What is cryo-EM?
- Cryo-electron microscopy is a very complex method to view the 3D shapes of biological molecules and complexes in high resolution. The technique rapidly freezes samples to temperatures below -150 °C, which traps them in glass-like ice. Frozen samples are then viewed using an electron microscope. This process basically defines a phenomenon wherein a beam of electrons is passed through the sample to produce several 2D pictures from different angles. A computer program constructs the 3D model from these 2D images.
- The key difference of cryo-EM from standard electron microscopy (EM) is its operation at extremely low temperatures. This means that delicate samples can be observed without destroying them too much, which is a major advantage over older techniques. Cryo-EM comprises several techniques: cryo-electron tomography, single-particle cryo-EM, and electron crystallography. Each of these techniques enables the observation of different biological scenarios, ranging from whole cells and viruses to single protein molecules.
- Cryo-EM can be applied in many areas of biological research. For example, cryo-electron tomography produces 3D images of whole cells and viruses, while single-particle cryo-EM views isolated large molecules by reconstructing many 2D images into clear 3D shapes. Additionally, cryo-EM is often used in combination with other techniques like X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy to get a better understanding of molecular structures.
- The scientists write about the latest progress in cryo-EM in research papers. They talk about better methods and more types of biological systems that can be studied. For beginners, learning the basics of cryo-EM and how it is used helps to understand its importance in studying structures in biology. Important resources are the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB), which host large collections of cryo-EM data that help in research and new findings in this constantly changing field.
Principle of cryo EM
- The working principle of cryo-EM is based on advanced imaging of biological specimens using electron beams in the cryogenic state. This technique extends from the foundational principles of electron microscopy but resolves very real problems with radiation damage to biological materials.
- In electron microscopy, images are created by passing an electron beam through a sample. Unlike light microscopy, which uses visible light and optical lenses to create images, electron microscopy uses electrons accelerated in a high vacuum at voltages usually between 80 and 300 kV. The electrons interact with the sample, and their scattering is collected and focused by electromagnetic lenses to form an image. The primary advantage of electron microscopy over light microscopy is that it has a better resolving power. This is because electrons have a shorter wavelength than visible light, which allows for the resolution of smaller structures in greater detail.
- Nonetheless, biological samples are extremely sensitive to electron irradiation. Interactions of electrons with organic materials may result in bond breakage and the creation of free radicals, which generate vital structural changes. To avoid such damage, the cryo-EM approach incorporates a cryogenic procedure by which samples are frozen rapidly, generally in liquid ethane or nitrogen, so that the specimen becomes locked in a glassy state. This fast vitrification of the sample prevents the sample from forming ice crystals, which would otherwise destroy the specimen.
- The extent of radiation damage is reduced to a large extent at cryogenic temperatures so that higher electron doses can be given without degrading the structural integrity of the specimen. This is because the slowing down of molecular movements reduces the formation of damaging free radicals at lower temperatures. Further, imaging at temperatures close to liquid nitrogen or liquid helium minimizes the effects of radiation even further, thus allowing high-resolution images to be obtained.
- Cryo-EM uses computer methods to make images better, along with cryogenic preservation. One of the ways of doing this is by averaging many images of the same particles or structures. This method is called single-particle reconstruction, and it improves the quality of the images by mixing data from several low-dose pictures. By averaging these images, unwanted noise is lessened, leading to clearer and more accurate 3D pictures.
- Cryo-EM employs low temperatures in preserving samples, and it aggregates multiple images for clear details regarding biological molecules. This method represents a significant stride in structural biology, as scientists can now observe and study biological objects much better than they used to.
