The oxidase test is the process used to detect whether a bacterium contain the enzyme cytochrome c oxidase, and it is applied as an early biochemical test in microbiology.
It is mainly helpful to separate oxidase-negative Enterobacteriaceae from organisms like Pseudomonas, Vibrio, Campylobacter and Neisseria which are usually oxidase-positive. It is a rapid assay and the result is used for directing the identification steps of Gram-negative bacteria in routine laboratory work. The reaction is based on the ability of cytochrome oxidase to transfer electrons to oxygen during aerobic respiration.
When the reagent (N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride) is added, it acts as an artificial electron donor, and if the enzyme is present the reagent is oxidised into a deep blue or purple compound. This is referred to as the formation of indophenol blue.
The test is performed by taking a fresh culture and touching it on filter paper or a strip moistened with reagent, and non-metallic tools are used because metal loops is causing false positive reactions. In this step the colour change which appears within 10–30 seconds is taken as a positive result. If no colour is produced within the proper time, the organism is oxidase-negative. It is important because the reagent auto-oxidises in air and delayed reading can give an incorrect interpretation.
Purpose of Oxidase Test
- To differentiate pseudomonads from related species.
- To determine if an organism possesses the cytochrome oxidase enzyme.
- To differentiate Neisseria, Moraxella, Campylobacter and Pasteurella species (oxidase positive).
Principle of Oxidase Test
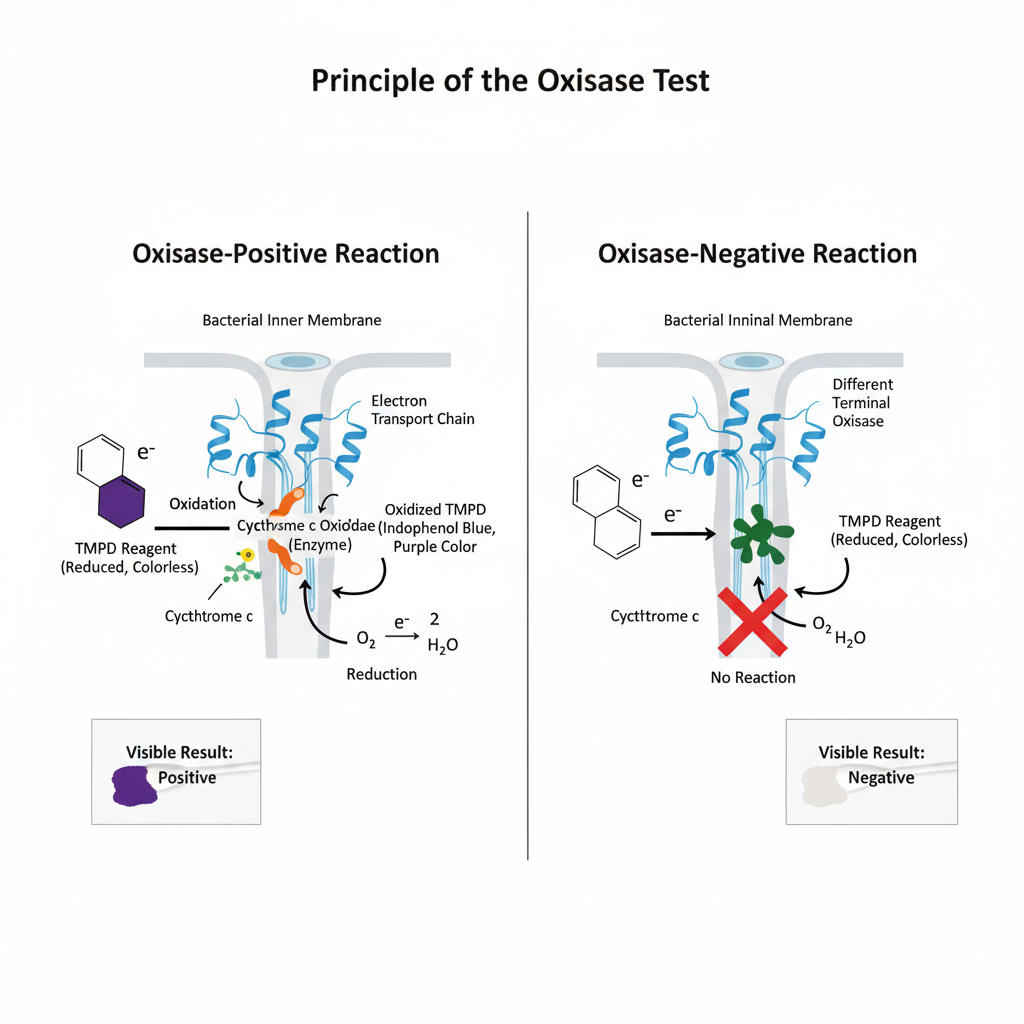
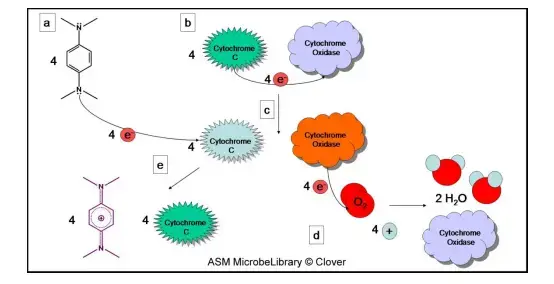
The oxidase test is based on the activity of cytochrome c oxidase, and it is the enzyme that takes part in the electron transport chain of aerobic bacteria. It is the process where electrons are transferred from cytochrome c to molecular oxygen, and the oxygen is reduced to water or sometimes hydrogen peroxide. A redox dye is used in this reaction, and the most common reagent is N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) which acts as an artificial electron donor. When the enzyme is present in the cell, the reagent is oxidised quickly as its electrons are removed, and the oxidised form appears as a deep purple or blue compound known as indophenol blue. This colour formation is the indication that cytochrome c oxidase is present. If the organism is lacking this enzyme, the reagent stays in its reduced and colourless state. It is the principle that only cytochrome c oxidase can oxidise the reagent in this way, and therefore the test is helpful in separating bacteria which is using different types of terminal oxidases.
Requirements for Oxidase Test
- Bacterial Culture Requirements
- The culture must be young and active, usually 18–24 hours old. It is used because older culture sometimes shows reduced enzyme activity.
- It is taken from well isolated colonies so the purity is maintained.
