What is Collagen?
- Collagen is a crucial structural protein found throughout the human body, comprising approximately 25% to 35% of the total protein content in mammals. This protein forms the fundamental framework for various tissues, including skin, bones, ligaments, tendons, cartilage, and even organs such as the cornea and blood vessels. The name “collagen” is derived from the Greek word “kolla,” meaning glue, which aptly reflects its role in providing structural integrity and cohesion among different tissues.
- The molecular structure of collagen consists of a unique triple helix formation, where amino acids are intricately woven together to create elongated fibrils known as collagen helices. This structural arrangement contributes to the rigidity and strength of collagenous tissues. Depending on the degree of mineralization, collagen can exhibit varying degrees of compliance and rigidity; for instance, bone is characterized by high mineralization and rigidity, while tendons are more compliant. Cartilage presents an intermediate property, combining both rigidity and flexibility, allowing it to serve its function effectively in joint support and cushioning.
- Collagen’s function extends beyond providing structural support. It plays a vital role in various physiological processes, including blood clotting. The presence of collagen in blood vessels aids in maintaining their integrity and functionality. Furthermore, collagen synthesis is closely linked to specific vitamins, particularly Vitamin C, which is essential for the formation of collagen fibers, while Vitamin E enhances collagen production.
- Fibroblasts are the predominant cells responsible for collagen production in the body. These specialized cells synthesize and secrete collagen fibers, contributing to the maintenance and repair of connective tissues. As individuals age, the body experiences a decline in both the quantity and quality of collagen production, leading to common age-related conditions such as skin wrinkling and joint stiffness.
- In addition to its biological significance, collagen also has practical applications. Gelatin, a widely used substance in culinary and industrial contexts, is derived from collagen that has undergone irreversible hydrolysis through processes involving heat or acid. This transformation retains some functional properties of collagen while rendering it suitable for various uses.
Definition of Collagen
Collagen is the most abundant protein in the body, providing structural support to connective tissues such as skin, bones, tendons, and ligaments. It consists of a triple helix of amino acids, forming a framework that maintains the integrity and elasticity of various tissues. Collagen plays crucial roles in wound healing, tissue repair, and overall cellular function.
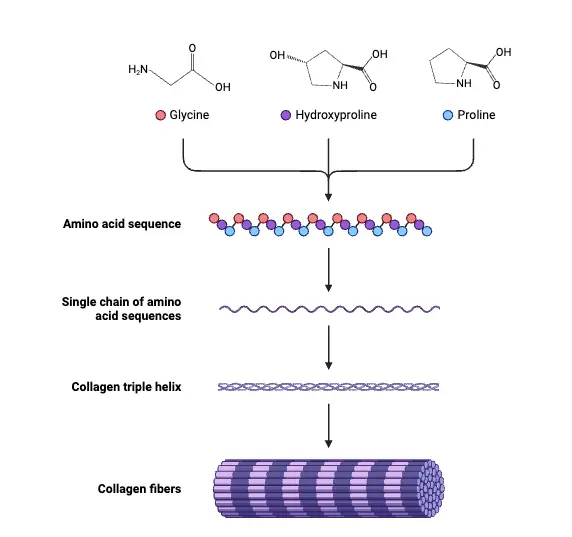
Types of Collagen
Collagen is classified into various types based on its structure and function within the body. The most prominent types include I, II, III, and IV, although there are at least 28 distinct types identified. Each type serves specific roles in maintaining the integrity and function of different tissues.
- Type I Collagen
- Comprising approximately 90% of the collagen in the body, Type I collagen forms large, eosinophilic fibers that provide significant structural support.
- It is primarily found in bones, skin, fibrous cartilage, teeth, connective tissues, and tendons.
- Its robust nature contributes to the tensile strength and durability of these structures, facilitating their ability to withstand mechanical stress.
- Type II Collagen
- This type is characterized by more loosely packed fibers, making it suitable for specific functional requirements.
- Type II collagen is predominantly located in elastic cartilage, which cushions joints, thereby aiding in shock absorption during movement.
- It plays a crucial role in maintaining the integrity of cartilage, ensuring smooth joint function and mobility.
- Type III Collagen
- Type III collagen supports the structure of various organs, muscles, and arteries.
- It is often found alongside Type I collagen, particularly in tissues requiring flexibility and resilience, such as skin and blood vessels.
- This collagen type is vital for the development and repair of connective tissues, contributing to overall tissue health.
- Type IV Collagen
- Located in the basement membranes, Type IV collagen serves as a crucial component of the skin’s structural layers.
- It plays a significant role in filtration processes within tissues, particularly in kidneys, where it helps maintain the barrier function.
- By providing a supportive network, Type IV collagen aids in the anchoring of cells to the extracellular matrix, facilitating proper tissue organization.