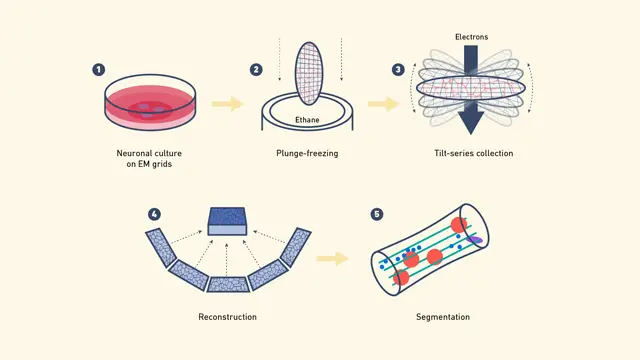
Types of cryo EM
Cryo-electron microscopy (cryo-EM) is a collection of special methods. These are broadly categorized into two types, mainly cryo-transmission electron microscopy (cryo-TEM) and cryo-scanning electron microscopy (cryo-SEM). Each has its own advantage in the analysis of biological samples in their native state, frozen by cryo-preparation.
- Cryo-Transmission Electron Microscopy (Cryo-TEM):
- Principle: In cryo-TEM, the sample is frozen rapidly and put into a transmission electron microscope (TEM). An electron beam passes through the sample, and the electrons interact with it, scattering and absorbing.
- Imaging: This interaction creates a set of two-dimensional (2D) images of the sample. To make a three-dimensional (3D) image, the sample is tilted at different angles, and many 2D images are taken.
- Processing: Such projection images are processed by using advanced computer techniques to provide a 3D density map. This map defines where the electrons are, and hence it can clearly give the idea of the arrangement of atoms in the sample.
- Applications: Cryo-TEM is appropriate for observing the inner structure of biological macromolecules, viruses, and cell components; thus, it delivers high-quality 3D models.
- Cryo-SEM:
- Principle: Cryo-SEM examines the frozen sample surface topography. Typically, a scanning electron microscope (SEM) scans a broken or split sample so its inside surfaces can be examined.
- Imaging: In SEM, a beam of focused electrons sweeps over the surface of the sample. Secondary and backscattered electrons, generated from the way the electron beam interacts with the sample, are collected to generate the surface image.
- Applications of Cryo-SEM: Cryo-SEM is very useful for illustrating the surface details and shape of biological samples. It allows researchers to view the outer features and surface structures of samples, which helps them in understanding how surfaces interact and their shape.
How does cryo EM work? – Protocol of cryo EM
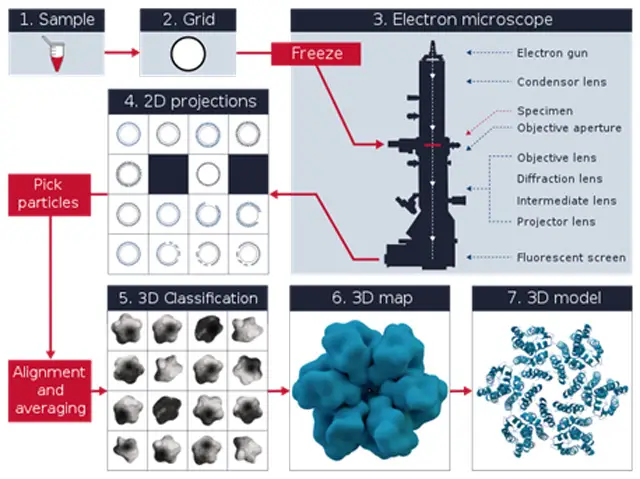
Cryo-electron microscopy (cryo-EM) works by following several careful steps to take clear images of biological samples as they are, frozen in a glass-like state. The cryo-EM process can be explained like this:
- Preparing the Sample:
- Sample Solution: It starts with a clean biological sample, usually a protein or a group of molecules mixed in a liquid.
- Vitrification: A small drop of the sample solution is placed on a special grid. This grid is plunged into a very cold liquid, such as liquid ethane, to freeze the sample rapidly. This rapid freezing converts the water in the sample into a form of ice that does not have a sharp shape, thus preserving the native structure of the biological molecules.
- Cryo-EM Microscopy:
- Sample Handling: The frozen sample is mounted on the microscope stage and kept at very low temperatures to prevent any damage or alteration.
- Cryo-EM Microscope Calibration: The cryo-EM microscope is aligned and calibrated to ensure that the images are clear and accurate. Data
- Acquisition: An electron beam is directed at the sample, producing a set of two-dimensional (2D) images from various angles. These images reveal different components of the sample’s structure.