- Non-selective and non-differential media is used (nutrient agar, trypticase soy agar).
- Media rich in glucose is avoided because acid formation can inhibit oxidase activity.
- Media having dyes like MAC or EMB is not used as these interfere with the reaction.
- Media containing tellurite is also avoided.
- The test is done for aerobic or facultative anaerobic organisms but strict anaerobes is not suitable.
- Reagents
- One reagent among the commonly used oxidase reagents is required.
- Kovács reagent (1% TMPD in water) is mostly used as it is very sensitive.
- Gordon and McLeod reagent (1% DMPD in water) can also be used.
- Gaby and Hadley reagents include reagent A (1% α-naphthol in ethanol) and reagent B (1% p-aminodimethylaniline oxalate).
- Commercial oxidase discs or strips is available which can be used directly.
- These reagents are light-sensitive so it is stored in dark bottles and kept in refrigeration (4°C).
- Reagents that already become blue or dark before use is discarded.
- Equipment and Tools
- Platinum loops, plastic loops or sterile wooden sticks is used for transferring colonies.
- Nichrome or steel loops is not used because iron may catalyze the reagent and gives false positive.
- For the filter paper method, Whatman No.1 filter paper is required.
- A clean petri dish is used to hold the filter paper.
- A timer or clock is required because the reading is taken within 10–60 seconds.
- PPE and waste container is used for safety and disposal.
- Quality Control
- A positive control organism like Pseudomonas aeruginosa is used to check reagent activity.
- A negative control organism like Escherichia coli is used to ensure no false positivity.
Procedure of Oxidase Test
Preparation and Materials
- A fresh bacterial culture (18–24 hours old) is used for the test. It is important that cultures from glucose-containing or dye-containing media is avoided as these interfere with enzyme activity.
- The oxidase reagent (1% Kovács reagent) must be fresh, and any reagent already turned blue is discarded.
- A sterile wooden stick, plastic loop, or platinum loop is used in inoculation. Nichrome or iron wire loops is not used because surface oxidation products can form false-positive reactions.
Method 1: Filter Paper Method
- A piece of qualitative filter paper is placed inside a sterile petri dish.
- It is moistened with 1–2 drops of oxidase reagent.
- A well-isolated colony is taken with a sterile wooden stick or platinum loop.
- The colony is smeared on the moistened portion of the filter paper.
- A change into deep blue or purple color within 10–30 seconds is observed.
Method 2: Direct Plate Method
- Bacteria is grown on a non-selective agar plate (nutrient agar or trypticase soy agar).
- Two to three drops of oxidase reagent is added gently on the suspected colonies.
- The plate is tilted slightly to expose colonies to oxygen and avoid flooding.
- A dark purple coloration on colony surface within 10 seconds is observed.
– The reagent kills microorganisms, so sub-culturing is done earlier if further analysis is needed.
