phase material used. Common stationary phase materials are silica gel, alumina, or even specialty gels and sheets made for this use. These materials’ porous character is rather important as it gives the components of the mixture a surface on which to interact and thereby helps the separation process.
- Conversely, the mobile phase in chromatography is the dynamic component meant to move the mixture over the stationary phase. Depending on the used chromatographic method, it could be a liquid or a gas. Acting as a carrier, this phase drives the mixture along the stationary phase thereby facilitating differential component movement. The kind of combination and the separation criteria define the mobile phase option; popular possibilities for liquid chromatography include solvents like water, alcohol, and acetone; for gas chromatography, gases like helium or nitrogen.
- The chromatographic separation process depends fundamentally on the interaction of the mobile and stationary phases. The components of the mixture are carried at different rates when the mobile phase passes either past or over the stationary phase. These variations in migration rates arise from different degrees of contact between every component and the stationary phase, therefore producing the effective separation and analysis of the components of the mixture.
Types of Chromatography
Chromatography refers to a variety of processes used to separate chemicals from a mixture. The interplay of the mobile and stationary phases applied in the process distinguishes these techniques mostly.
- Affinity Chromatography: Using the particular interactions between a molecule and a partner it binds with, such an enzyme and its substrate or an antibody and its antigen, affinity chromatography The stationary phase features a ligand from which particular mixture molecules will bind. Unbound compounds are eliminated, and under particular conditions the bonded molecules are later released.
- Ion Exchange Chromatography: Separating ions and polar compounds depending on their charge is especially benefited by ion exchange chromatography. It binds molecules with opposing charge by use of a charged stationary phase. One can eluate the bound components by changing the pH or ionic strength of the mobile phase.
- Size Exclusion Chromatography (SEC): Separating molecules depending on their size and form, Size Exclusion Chromatography (SEC), often referred to as Gel Filtration Chromatography, This technique employs a porous stationary phase; although bigger molecules avoid the pores and elute more quickly, smaller molecules enter the pores and transit across the column more slowly.
- Partition Chromatography: Differential partitioning between the mobile and stationary phases bases separation in partitioning chromatography. Comprising a liquid coating a solid support, the stationary phase causes chemicals in the mixture to disperse themselves between the two phases based on their solubility.
- Adsorption Chromatography (or Normal Phase Chromatography): Usually including a solid substance on which the sample components adsorb, adsorption chromatography—also known as normal phase chromatography—is Polarity of the analyte determines the degree of adsorption either physically or chemically. Analytes from the column are extracted using nonpolar solvues.
- Reverse Phase Chromatography (RPC): Reverse phase chromatography, or RPC, is practically the reverse of conventional phase chromatography. Polar chemicals elute early; nonpolar compounds have a stronger contact and elute later; hence, the stationary phase is nonpolar.
- Gas Chromatography (GC): Volatile substances are handled in gas chromatography (GC). Usually either helium, nitrogen, or hydrogen, the mobile phase is a carrier gas; the stationary phase is a high-boiling liquid covered inside a column. Boiling temperatures and affinity for the stationary phase help to determine separation.
- High-Performance Liquid Chromatography (HPLC): Often used extensively in biochemistry and analytical chemistry to separate, identify, and measure chemicals, high-performance liquid chromatography (HPLC) is an enhanced variation of column chromatography. By pushing the solvent over the column using high pressure, HPLC makes advantage of smaller particle size for the stationary phase, hence increasing surface area for interactions between the stationary phase and molecules in the sample.
- Thin-Layer Chromatography (TLC): TLC, or thin-layer chromatography, is the technique wherein a stationary phase is immobilized on a glass or plastic plate as a thin layer. Developed by capillary action moving a solvent front up the plate, the sample is spotted close to the base and causes the compounds to migrate at varying speeds.
- Paper Chromatography: Using a strip of paper as the stationary phase, this technique known as paper chromatography moves the mobile phase via capillary action. Different speeds of movement of the components in the mixture cause their separation.
- Hydrophobic Interaction Chromatography (HIC): Hydrophobic Interaction Chromatography (HIC) sorts molecules according to hydrophobicity. Usually covered with hydrophobic groups, the stationary phase is kept in place by high salt concentrations to induce hydrophobic interactions. Greater hydrophobic surfaces help molecules stick more firmly and elute later when the salt concentration is progressively lowered.
- Hydrophilic Interaction Liquid Chromatography (HILIC): Particularly helpful for polar component separation, hydrophilic interaction liquid chromatography (HILIC) is a variation on normal phase chromatography. Encouraging polar chemicals to interact with the stationary phase and be maintained longer, the stationary phase in HILIC is polar while the mobile phase is a combination of water and a less polar solvent.
- Chiral Chromatography: Enantiomers—miracle pictures of each other—can be distinguished using chiral chromatography, a type of chromatography whose identical physical and chemical characteristics in a symmetrical environment cannot be differentiated by standard techniques. A chiral stationary phase interacting differently with the enantiomers results in their separation in chiral chromatography.
- Supercritical Fluid Chromatography (SFC): SFC, or supercritical fluid chromatography, employs a material at a temperature and pressure above its critical point—where it exhibits characteristics of both gases and liquids—as the mobile phase. Effective for a broad spectrum of chemicals, particularly those that are thermally labile or have a large molecular weight, this approach combines the high diffusion rates of gas chromatography with the great solvating capacity of liquid chromatography.