- Image Processing:
- Correction and Improvement: The collected 2D images are processed to correct any artifacts and also to enhance the signal-to-noise ratio.
- Particle Identification: Individual particles within the images are identified through the process of particle picking, where specific features of interest are isolated to a particular region on the micrograph.
- 3D Reconstruction:
- Data Collection: A massive quantity of particle images are collected and analyzed.
- Computational Reconstruction: High-speed computer techniques allow for the reconstruction of a 3D density map from a set of 2D images. The 3D map illustrates the spread of electron density within the sample.
- Model Fitting and Analysis
- Atomic Modeling: Atomic models are positioned into the 3D density map. These models are iteratively refined to optimally fit the experimental data.
- Validation and Interpretation: Validation and interpretation are carried out with the final models to describe the structural properties of the molecules investigated.
Cryo-EM may be conducted using various electron microscopes. Some of them are;
- Cryo-Transmission Electron Microscopy (Cryo-TEM)
- Imaging Technique: Cryo-TEM is a type of imaging in which the vitrified specimen is observed with an electron beam. A sequence of 2D projection images are recorded while the sample is tilted at a series of different angles.
- 3D Reconstruction: These images create a 3D density map that indicates what is going on inside the sample.
- Cryo-Scanning Electron Microscopy (Cryo-SEM)
- Surface Imaging: Cryo-SEM is applied to obtain images of the surface morphology of frozen samples. Often, the sample is fractured to expose its inner surfaces.
- Surface Analysis: The surface of the sample is scanned with a focused electron beam, and secondary and backscattered electrons are detected to produce a surface image.
Advantages of Cryo-Electron Microscopy (cryo-EM)
- Imaging with a high, near atomic resolution.
- In fact, Cryo-EM has such an incredible resolution that it is nearly at the atomic level. The high resolution enables biological macromolecules to be visualized at the level of individual amino acid side chains, illustrating structural features that are invisible using nearly any other biophysical technique and that provide fundamental insights into molecular function and interactions.
- Storing the sample in a form that is as close to its natural condition as possible.
- The method utilizes vitrification, the quick-freeze process that preserves the samples in a life-like form. Which is imperative for the examination of a biological specimen as the structure changes take place during sample preparation in other techniques.
- Broad array of biological specimens.
- Helical complexes
- It is especially useful for studying large, dynamic molecular assemblies that are difficult, if not impossible, to study with other methods. Its capacity to house large structures and dynamic assemblies is therefore crucial for studying complex biological systems.
- One particle analysis limitless.
- Because it does not require crystallization, cryo-EM allows single particle analysis since the sample has no size limitations. This provides a means of studying macromolecules and assemblies in their native, non-crystalline state, and thereby extends X-ray methods in structural biology.
Limitations of Cryo-Electron Microscopy (cryo-EM)
- Required Homogeneity– Cryo-EM takes use of sample particle homogeneity to generate high-resolution findings. Sample homogeneity is needed for imaging and structural determination. Reconstructed pictures might suffer from sample variability.
- Computational Demands– Imaging creates large datasets that require computer power to process. These big data sets require advanced computer processes and a lot of work.
- Limitations in resolution – Cryo-EM may struggle to resolve finer structural features despite its great resolution. When working with complicated or diverse structures, sample characteristics may stay unclear.
- Challenges with Larger Samples – Larger samples are difficult to analyze. Larger specimens may make sample integrity and imaging conditions challenging, hence the procedure is best for smaller specimens.
- Cost, Expertise– Cryo-EM equipment is costly and requires training. High technology costs and n
Applications of Cryo-Electron Microscopy (cryo-EM)
- Structural Biology
- Function: Cryo-EM has revolutionized structural biology because it gives us clear images of difficult biological targets.
- Applications: This technique is especially helpful for viewing membrane proteins, large groups of molecules, and flexible or varied complexes that are hard to view with standard techniques.
- Outcome: It helps us understand the protein structures, functions, mechanisms, and interactions, which are important for knowing different biological processes.