Method 3: Swab Method
- A sterile swab is dipped in the oxidase reagent to moisten.
- A well-isolated colony is touched with the swab.
- The swab tip is observed for deep blue or purple color within 10 seconds.
Method 4: Test Tube Method (Gaby-Hadley)
- A nutrient broth with low sugar concentration is inoculated and incubated for 18–24 hours.
- About 0.2 mL of 1% α-naphthol (Reagent A) and 0.3 mL of 1% p-aminodimethylaniline oxalate (Reagent B) is added.
- The tube is shaken vigorously for proper oxygenation and mixing.
- A blue coloration within 15–30 seconds is observed.
Result and Interpretation of Oxidase Test
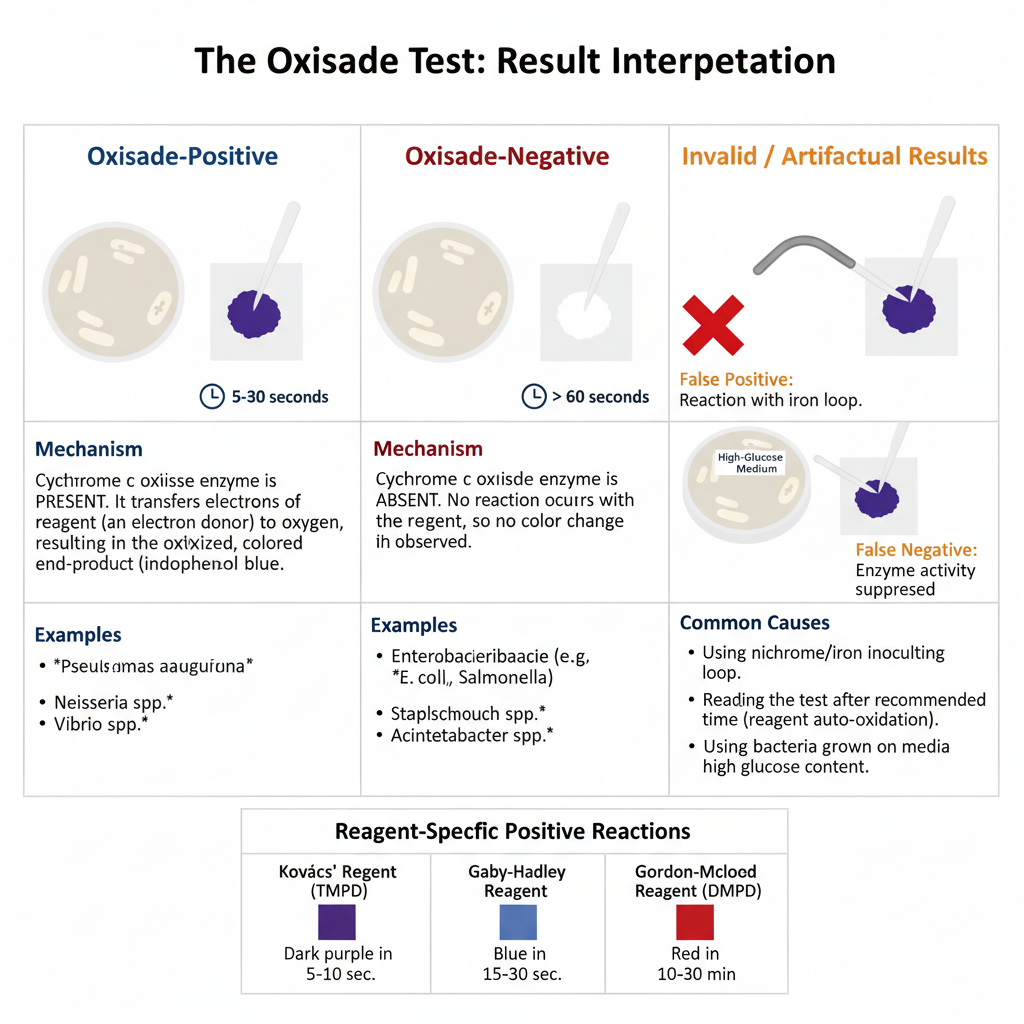
1. Positive Result (Oxidase-Positive)
- The colonies or reagent area turn deep blue or dark purple in appearance.
- A rapid reaction is seen usually within 5–10 seconds and sometimes up to 30 seconds.
- A delayed type reaction can appear between 10–60 seconds, and in some protocols this is extended to 90 seconds.
- It is the indication that cytochrome c oxidase enzyme is present and it helps in transferring electrons to oxygen forming the oxidized indophenol blue.