1. Gas chromatography
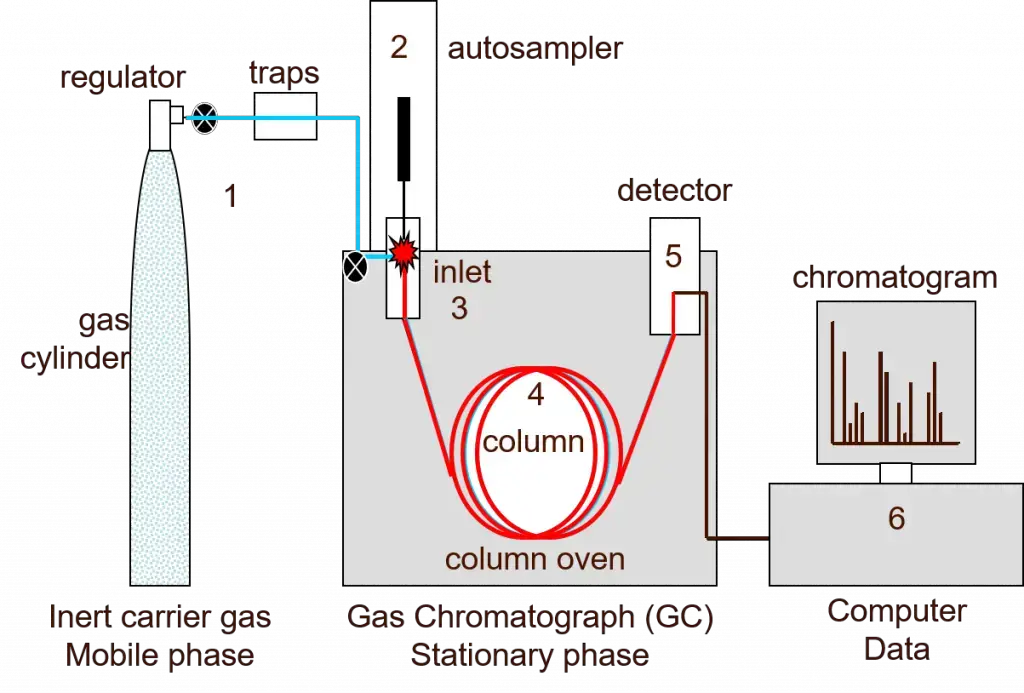
Separating and analyzing chemicals depending on their volatility and the variations in their interactions with a stationary phase is the analytical method known as gas chromatography. with gas chromatography, a little sample is injected and vaporized then transported by an inert gas across a long, thin column covered with a liquid stationary phase. Gas chromatography’s basic idea is that each chemical travels down the column at a distinct pace by use of differential partitioning of the sample components between the mobile gas phase and the stationary liquid phase. A detector notes the separated compounds as they depart the column at different retention durations, producing a chromatogram whereby every peak denotes a unique component. By matching retention periods and peak regions, this technique allows both qualitative identification and quantitative study of difficult combinations.
Steps
- To make sure the analytes are in the right form for vaporization, dissolve or dilute the sample in an appropriate solvent.
- Using a syringe or an autosampler, precisely measure the sample and inject it into the heated injection port.
- Quickly vaporize the sample in the injection port so that it creates a gaseous mixture entering the carrier gas stream.
- Using an inert carrier gas, such helium or nitrogen, move the vaporized sample into the chromatographic column.
- Sort the sample components inside the column according to their interactions with the stationary phase covering the inner column walls.
- Depending on their affinity to the stationary phase, let each component elute from the column at different retention durations.
- Use an appropriate detector to identify the separated chemicals as they leave the column by turning the chemical signal into an electrical one.
- Record the detector response to generate a chromatogram whereby every peak represents a particular component in the sample.
- Compare retention times and peak regions in the chromatogram to find and measure each individual chemical in the sample.
Uses
- In chemical and petrochemical sectors, gas chromatography is used to monitor the composition of complicated combinations and confirm the purity of compounds.
- Environmental monitoring uses it to find and measure toxins in soil, water, and air.
- In arson investigations and for trace level drug analysis, forensic labs use gas chromatography to examine evidence including accelerants.
- By use of gas chromatography, the pharmaceutical sector guarantees quality control by means of active component concentration and composition determination.
- For quality control, food and beverage companies use gas chromatography to evaluate tastes, perfumes, and pollutants.
- For diagnostic and safety testing, clinical and toxicology labs employ the method to identify trace chemicals in biological samples.
- Gas chromatography is used in research laboratories across many scientific disciplines to investigate reaction kinetics, metabolic profiles, and the detection of volatile substances.
2. Affinity chromatography
- Affinity chromatographic is a liquid chromatographic method based on certain binding interactions that separates and purifies biomolecules.
- It exploits an immobilized ligand that binds a target molecule from a complex mixture specifically.
- The approach depends on reversible interactions, including those between an antibody and its antigen or an enzyme and its substrate.
- While the target molecule is attached to the stationary phase, non-target components wash away.
- Changing the circumstances to disturb the binding relationship then helps the target biomolecule to elute.
- Protein purification, drug development, and molecular interactions research all make extensive use of this method.
Steps
- Wash the affinity column with a binding buffer to balance the circumstances for the particular interaction between the immobilized ligand and the target molecule.
- Load the sample onto the column so that under ideal conditions the target molecule has the chance to attach to the immobilized ligand.
- Let an incubation period allow the target biomolecule to attach firmly to the ligand while non-specific components pass through
- Remove any unbound or lightly bound contaminants by washing the column with the binding buffer.
- Change the buffer conditions, for instance by introducing a competing ligand or adjusting the pH or ionic strength, therefore eluting the bound target molecule.
- Gather the eluted fractions including the pure target molecule.
- Eliminating any remaining material and re-equilibrating the column with the binding buffer will help it to regenerate for further purification runs.
Uses
- Target biomolecules like proteins, antibodies, and enzymes from complicated mixtures are specifically purified using affinity chromatography.
- It is often used to separate recombinant proteins created with affinity tags like GST or His-tag therefore facilitating effective purification.
- The technique is used in immunoaffinity chromatography to immobilize ligands including Protein A, Protein G, or certain antigens, therefore capturing antibodies or antigens.
- It is employed in lectin affinity chromatography to concentrate glycoproteins according to their particular carbohydrate moieties.
- Chemoproteomics and drug development by fishing for protein–ligand interactions from complicated cell lysates depend much on affinity chromatography.
- Pull-down tests and co-immunoprecipitation studies using the method examine protein–protein interactions and signaling networks.
- It also helps to deplete plentiful contaminating proteins from samples, therefore enhancing the detection of low-abundance targets.

3. Gel filtration chromatography/ Gel permeation chromatography/ Size exclusion chromatography/ Molecular sieve chromatography
- Gel filtration chromatography, often referred to as size exclusion chromatography, gel permeation chromatography, or molecular sieve chromatography, is a method of molecular size and shape based separation of molecules.
- Smaller molecules enter the pores and follow a longer, more convoluted path whereas bigger molecules are rejected and elute quickly in the process using a column filled with porous beads as the stationary phase.
- The method is mild and preserves the natural structure of the analytes as the separation depends just on the physical dimensions of the molecules instead of chemical interactions.
- In biochemistry, gel filtration chromatography is extensively utilized for desalting, buffer exchange, and molecular weight distribution analysis in addition to for protein, polysaccharide, and other macromolecule purification.
- Because of its great resolution, simplicity, and capacity to handle samples in moderate circumstances, it is a necessary instrument in both research and industry environments.
Steps
- Fill the column with a gel filtration resin, such Sephadex or Superdex, therefore guaranteeing consistent bead distribution.
- Run the column with a running buffer suitable for your sample and intended conditions.
- To keep great resolution during separation, load a tiny volume of the sample on top of the column.
- By running the same buffer continually down the column at a regulated flow rate, elute the sample allowing smaller molecules to penetrate the resin pores and bigger molecules to avoid them.
- As the individual components come out of the column, gather the eluate in fractions; bigger molecules elute first and smaller ones later.