- Virology
- Function: Cryo-EM provides clear images of virus structures including capsids, envelopes, and surface proteins.
- Applications: This capability is highly relevant in vaccine production, designing antiviral drugs, and in studying the process of how viruses assemble, replicate, and infect.
- Outcome: Recent advancements include sharp structural information of viruses such as the SARS-CoV2 omicron variant that assist in devising specific treatment options.
- Cell Biology
- Function: Cryo-EM offers sharp images on how cells are structured and assembled.
- Applications: It is used to visualize cellular organelles, cytoskeletal networks, and molecular complexes, which allow the study of basic cellular processes such as mitosis, endocytosis, intracellular transport, and organelle biogenesis.
- Outcome: This technique helps in having a better understanding of cellular mechanisms and the spatial organization of cellular components.
- Drug Discovery
- Function: Drug discovery is significantly involved with the help of cryo-EM that reveals the structures of drug targets.
- Applications: This technique is applied to the study of key targets such as G protein-coupled receptors (GPCRs) and ion channels, which are crucial for smart drug design and optimization.
- Outcome: It enables us to understand how ligands bind and helps in designing drugs that work better and are more specific.
- Neurobiology
- Function: Cryo-EM is applied to explore nerve structures and large molecules associated with neurodegenerative diseases.
- Applications: It is used to study synaptic vesicles, neurotransmitter receptors, and how synaptic transmission and neuronal signaling work at the molecular level.
- Outcome: This research helps us understand neurological disorders better and may lead to new treatments.
- Biochemistry and Molecular Biology
- Function: Cryo-EM helps in studying large molecules that play important roles in cellular processes.
- Applications: This technique is used to look into processes like DNA replication, transcription, translation, and protein folding.
- Outcome: It gives the critical structural information concerning these essential biological mechanisms at the molecular level.
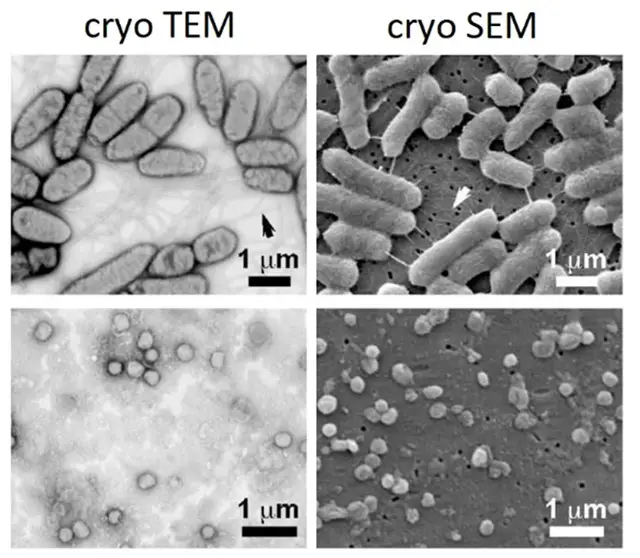
Differences Between cryo TEM and cryo SEM
Cryo electron microscopy (cryo-EM) comprises multiple techniques, and cryo transmission electron microscopy (cryo-TEM) and cryo scanning electron microscopy (cryo-SEM) are the major two techniques. These techniques have unique properties and applications that make them uniquely suitable for particular applications. The main features of cryo-TEM and cryo-SEM are compared as follows:
Cryo-TEM
- Equipment:
- Transmission Electron Microscope: This transmits electrons through the sample with the help of a high-vacuum transmission electron microscope.
- Electron Beam Interaction:
- Transmission Through the Sample: The electrons pass through the sample to interact with it in creating images.
- Sample Thickness:
- Needs to be Thin: So that electrons can pass through it, the sample preferably has to be 100 nm or thinner.
- Information Obtained:
- Internal Structure: Gives clear information about the internal structure of the sample, including how molecules are arranged and how they interact.
- Resolution:
- High Resolution: Reaches nearly atomic resolution, making it possible to see molecular structures in detail.