- Some common examples are– Pseudomonas aeruginosa, Neisseria spp., Vibrio spp., Campylobacter, Helicobacter, Moraxella, Pasteurella.
2. Negative Result (Oxidase-Negative)
- No visible change in color is seen and the area remains the same as the reagent or paper.
- No characteristic deep coloration appears within 60 seconds to 2 minutes.
- It is the indication that cytochrome c oxidase is absent and the organisms may use alternative terminal oxidases which do not react with the reagent.
- Common examples are– Enterobacteriaceae members (E. coli, Klebsiella, Salmonella, Proteus), Staphylococcus, Streptococcus, Acinetobacter spp.
3. Reagent-Specific Interpretations
- Kovács Reagent (TMPD): A positive reaction shows dark purple within 5–10 seconds. A delayed reaction shows purple coloration within 60–90 seconds.
- Gaby–Hadley Reagents: A blue color within 15–30 seconds is considered positive. Delayed purple coloration may appear within 2–3 minutes.
- Gordon–McLeod Reagent (DMPD): The reaction is slower, with red color in 10–30 minutes or sometimes black color in 60 minutes.
4. Invalid or Artifactual Results
- Any color appearing after 60 seconds or after 2 minutes (based on the protocol) is not taken because auto-oxidation of reagent in air can produce false positive reactions.
- If nichrome or iron loops is used, an immediate false-positive may occur due to metal oxidation, so plastic or platinum loops is required.
- Growth on glucose-rich medium can suppress oxidase activity and may produce a false negative reaction.



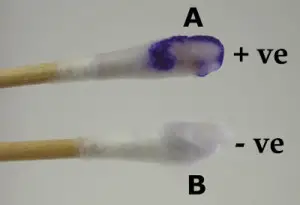
Quality Control of Oxidase Test
Control Organisms
A known positive and negative organism is always included to confirm the accuracy of the reagent or disc.
- Positive control: Pseudomonas aeruginosa (ATCC 27853 or similar). It gives a rapid deep blue or purple color within 10–30 seconds.
- Negative control: Escherichia coli (ATCC 25922 or similar). It shows no color change within 60 seconds and any later color is not considered.
- Fungal controls: Candida albicans acts as positive and Saccharomyces cerevisiae acts as negative.
Reagent Integrity Checks
- The reagent or oxidase paper is inspected before use, and if it has turned blue or dark it is discarded.
- It is prepared fresh because the reagent is unstable and gets deactivated rapidly.
- It is stored at 4°C and kept protected from light to reduce auto-oxidation.
Procedural Controls
- Nichrome or iron loops is not used for inoculation because metal oxidation can give false-positive reaction. Wooden sticks, plastic loops, or platinum loops is preferred.
- Cultures from glucose-rich or dye-containing medium is avoided as these may inhibit oxidase activity or give irregular reactions.
- A fresh culture (18–24 hours old) is taken because older cultures may produce unreliable negative reactions.
- The result is always read within the proper time window (usually below 60 seconds). Any color after 2 minutes is invalid because of reagent auto-oxidation.
Precautions during Oxidase Test
- Freshly prepared oxidase reagent must be used as it is unstable and it is easily auto-oxidized when exposed to light and air.
- The reagent should be kept in dark bottles and stored at low temperature to prevent early oxidation.
- Any reagent that has turned blue before use is discarded as it is already oxidized.
- Fresh cultures (18–24 hours) are selected because older cultures show reduced enzyme activity.
- Colonies from media containing excess glucose are not used as it is the major cause for false-negative reaction.
- Colonies grown on media with dyes like MacConkey or EMB are not selected because these interfere with the colour formation.
- Tellurite-containing media is also avoided in this test.
- The sample must be taken from well isolated colonies so that mixed cultures do not affect the result.
- Nichrome or steel loops are not used as these metals can catalyse oxidation of reagent giving false-positive reaction.
- Platinum or plastic loops or sterile wooden sticks are used for picking colonies in this step.
- The colour change must be read within the specific time (usually within 10–60 seconds) because colour after this time is due to auto-oxidation.
- Different reagents have different reaction times and these instructions must be followed as given.
- The oxidase reagent kills organisms so the colony is picked before reagent addition if further culture is required.
- Strict anaerobes are not tested because this enzyme system is not present in anaerobic organisms.
- The reagent is not flooded on the plate and only a few drops are used.
- Work is done carefully with PPE and inside safety cabinet if aerosols may occur.
Application of Oxidase Test
- It is used to differentiate the Enterobacteriaceae group which is oxidase-negative from other Gram-negative rods which are oxidase-positive.
- Non-fermenting Gram-negative rods like Pseudomonas aeruginosa are identified quickly because these organisms show strong oxidase activity.