- Track the fractions with a detector—for instance, UV absorbance—to find which ones contain your target molecule.
- Run standard molecules of known molecular weight to link elution volumes with molecular sizes, hence calibrating the column.
- Combine the fractions including the targeted pure molecule and, if necessary, concentrate or further treat them.
- To eliminate any last traces of the sample and renew it for next usage, lastly wash the column with buffer.
Uses
- Based on size, proteins and other major macromolecules are filtered from complicated mixtures using gel filtration chromatography.
- Desalting and buffer exchange make frequent use of it as it removes tiny molecules and salts from biological samples.
- The method is used to ascertain protein and polymeric oligomeric state as well as molecular weight distribution.
- Separating monomeric proteins from aggregates, it is a polishing stage in protein purification.
- The polymer business makes extensive use of gel permeation chromatography to examine synthetic polymer molecular weight and distribution.
- Purification of polysaccharides, nucleic acids, and other macromolecules use size exclusion chromatography.
- Research and industry quality control applications of the technique evaluate macromolecule homogeneity and conformational state.
- It offers a mild separation mechanism preserving the natural structure and activity of the target compounds.
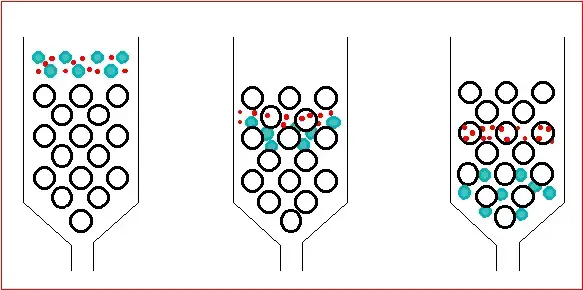
4. Ion exchange chromatography
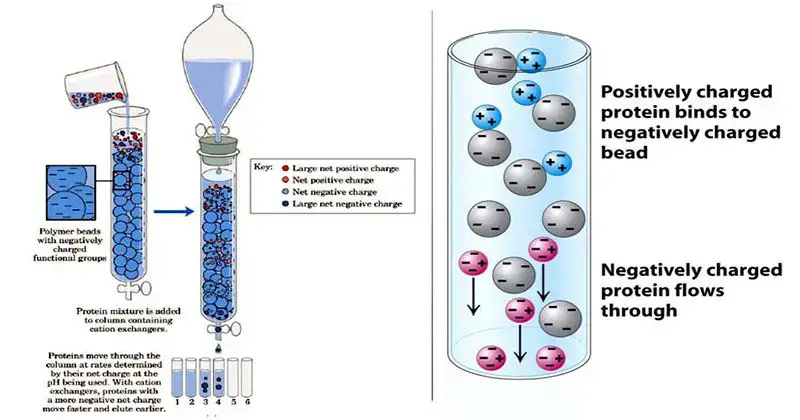
- A method of separating molecules depending on variations in their net charge and distribution of charged groups is ion exchange chromatography.
- It makes use of a stationary phase functionalized with charged groups either positively charged for anion exchange or negatively charged for cation exchange.
- While molecules with same charges move through the column, molecules with opposing charges to the stationary phase bond to the resin.
- Rising the salt content or changing the pH of the mobile phase helps to break the ionic connections, thereby progressively eluting bound molecules.
- Purifying proteins, nucleic acids, and other macromolecules as well as for analytical objectives like evaluating protein isoforms and charge heterogeneity, ion exchange chromatography is extensively applied.
- Its great resolution, scalability, and capacity to distinguish closely related compounds make it a necessary instrument in industrial and scientific domains.
Steps
- Choose a suitable ion exchange resin—cation or anion—based on the net charge of the target molecule.
- For best binding to the resin, balance the column with a binding buffer at a pH that supports the intended charge on the target molecule.
- Load the clean sample—such as a cell lysate or protein solution—onto the equilibrated column such that the target molecules attach to the resin.
- Retaining the target molecule, wash the column with the binding buffer to eliminate unattached and loosely bound contaminants.
- Gradually raise the ionic strength (by adding salt) or change the pH to disturb the ionic connections, hence eluting the bound target molecule.
- Gather the eluted fractions and track them usually using absorbance to find those including the target molecule.
- Use SDS-PAGE and other methods to verify the target protein’s molecular weight and purity from the gathered fractions.
- Wash the column using a high-salt buffer or other regeneration solution, then re-equilibrate with the binding buffer for further runs.
Uses
- By separating proteins depending on net charge, ion exchange chromatography is extensively applied in protein purification.
- It is routinely used to separate other biomolecules, enzymes, and antibodies from complicated mixtures including cell lysates.
- Protein isoforms and charge variability in purified samples are investigated using this method.
- It is used for the general charge properties-based purification and separation of nucleic acids and peptides.
- Biopharmaceutical production for quality control and therapeutic protein purification depends critically on ion exchange chromatography.
- In desalting and buffer exchange systems, it helps eliminate tiny charged particles from macromolecular samples.
- Environmental and clinical laboratories also use the technique to monitor and describe charged molecules in different sample systems.
5. Anion exchange chromatography
- Anion exchange chromatography is a particular kind of ion exchange chromatography whereby a positively charged stationary phase preferentially binds negatively charged molecules.
- Usually functionalized with quaternary ammonium groups, which provide a steady positive charge across a broad pH range and guarantee efficient binding of anions, the resin is
- Under appropriate buffer conditions, a sample including negatively charged biomolecules loads into the column and these anionic species bond to the resin while neutral or positively charged molecules pass through.
- Then, either changing the pH or progressively raising the salt content elutes the bound molecules, therefore upsetting the ionic connections and enabling the collecting of the negatively charged molecules.
- Widely utilized for purifying proteins, nucleic acids, and other biomolecules displaying a net negative charge, this method is a fundamental tool in both academic and industrial uses.
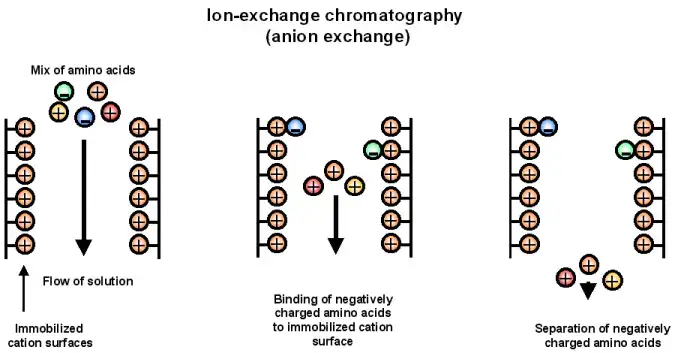
Steps
- Choose an anion exchange resin whose positively charged functional groups—such quaternary ammonium groups—will bind negatively charged molecules.