- Applications:
- Molecular Structures and Interactions: Mainly used to study the structures of individual large molecules and groups of molecules, good for analyzing single particles.
Cryo-SEM
- Equipment:
- Scanning Electron Microscope: Uses a scanning electron microscope to look at the surface of the sample with a focused beam of electrons.
- Electron Beam Interaction:
- Focused on the Surface: Electrons impinge on the surface of the sample and produce secondary and backscattered electrons to form images.
- Sample Thickness:
- Any Thickness: Samples may be any thickness; in fact, samples can be much thicker than those used in cryo-TEM.
- Information Obtained:
- Surface Topography and Morphology: Provides sharp image of features of structure and shape at the surface of the sample.
- Resolution:
- Lower Resolution: It usually provides a lower resolution compared with cryo-TEM, but it’s good for looking at surfaces.
- Applications:
- Surface Characterization: It is good for examining large structures or materials and for analyzing the surface details of samples.
Comparison
- Technique: Cryo-TEM employs transmission electron microscopes in seeing inside structures, whereas cryo-SEM utilizes a scanning electron microscope in photographing the surfaces.
- Sample Preparation: Cryo-TEM requires thin samples while cryo-SEM can work on samples of different thicknesses.
- Resolution: Cryo-TEM has a high resolution that is excellent for molecular-level details, whereas cryo-SEM provides surface-level imaging with generally lower resolution.
- Applications: Cryo-TEM is suited to molecular structures and interactions, whereas cryo-SEM excels in surface characterization and large-scale structural analysis.
| Feature | Cryo-TEM | Cryo-SEM |
|---|---|---|
| Equipment | Transmission Electron Microscope | Scanning Electron Microscope |
| Electron Beam | Transmitted through the sample | Focused on the surface |
| Sample Thickness | Thin samples (typically ≤100 nm) | Any thickness |
| Information Obtained | Internal structure and molecular arrangements | Surface topography and morphology |
| Resolution | High resolution (near-atomic) | Lower resolution |
| Applications | Molecular structures, single-particle analysis | Surface characterization, large-scale structures |
Comparison of the Main Features of Conventional EM and Cryo-EM
- Sample Preparation
- Conventional EM:
- Process: A number of stages are involved in this process, which includes fixation, staining, dehydration, and chemical compounds or polymers treatment.
- Impact: These methods distort the native structures and thus introduce artifacts that will affect the consistency of the structural interpretation.
- Cryo-EM:
- Process: Flash-freezing, also referred to as vitrification, is utilized in the rapid freezing of the sample under its native state.
- Impact: This does not distort the native structures; therefore, this method reduces the formation of artifacts.
- Conventional EM:
- Sample Integrity
- Conventional EM:
- Condition: The native structures of samples can be altered due to the fixation and staining processes.
- Result: The preservation of sample integrity is compromised, which may affect the accuracy of the structural data obtained.
- Cryo-EM:
- Condition: Samples are well-preserved as they are rapidly frozen, maintaining their native state.
- Result: This method ensures that the sample’s native structures are retained, providing more accurate structural information.
- Conventional EM:
- Resolution
- Conventional EM:
- Resolution: Achieves high resolution in the nanometer range (nm).
- Detail: It is appropriate for viewing fine cellular and subcellular structures but will not necessarily reveal atomic details.
- Cryo-EM:
- Resolution: Provides higher resolutions that are capable of reaching near-atomic levels (Å).
- Detail: This resolution gives a clear view of biomolecules and complex assemblies at the atomic level.
- Conventional EM:
- Specimen Types
- Conventional EM:
- Suitability: Will be ideal to view cellular and subcellular structures.
- Scope: This technique is excellent for imaging larger biological structures, but it may not be the best suited for complicated biomolecular assemblies.
- Cryo-EM:
- Applicability: It is excellent for the study of biomolecules and complex macromolecular groups.
- Scope: Cryo-EM is very useful for studying smaller or more fragile biological samples that must be preserved in their native state.
- Conventional EM:
- Amount of Sample
- Conventional EM:
- Demand: Requires greater quantities of sample material.