- Neisseria and Moraxella species are identified by this test as these cocci give oxidase-positive reaction while Staphylococcus and Streptococcus are oxidase-negative.
- Curved rods like Vibrio cholerae, Campylobacter and Helicobacter are detected because these organisms show positive oxidase reaction.
- Some other genera like Aeromonas, Alcaligenes, Brucella and Pasteurella are also recognized due to their oxidase-positive nature.
- It is used for Gram-positive cocci where Micrococcus species is oxidase-positive while Staphylococcus species is oxidase-negative.
- A modified oxidase test is used with DMSO solution for distinguishing Micrococcus from Staphylococcus.
- It can help in the study of yeasts where Candida, Saccharomyces and Torulopsis show different oxidase reactions depending on the medium used.
Limitations of Oxidase Test
- The oxidase reagent is chemically unstable and it is easily auto-oxidized by light, heat or air which causes false-positive reaction.
- The reagent must be freshly prepared and stored in dark bottles because old reagent turns blue on its own.
- Iron containing loops like nichrome or steel are not used as these metals oxidize the reagent chemically and give false-positive results.
- Only inert loops like platinum, plastic or wooden sticks are suitable for transferring colonies.
- Colonies from media with high glucose concentration may show false-negative reaction because oxidase activity is suppressed.
- Media containing dyes like MacConkey or EMB are not used since these dyes interfere with the colour development.
- Old cultures do not show proper enzyme activity and these can give false-negative results.
- The colour change must be read within the fixed time because colour after that time is due to reagent oxidation in air.
- The reagent kills the organisms so the colonies tested cannot be used again for further culture or tests.
- This test is only a screening tool because it cannot identify an organism completely and further biochemical tests is needed.
- AAT Bioquest. (2024, May 9). What precautions should I take during oxidase tests? https://www.aatbio.com/resources/faq-frequently-asked-questions/what-precautions-should-i-take-during-oxidase-tests
- Aryal, S. (2022, August 10). Oxidase test- Principle, uses, procedure, types, result interpretation, examples and limitations. Microbiology Info. https://microbiologyinfo.com/oxidase-test-principle-uses-procedure-types-result-interpretation-examples-and-limitations/
- Centers for Disease Control and Prevention. (n.d.). How to perform an oxidase test [Job aid]. https://reach.cdc.gov/sites/default/files/video-transcripts/How%20to%20Perform%20an%20Oxidase%20Test.pdf
- Centers for Disease Control and Prevention. (n.d.). How to perform the oxidase test [Video]. YouTube. https://www.youtube.com/watch?v=YGaC6mZgOjA
- Dahal, P. (2023, April 4). Oxidase test- Principle, procedure, types, results, uses. Microbe Notes. https://microbenotes.com/oxidase-test-principle-procedure-and-results/
- Franco-Duarte, R., Černáková, L., Kadam, S., Kaushik, K. S., Salehi, B., Bevilacqua, A., Corbo, M. R., Antolak, H., Dybka-Stępień, K., Leszczewicz, M., Tintino, S. R., de Souza, V. C. A., Sharifi-Rad, J., Coutinho, H. D. M., Martins, N., & Rodrigues, C. F. (2019). Advances in chemical and biological methods to identify microorganisms—From past to present. Microorganisms, 7(5), 130. https://doi.org/10.3390/microorganisms7050130
- Hafezi, A., & Khamar, Z. (2024). The method and analysis of some biochemical tests commonly used for microbial identification: A review. Comprehensive Health and Biomedical Studies, 3(2), e160199. https://doi.org/10.5812/chbs-160199
- Shields, P., & Cathcart, L. (2010, November 11). Oxidase test protocol. American Society for Microbiology. https://asm.org/getattachment/00ce8639-8e76-4acb-8591-0f7b22a347c6/oxidase-test-protocol-3229.pdf
- The oxidase test: Protocol standardization, biochemical mechanism, and diagnostic utility in clinical microbiology. (n.d.). [Review article].
- UK Health Security Agency. (2025). UK standards for microbiology investigations: Oxidase test (TP 26, Issue 4.1). Royal College of Pathologists. https://www.rcpath.org/static/50481369-aea7-4183-9d6c83de6d9c8445/uk-smi-tp-26i4-oxidase-test-march-2025-pdf.pdf
- Watson, R. (n.d.). Tests used to identify Gram negative bacteria. University of Wyoming. https://www.uwyo.edu/molb2210_lab/info/biochemical_tests.htm
- Wikipedia. (n.d.). Oxidase test. Retrieved from https://en.wikipedia.org/wiki/Oxidase_test