- Equilibrate the column using a low-salt binding buffer at a pH where your intended biomolecules have a net negative charge.
- Under these binding circumstances, load your cleared sample onto the column such that the negatively charged molecules bond to the resin.
- Retaining the bound target molecules, wash the column with the binding buffer to eliminate unbound proteins and other impurities.
- Either progressively raise the salt content or change the pH to disturb the ionic connections between the resin and the target molecules, hence eluting the bound molecules.
- While tracking absorbance (or another suitable detector), gather the eluted fractions to find the ones including the target biomolecule.
- Use spectrophotometry or SDS-PAGE to evaluate the purity and identification of the eluted proteins by analyzing the gathered fractions.
- Wash the column with a high-salt solution or another regeneration buffer, then re-equilibrate using the binding buffer for further runs.
Uses
- Negative charged biomolecules including proteins, peptides, and nucleic acids EN.WIKIPEDIA.ORG are purified and examined via anion exchange chromatography.
- It is extensively used in biopharmaceutical production to separate recombinant proteins and monoclonal antibodies displaying net negative charges under regulated pH settings EN.WIKIPEDIA.ORG.
- The method is applied to evaluate charge heterogeneity and resolve protein isoforms, therefore supporting quality control and therapeutic protein characterisation EN.WIKIPEDIA.ORG.
- Small ionic impurities from macromolecular samples EN.WIKIPEDIA.ORG are removed for desalting and buffer exchange applications.
- Environmental and clinical studies also employ anion exchange chromatography to separate and measure anionic molecules including organic acids and other charged metabolites.
6. Cation exchange chromatography
- Cation exchange chromatography is a variation of ion exchange chromatography in which molecules are separated according to net positive charge.
- It attracts and bonds positively charged (cationic) biomolecules using a negatively charged stationary phase usually resin beads functionalized with acidic groups.
- Whereas negatively charged or neutral molecules flow through the column, molecules with a net positive charge interact with the resin and are held under suitable buffer conditions.
- Increasing the ionic strength (typically by adding salt) or changing the pH then elutes the bound cationic species, therefore upsetting the ionic connections between the molecules and the stationary phase.
- Protein purification makes extensive use of cation exchange chromatography to separate proteins depending on variations in their surface charge, hence resolving charge variants and isoforms.
Steps
- Select a cation exchange resin including carboxyl or sulfonate groups, having negatively charged functional groups.
- Pack the column with the chosen resin and equilibrate it under a low ionic strength binding solution at a pH where your target proteins carry a net positive charge.
- Under these binding circumstances, load the cleared sample onto the column such that non-binding components pass through while the positively charged molecules bond to the resin.
- Using the same binding buffer, wash the column to eliminate any loosely bound or unbound contaminants.
- Gradually raise the salt content or change the buffer’s pH to elute the bound proteins and disturb the ionic connections between the proteins and the resin
- Gather the eluted fractions under observation of absorbance or another suitable detection technique to find fractions including the target proteins.
- Verify the purity and molecular weight of the eluted proteins by analyzing the gathered fractions with methods like SDS-PAGE.
- Wash the column using a high-salt solution or a regeneration buffer; then, re-equilibrate using the binding buffer for further runs.
Uses
- Useful for separating enzymes and other biomolecules from complicated mixtures, cation exchange chromatography is extensively applied for purifying proteins carrying a net positive charge at a specific pH.
- By guaranteeing the quality of therapeutic proteins and resolving protein charge variations, it is crucial in biopharmaceutical production.
- The method is used to differentiate isoforms and post-translational changes of proteins, therefore helping to define protein structure and function.
- Downstream processing also uses cation exchange chromatography to concentrate target proteins and eliminate tiny charged impurities and pollutants including salts.
- In research environments, it is applied for analytical investigations of protein–protein interactions and protein purity evaluation by means of closely related cationic species’ separation.
- The technique also finds and quantifies positively charged biomolecules in biological samples in clinical and diagnostic laboratories.
7. Paper Chromatography
- A form of partition chromatography, paper chromatography employs a solvent moved by capillary action as the mobile phase and paper as the stationary phase.
- A tiny area of the sample is placed close to one end of the paper; as the solvent rises, the components of the mixture move at varying rates depending on their relative solubility in the solvent against their affinity for the paper.
- Larger or less soluble molecules tend to move more slowly, whereas smaller or more soluble molecules migrate farther up the paper, so separating happens.
- Following the run, separate areas show up on the paper that match various components of the original mixture; these may then be seen with suitable detection techniques.
- Commonly employed for the qualitative investigation of dyes, pigments, amino acids, and tiny organic compounds, this method is preferred for its simplicity and inexpensive cost in educational and research facilities.
Steps
- About 1–2 cm from the bottom border, draw a light pencil line (baseline) on a strip of chromatography paper.
- Using a capillary tube or micropipette, apply a tiny, focused area of the sample solution to the baseline and let it dry.
- To guarantee the solvent level is below the baseline, place the paper in a sealed developing chamber with a tiny volume of the selected solvent (mobile phase).
- Allow the solvent to ascend by capillary action up the paper until it approaches the top, transporting the components of the sample at varied speeds.
- Take the paper out of the chamber and pencil instantly indicate the solvent front.
- After the paper dries totally, use the suitable detection technique (like UV light or iodine staining) to see the separated spots.
- To find their Rf values for additional study, measure the lengths each spot and the solvent front have travelled.
Uses
- A cheap, basic technique used extensively to separate and examine minute amounts of compounds in mixtures is paper chromatography.
- In educational environments, it functions as a teaching tool illustrating ideas of separation and chromatography.
- Food companies use it to examine food pigments and guarantee food items’ authenticity and purity.
- In criminal investigations, forensic science detects and identifies inks, dyes, and other trace chemicals using paper chromatography.
- Environmental analysis makes use of this method to track toxins and pollutants in samples of air, soil, and water.
- In biochemistry, paper chromatography is applied for the qualitative study of amino acids, carbohydrates, and tiny chemical compounds.
- By matching Rf values and viewing distinct spots with different detection reagents, it helps in first identification of substances.
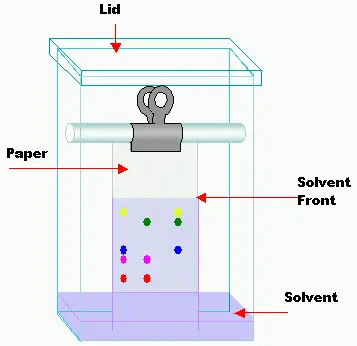
8. Flash chromatography
- Usually silica gel, flash chromatography is a preparative variant of column chromatography in which the mobile phase is forced through a column packed with a stationary phase under compressed gas.