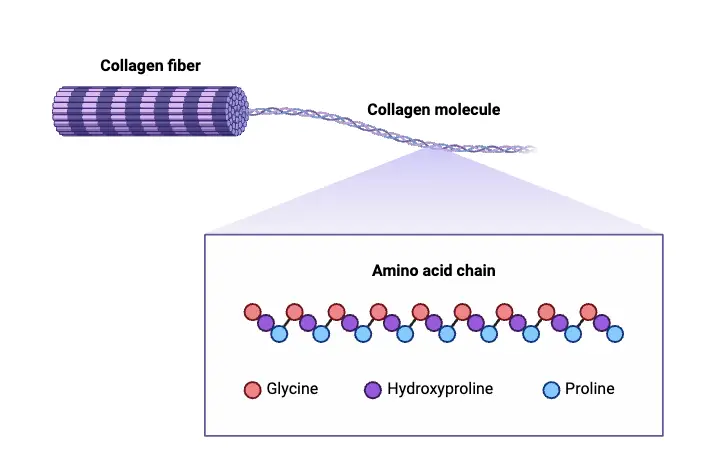
Structure and Composition of Collagen
Collagen is a fundamental structural protein found in various tissues throughout the body, characterized by its unique triple helical structure. The understanding of collagen’s composition and organization has evolved significantly, leading to insights into its functional properties and roles in biological systems.
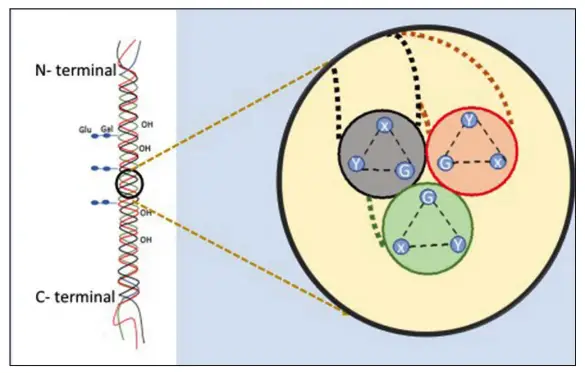
- Molecular Composition: Collagen is primarily composed of three polypeptide chains, which can be either identical (homotrimers) or different (heterotrimers). This variability is observed in various types of collagen, such as Types I, II, and IV. The polypeptide chains exhibit a repetitive amino acid sequence that is crucial for their structural integrity. Specifically, every third amino acid is glycine (Gly), while the X and Y positions are often occupied by proline and hydroxyproline.
- Triple Helix Structure: The widely accepted model, known as the Madras model, describes collagen as a right-handed triple helix formed by three coiled α-polypeptide chains. This helical structure is stabilized by intramolecular hydrogen bonds, primarily involving 4-hydroxyproline. The arrangement of these hydrogen bonds contributes to the overall stability and resilience of the collagen molecule.
- Hydrogen Bonding: Initial studies by Astbury and Bell suggested a simpler structure with amide bonds, while later work by Pauling and Corey indicated the involvement of hydrogen bonds. More recent research has shown that each triplet in the collagen sequence is associated with two hydrogen molecules, contributing to its structural integrity.
- Staggered Arrangement: Collagen molecules exhibit a stagger of approximately 65 nm between adjacent rows. This quarter stagger arrangement is essential for the characteristic 65 nm banding seen under an electron microscope, providing insight into the organization of collagen fibers in tissues.
- Carbohydrate Content: Collagen also contains a small amount of carbohydrates, specifically glucose-galactose disaccharides, although the amount varies depending on the type of collagen. This carbohydrate presence is significant for the overall function and stability of the collagen structure.
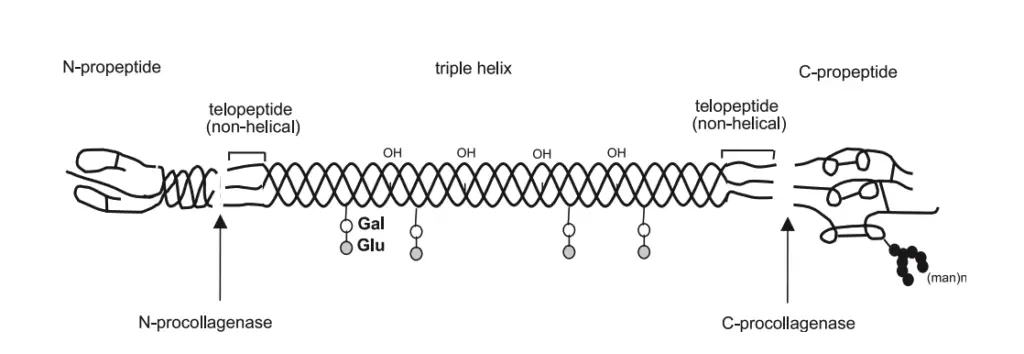
- Non-collagenous Domains: The collagen structure includes non-collagenous domains, which play a critical role in maintaining the stability of the molecule. These domains are located at the C-terminal and N-terminal ends of the molecule. The C-terminal is involved in initiating the formation of polypeptide chains, while the N-terminal regulates the diameter of collagen fibrils, thereby influencing the overall mechanical properties of collagen-rich tissues.
- Molecular Characteristics: The molecular weight of tropocollagen is approximately 300,000 Daltons, and it measures around 260 nm in length. The coiled structure of the polypeptide chains has a pitch of about 0.858 nm, with individual α-helices displaying a pitch of approximately 0.54 nm.