- Implication: This may be a problem when dealing with small or valuable samples.
- Cryo-EM:
- It can analyze small amounts of biomolecules or complexes. This makes it possible to study rare or valuable samples very accurately.
- Conventional EM:
| Feature | Conventional EM | Cryo-EM |
|---|---|---|
| Sample Preparation | Multiple steps: fixation, staining, dehydration; potential artifacts | Flash-freezing (vitrification) to preserve native state |
| Impact on Sample | Can alter native structures, introducing artifacts | Preserves native structures with minimal distortion |
| Sample Integrity | Compromised due to fixation and staining | Well-preserved, maintaining native state |
| Resolution | High resolution in the nanometer range (nm) | Higher resolution, near-atomic levels (Å) |
| Detail | Suitable for fine cellular and subcellular structures | Detailed visualization of biomolecules and complex assemblies |
| Specimen Types | Ideal for cellular and subcellular structures | Best for biomolecules and complex macromolecular assemblies |
| Amount of Sample | Requires larger amounts of material | Can analyze minimal amounts of biomolecules or complexes |
Images of Cryo-Electron Microscopy (cryo-EM)
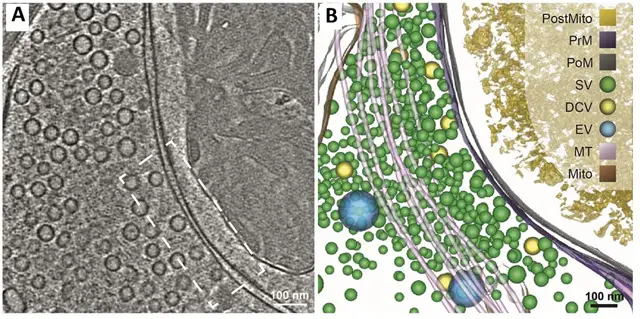
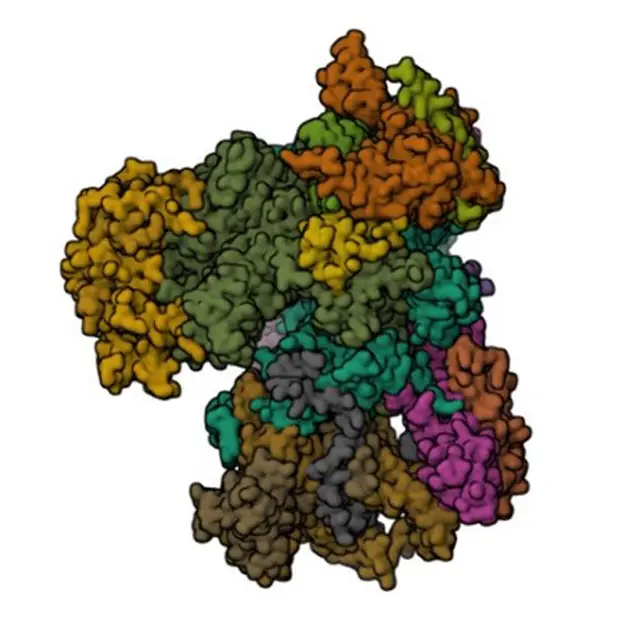
- Milne JL, Borgnia MJ, Bartesaghi A, Tran EE, Earl LA, Schauder DM, Lengyel J, Pierson J, Patwardhan A, Subramaniam S. Cryo-electron microscopy–a primer for the non-microscopist. FEBS J. 2013 Jan;280(1):28-45. doi: 10.1111/febs.12078. Epub 2012 Dec 17. PMID: 23181775; PMCID: PMC3537914.
- https://www.technologynetworks.com/analysis/articles/cryo-electron-microscopy-principle-strengths-limitations-and-applications-377080
- https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy
- https://www.chemistryworld.com/news/explainer-what-is-cryo-electron-microscopy/3008091.article
- https://www.lsi.umich.edu/science/centers-technologies/cryo-electron-microscopy
- https://www.thermofisher.com/in/en/home/electron-microscopy/life-sciences/cryo-em.html