- W. Clark brought it forward. still in the late 1970s, greatly lowering separation time relative to conventional gravity-driven column chromatography
- The method works by exerting positive pressure, which speeds up solvent flow and enhances compound separation from reaction mixtures’ resolution and efficiency.
- Organic synthesis and pharmaceutical research make extensive use of flash chromatography for the quick preparation of crude mixtures and reaction products on a preparative level.
- Its benefits include faster run times, less solvent use, and automation of the process, which makes it a useful tool in industrial uses as well as research facilities.
Steps
- Usually silica gel, load the flash chromatography column with a suitable stationary phase using a slurry technique to guarantee homogeneous packing.
- Establish the intended separation conditions by pre-equilibrating the packed column with the selected mobile phase or solvent solution.
- Form a concentrated solution by dissolving the crude sample in a minimum amount of solvent suitable for the mobile phase.
- Load the sample solution gently onto the top of the column without upsetting the stationary phase.
- Force the mobile phase across the column at a predetermined flow rate by applying positive pressure—using compressed air or a pump.
- Run the mobile phase—or a gradient of solvents—through the column to elute the mixture thereby separating distinct compounds according on their affinities to the stationary phase.
- As the eluent leaves the column, gather it in fractions; bigger compounds usually elute before smaller ones.
- Using thin-layer chromatography or another analytical technique, examine the obtained fractions to find the ones including the target molecule.
- Combine the fractions with the intended product and eliminate the solvent—often by evaporation—to get the pure chemical.
- Re-equilibrate the column with the mobile phase for further purification runs after washing and rejuvenating it with suitable cleaning solutions.
Uses
- Organic synthesis makes extensive use of flash chromatography to quickly separate desired products from reaction mixtures.
- Medical chemistry and pharmacological research use it to effectively separate bioactive molecules and medication prospects.
- Natural product isolation is used here to separate intricate combinations from plant extracts and other natural sources.
- Industrial settings use flash chromatography for scale-up and process development in order to manage quality and maximize purification techniques.
- In research labs, its rapid run times, low solvent use, and simplicity of automation make it a useful preparative tool.
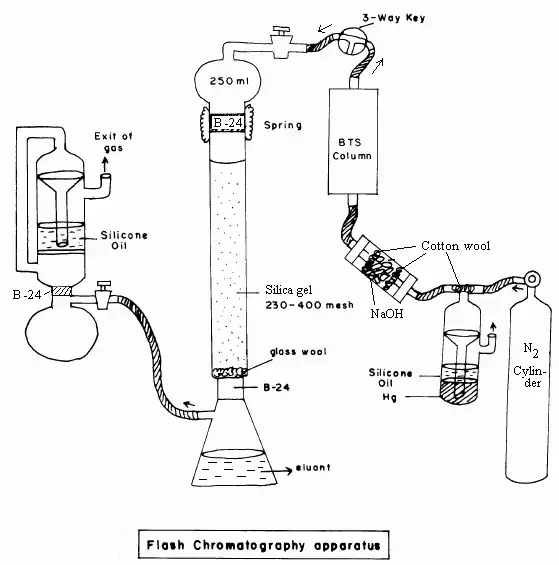
9. Liquid chromatography
- Liquid chromatography is a method of separating the components of a mixture depending on their differential interactions using a liquid mobile phase and a solid or liquid stationary phase.
- The basis of the idea is the separation of analytes between the stationary phase and the mobile phase; components with weaker connections elute faster while those with more strongly interacting contacts are maintained longer.
- Each chemical moves at a different pace while the mobile phase is constantly cycled through the column under regulated circumstances, therefore separating them and enabling later identification and analysis.
- A flexible method for both analytical and preparative use, the process may be modified by changing elements like the composition and flow rate of the mobile phase, the type of the stationary phase, and the temperature.
- This basic idea of differential partitioning across phases guides several forms of liquid chromatography (e.g., reversed-phase, normal-phase, ion exchange, and size exclusion).
Steps
- Choose and set up a chromatography column loaded with the suitable stationary phase, like reversed-phase C18, therefore guaranteeing consistent packing for repeated separations.
- Flushing the column with a mobile phase that controls the target analytes’ ideal pH, polarity, and ionic strength will help to equilibrium it.
- Filter and degas the mobile phase to eliminate dissolved gases and particulate particles that can compromise flow or influence detection.
- Using an autosampler or manual injection, dissolve the sample in a solvent compatible with the mobile phase then inject a precise volume into the column.
- Under regulated pressure and flow rate, let the mobile phase pass through the column carrying the sample over the stationary phase where differential interactions induce separation.
- Discrete peaks arise from the analytes separating and eluting at different retention durations as they vary in interaction with the stationary phase.
- With an appropriate detector—such as a UV-Vis, fluorescence, or mass spectrometer—detect the eluting components and record the signals to generate a chromatogram.
- Using recognized criteria, compare retention durations and peak regions in the chromatogram to find and measure the separated chemicals.
- To get the column ready for further runs, lastly wash it with a strong solvent and re-equilibrate using the mobile phase. Remove any remaining sample.
Uses
- In pharmaceutical research, liquid chromatography is extensively applied in development, analysis, and quality control of medication formulations.
- Clinical labs use it to find and measure biomarkers, metabolites, and other analytes in biological fluids.
- Monitoring pollutants, pesticides, and organic contaminants in water, soil, and air helps the method to be very important in environmental analysis.
- Food and beverage companies use liquid chromatography to evaluate additives, preservatives, and pollutants thereby guaranteeing product safety.
- Separating and identifying proteins, peptides, and tiny metabolites is fundamental in proteomics and metabolomics research.
- In forensic science, the technique is applied to examine complicated mixes in criminal investigations—such as those containing narcotics or poisons.
- In chemical research and industrial environments for the purification and analysis of complicated mixtures, liquid chromatography is absolutely vital.
- Found in basic form in hyphenated methods such as LC-MS, which provide great sensitivity and specificity for chemical identification, it is
- Polymers, vitamins, and other tiny molecules as well as other techniques are applied for separation and analysis.
- In biotechnology, liquid chromatography is used to purify proteins, nucleic acids, and other biomolecules from complicated materials.
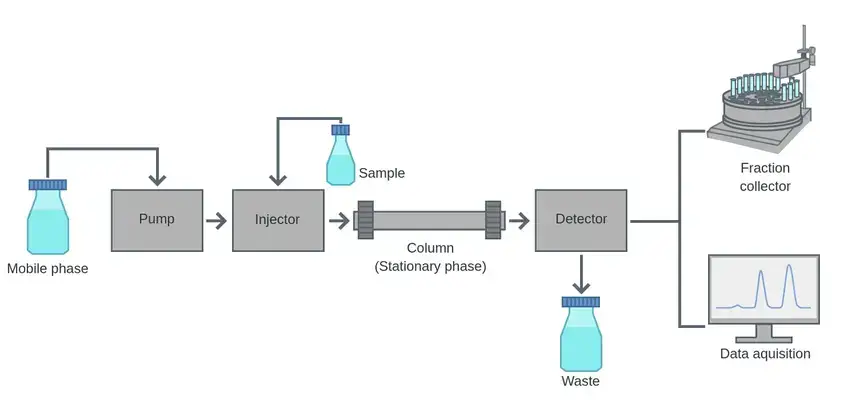
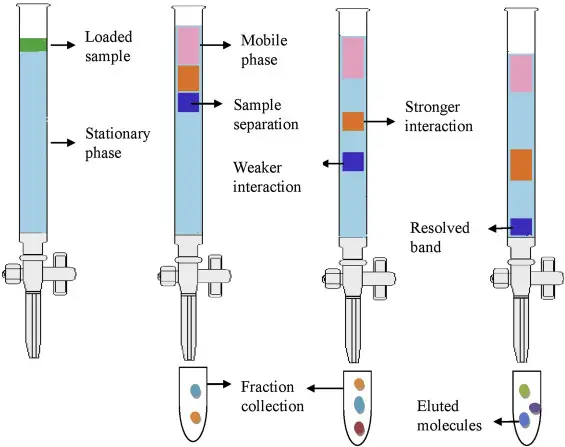
10. Column chromatography
- By running a mixture through a tube (column) filled with a solid stationary phase, column chromatography separates its components.
- Its basis is the differential affinity of the stationary phase’s sample components for the mobile phase versus one another.
- Compounds that interact strongly with the stationary phase are held longer while the mobile phase passes through the column; those with less interactions go quicker.
- This differential retention lets the components elute at various periods so that they may be separated, identified, and isolated.
Steps
- Given the characteristics of the combination, choose an appropriate column and stationary phase (like silica gel or alumina).
- To prevent channeling, slurry the stationary phase in a suitable solvent and evenly load it into the column.
- Let the column settle then gently tap or use a mild vacuum to release any trapped air.
- Flushing the column with the selected mobile phase will help to equip it so that a stable baseline is obtained.
- Dissolve the sample in a minimum volume of a solvent appropriate for the mobile phase.
- Load the sample gently onto the top of the column such that the packed stationary phase is not disturbed.
- Using either an isocratic or gradient elution as required, pass the mobile phase over the column at a regulated flow rate to elute the sample.
- Gather the eluent in fractions since various components elute at varied retention durations.
- Use techniques include UV detection or thin-layer chromatography to examine the fractions and find those with the target molecule.
- If required, mix and focus the fractions including the desired component.
- Wash the column with a strong solvent to regenerate it; then, re-equilibrate using the mobile phase for further runs.
Uses
- In organic synthesis, column chromatography is extensively applied to extract reaction products from crude mixtures.
- It is used in natural product research to separate from plant extracts and other complicated sources bioactive molecules.
- Pharmaceutical development uses the method for active ingredient quality monitoring and purification.
- Analytical chemistry uses it to separate and examine elements in complicated mixtures for identification.
- Environmental analysis makes use of column chromatography to identify and count pollutants and contaminants.
- It is a prelude for increasing the isolation of pure chemicals in industrial operations.
- Biochemical research also uses the approach for the separation and purification of biomolecules like proteins and peptides.
- Consistentity and purity of chemical and biological goods depend on this indispensable instrument in quality control laboratories.
11. High-performance liquid chromatography (HPLC)
- By use of a liquid mobile phase forced through a column packed with a stationary phase under high pressure, high-performance liquid chromatography (HPLC) is a sophisticated analytical method used to separate, identify, and quantify components in a mixture.
- The differential partitioning of analytes between the mobile phase and the stationary phase forms the basis of HPLC; molecules that interact more strongly with the stationary phase are kept longer, whilst those with less interaction elute more rapidly.
- Faster flow rates and more defined, sharp peaks made possible by high pressure help to improve the separation process’s sensitivity and resolution.
- HPLC may be tuned for the separation of a broad spectrum of chemical and biological components by varying parameters like the mobile phase composition, flow rate, and temperature.
- Because this method offers exact, repeatable, and high-resolution separations, it is extensively applied in environmental analysis, biochemical research, and pharmacological development.
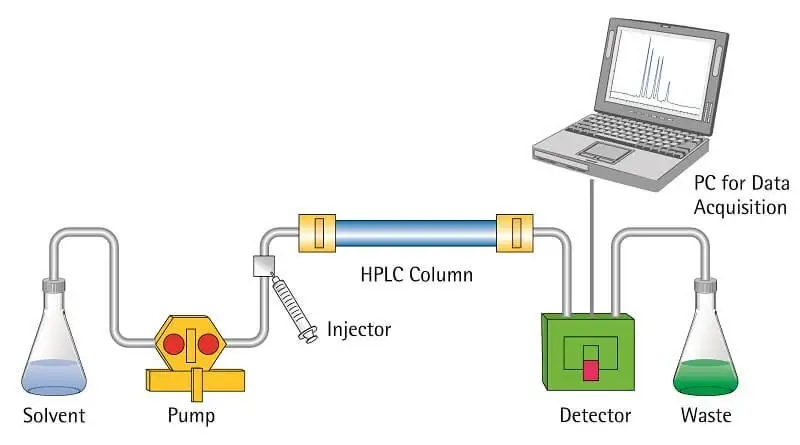
Steps
- First get the mobile phase ready by combining the suitable solvents; next, filter and degassing them to eliminate dissolved gases and particles.
- Mount the HPLC column in the instrument, then equilibrate it with the mobile phase within the specified flow rate and temperature range.
- Dissolve your sample in a solvent suitable for the mobile phase; filter it to remove any insoluble components likely to clog the column.
- Load an exact volume of the produced sample into the autosampler to inject it into the HPLC system at run beginning.
- Pump the mobile phase high-pressure through the column to start the run; as the sample goes through, the components segregate according on their chemical characteristics and interact differently with the stationary phase.
- With a suitable detector—such as UV-Vis, fluorescence, or mass spectrometry—which records the signal and generates a chromatogram with separate peaks—detect the eluting substances.
- Gather the information and examine the chromatogram to ascertain the relative amounts and retention durations of the separated elements.
- To be ready for next studies, flush the column with a strong solvent when the run is over then re-equilibrate it using the mobile phase to eliminate any last sample remains.
Uses
- In pharmaceutical research, HPLC is applied widely for drug formulation analysis, purity testing, and quality control analysis.
- Clinically, it is used to measure therapeutic medication levels in biological fluids and biomarkers.
- HPLC is used by environmental scientists in water, soil, and air samples to identify and measure toxins, pesticides, and other pollutants.
- Biochemical research depends critically on HPLC to separate and examine proteins, peptides, and nucleic acids.
- HPLC is used in the food and beverage sector to monitor additives, preservatives, and possible contaminants therefore guaranteeing product safety.
- In forensic research, the method is crucial to identify and measure trace quantities of metabolites, poisons, and pharmaceuticals in complicated samples.
- Industrial process monitoring and quality assurance for chemical production and polymer analysis depend heavily on HPLC.
- When combined with mass spectrometry (LC-MS), HPLC offers great sensitivity and specificity for chemical in complex mixtures identification.
12. Hydrophobic interaction chromatography
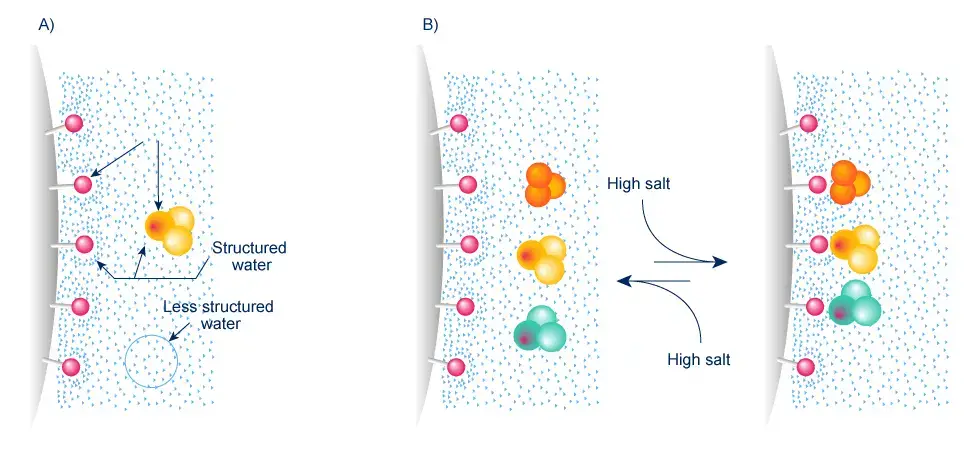
- Hydrophobic interaction chromatography (HIC) sorts molecules according to variations in surface hydrophobicity.
- Water molecules are less accessible to solvate hydrophobic areas under high-salt conditions, hence strengthening contacts between the molecule and the hydrophobic stationary phase.
- The high-salt environment reduces the solvation of hydrophobic regions on proteins or other macromolecules, therefore promoting binding.
- The hydrophobic interactions diminish when the salt concentration is progressively reduced, thereby eluting bound molecules.
- Protein purification makes extensive use of this idea to separate proteins depending on minute changes in hydrophobic properties.
Steps
- Equilibrate the HIC column with a high-salt binding buffer so that any storage solutions or impurities are washed out.
- Dissolving or diluting the sample in the same high-salt binding solution will help to retain protein solubility and encourage hydrophobic interactions.
- Load the sample onto an equilibrated HIC column; high salt conditions help proteins to bind to hydrophobic ligands on the stationary phase.
- Remove unattached or poorly bound contaminants by running the column using the high-salt binding buffer.
- Using a linear gradient or stepwise elution, start a slow reduction in salt concentration that reduces the hydrophobic interactions and leads proteins to elute in order of increasing hydrophobicity.
- Gather the eluted fractions; they might be investigated further to evaluate activity, protein purity, and yield.
- Wash the column using a low-salt buffer and, if needed, a cleaning solution to regenerate and re-equilibrate it thus ready the system for the next run.
Uses
- While maintaining their natural structures, hydrophobic interaction chromatography is extensively utilized to concentrate and clean proteins from complicated biological mixtures.
- It is routinely used for the purification of antibody-drug conjugates, monoclonal antibodies, and recombinant proteins.
- In biopharmaceutical manufacture, the method functions as a useful capture, intermediate purification, and polishing step.
- Protein isoforms and proteoforms are separated there depending on minute variations in surface hydrophobicity.
- By helping to eliminate aggregates and contaminants, hydrophobic interaction chromatography increases the stability of protein compositions.
- Research and therapeutic benefits of the technique also extend to the purification of viruses, plasmids, and exosomes.
- It facilitates technique development and high-throughput screening in order to maximize purifying conditions depending on hydrophobic interactions.
13. Reverse-phase chromatography
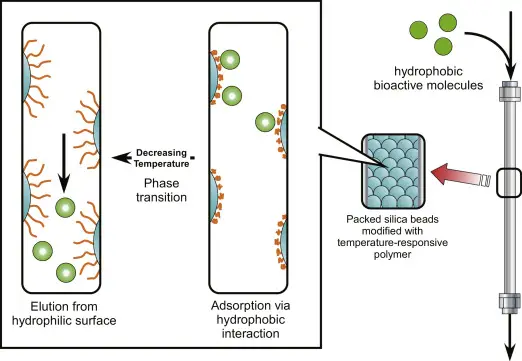
- Generally silica particles modified with long-chain alkyl groups like C18, reverse-phase chromatography is a form of liquid chromatography whereby a nonpolar stationary phase is used together with a somewhat polar mobile phase, generally water or an aqueous buffer combined with an organic solvent.
- Separating analytes between the polar mobile phase and the nonpolar stationary phase depends on hydrophobic interactions, in which case molecules with higher hydrophobicity interact more strongly with the stationary phase and are kept longer.
- Often by a gradient of increasing organic solvent concentration, the composition of the mobile phase is progressively altered, which reduces the polarity of the mobile phase and causes the bound analytes to elute depending on their relative hydrophobicity.
- With more polar chemicals eluting earlier and more hydrophobic compounds eluting later, this differential retention enables for the separation of complicated mixtures, therefore permitting exact characterization and quantification of the sample components.
Steps
- Using a polar mobile phase—usually a combination of water and an organic solvent such as methanol or acetonitrile—reverse-phase chromatography is carried out by passing the sample mixture through a column filled with a nonpolar (hydrophobic) stationary phase.
- The basis of the concept is hydrophobic interactions: although more polar molecules interact less and elute early, analytes with higher hydrophobicity have stronger interactions with the nonpolar stationary phase and are maintained longer.
- Usually increasing the proportion of the organic solvent, a gradient is used throughout the separation to progressively lower the polarity of the mobile phase, therefore weakening the hydrophobic contacts and enabling the analytes to elute based on their hydrophobicity differences.
- Different elution periods (retention times) for every analyte produced by this differential retention help to separate them and then enable their detection or collecting.
Uses
- Organic molecules in complicated mixtures are separated, identified, and quantified using reverse-phase chromatography most often.
- In pharmaceutical research including drug formulation analysis, purity testing, and quality control, it is a fundamental analytical technique.
- In proteomics and biochemistry, the method is fundamental for characterizing and purifying oligonucleotides, proteins, and peptides.
- Environmental analysis uses it to find and measure minute quantities of organic contaminants in air, soil, and water.
- Clinical laboratories utilize reverse-phase chromatography to analyze therapeutic medicines, metabolites, and biomarkers in biological fluids.
- Natural product research uses the approach to separate bioactive molecules from plant and microbial extracts.
- Usually combined with mass spectrometry (LC-MS), it offers great sensitivity and specificity for molecule identification.
- Large-scale purification of important chemical and biological compounds uses reverse-phase chromatography as a preparatory technique.
- The method finds use in the food and beverage sector to examine additives, taste compounds, and pollutants.
- In contemporary chemical and biochemical laboratories, it is absolutely important for method development and routine analytical testing.
14. TLC, or thin-layer chromatography (TLC)
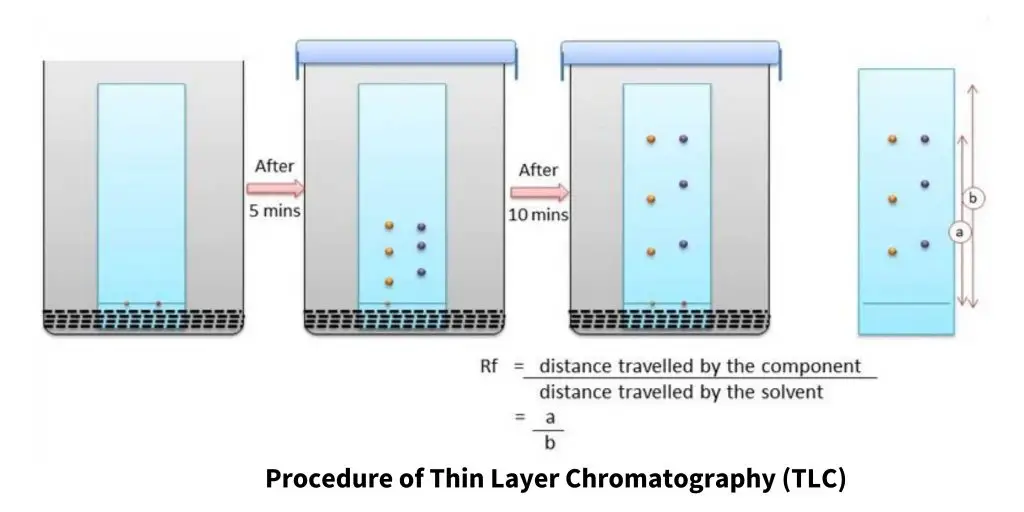
- TLC is a planar chromatographic method whereby the stationary phase is a thin layer of an adsorbent material, like silica gel, alumina, or cellulose, deposited on an inert substrate.
- Applied at the TLC plate’s bottom border, a tiny, concentrated sample is let to dry before development.
- The plate is set in a closed chamber with a thin layer of solvent acting as the mobile phase and rises by capillary action.
- Depending on each compound’s relative affinity for the mobile phase vs the stationary phase, the solvent travels up the plate carrying the sample components at varying speeds.
- Generally more polar compounds, compounds that interact strongly with the stationary phase migrate more slowly; those with less polarity go farther up the plate.
- To assist in compound identification in the combination, the distance each component travels relative to the solvent front is measured as the retention factor (Rf value).
Steps
- Get a ready-made TLC plate on an inert support covered in a small layer of adsorbent, such silica gel, alumina, or cellulose.
- Mark a horizontal line around one centimeter from the bottom of the plate using a pencil to use as the baseline for sample application.
- Dissolving the material in an appropriate volatile solvent will help you create a concentrated solution.
- Apply tiny, clearly defined sample spots evenly spaced between the baseline using a capillary tube or micropipette.
- Let the spots dry entirely so the solvent evaporates and the sample is tightly stuck to the plate.
- Transfer a tiny amount of the mobile phase—a solvent or combination of solvents—into a development chamber such that the solvent level falls below the baseline.
- To guarantee even development and fill the environment with solvent vapours, place a wet piece of filter paper inside the chamber.
- Keeping the spots above the solvent level, vertically insert the TLC plate with its spotted side toward the chamber.
- Close the chamber with a cover and use capillary action to allow the mobile phase rise the plate until it almost reaches the top.
- Before the solvent reaches the top, take the plate out of the chamber and quickly pencil note the location of the solvent front.
- Let the plate dry totally to remove any last traces of solvent.
- Calculating the ratio of the distance travelled by each spot to the distance travelled by the solvent front can help you to visualise the separated components using UV light or by adding an appropriate chemical stain and then comparing the locations of the spots to ascertain their relative movement (Rf values).
Uses
- While maintaining their natural structure, hydrophobic interaction chromatography is extensively applied to clean and concentrate proteins from complicated biological mixtures including cell lysates.
- Using minor variations in protein surface hydrophobicity, it is widely used in biopharmaceutical production to separate monoclonal antibodies and antibody-drug conjugates.
- The method helps to eliminate aggregates and contaminants from formulations by acting as a useful polishing step in protein purification methods.
- Applying HIC in the isolation and characterisation of protein isoforms helps to analyze structural variations and post-translational changes.
- Research for the purification of plasmids, viruses, and exosomes also exploits it to enable downstream usage in diagnostics and therapies.
- Furthermore used in technique development and process optimization—including high-throughput screening to choose ideal purifying conditions—hydrophobic interaction chromatography
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- https://mz-at.de/en/chromatography/further-categories/thin-layer-chromatography/
- https://en.wikipedia.org/wiki/Reversed-phase_chromatography
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/reversed-phase-chromatography
- https://unacademy.com/content/kerala-psc/study-material/bioinstrumentation/reversed-phase-chromatography/
- https://jcsmr.anu.edu.au/files/reverse-phase_handbook.pdf
- https://www.sciencedirect.com/topics/chemistry/reverse-phase-liquid-chromatography
- https://www.americanpharmaceuticalreview.com/Featured-Articles/177927-Hydrophobic-Interaction-Chromatography-for-Antibody-Drug-Conjugate-Drug-Distribution-Analysis/
- https://www.slideshare.net/GamalAbdulHamid/high-performance-liquid-chromatograph-hplc
- https://www.jove.com/science-education/10156/high-performance-liquid-chromatography-hplc
- https://www.chemguide.co.uk/analysis/chromatography/hplc.html
- https://ruo.mbl.co.jp/bio/e/support/method/chromatography.html
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.