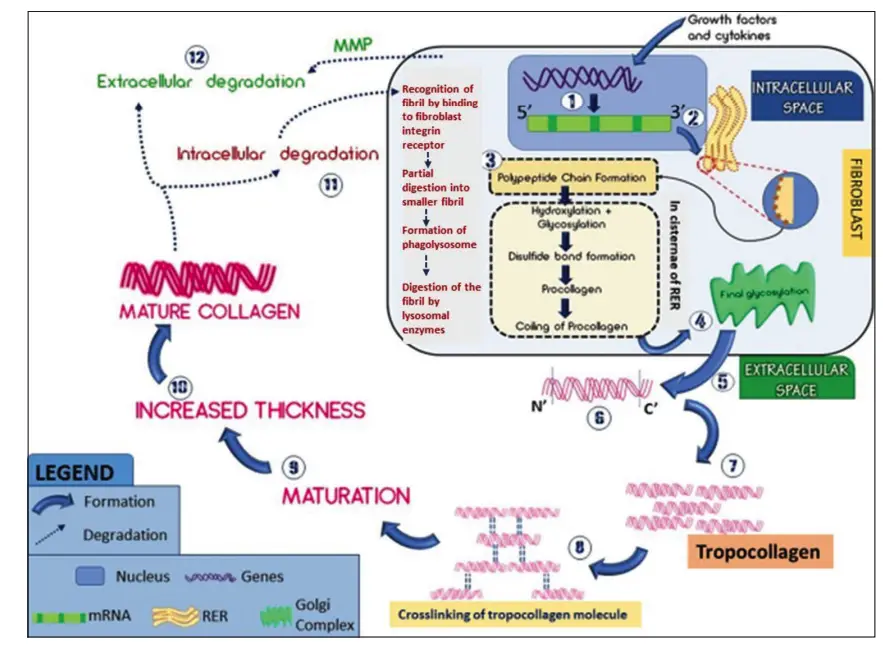
Synthesis and Degradation of Collagen
Collagen synthesis and degradation are vital processes that maintain the structural integrity and function of various tissues throughout the body. This dynamic interplay ensures that collagen is continually remodeled, which is crucial for normal physiological functions, as well as pathological conditions.
- Synthesis Process:
- Initiation: The synthesis of collagen begins in the nucleus, where various exons of collagen genes are transcribed into messenger RNA (mRNA). This process is regulated by growth factors and cytokines.
- mRNA Processing: The mRNA is then transported to the cytoplasm, where it is translated on the ribosomes of the rough endoplasmic reticulum (RER). This translation leads to the formation of three α-polypeptide chains.
- Post-Translational Modifications: Once formed, these polypeptide chains enter the cisternae of the RER, where they undergo critical hydroxylation of proline and lysine residues facilitated by the enzymes prolyl hydroxylase and lysyl hydroxylase. This step is dependent on the presence of Vitamin C. Additionally, some hydroxylysine residues are glycosylated through the action of galactosyltransferase.
- Formation of Procollagen: The chains are then aligned and stabilized by disulfide bonds, created with the help of disulfide isomerase. This results in the formation of procollagen, which is significantly longer than the final collagen product.
- Final Modifications in Golgi Complex: The procollagen is transported to the Golgi complex, where a final glycosylation occurs, adding glucose to O-linked galactose residues.
- Secretion: The modified procollagen is packaged into secretory granules and transported out of the trans face of the Golgi complex into the extracellular space.
- Cleavage of Procollagen: Extracellularly, the C-terminal and part of the N-terminal of the procollagen are cleaved by specific proteinases, resulting in the formation of tropocollagen, which features a 5-unit quarter stagger arrangement.
- Cross-Linking: Stabilization of the collagen molecules occurs through the oxidation of lysine and hydroxylysine residues by the enzyme lysyl oxidase, leading to cross-linking between the collagen fibrils.
- Degradation Process:
- Physiological Significance: The degradation of collagen is essential for tissue remodeling, development, and repair, as well as in pathological processes such as tumorigenesis and metastasis.
- Intracellular Degradation: This mechanism primarily involves the recognition of collagen fibrils by fibroblast integrin receptors. The process includes partial digestion into smaller fibrils, formation of phagolysosomes, and digestion of these fibrils by lysosomal enzymes.
- Extracellular Degradation: In the extracellular environment, collagen is degraded through the action of matrix metalloproteinases (MMPs), which are secreted by various cells, including fibroblasts and inflammatory cells. These enzymes selectively cleave different types of collagen and are categorized into specific classes, such as collagenases and gelatinases.
- Regulation of Degradation: The activity of MMPs is regulated by tissue inhibitors of metalloproteinases (TIMPs), which bind to the active sites of MMPs to inhibit their function, thus ensuring a balance between collagen synthesis and degradation.
Location of Collagen
Collagen is a fundamental structural protein found throughout the body, particularly within various tissues that contribute to the integrity and function of organs. It plays a vital role in both hard and soft tissues, with distinct types of collagen located in specific anatomical areas.
- Maxillofacial Region:
- This area encompasses a variety of hard and soft tissues, including bone, connective tissue, muscles, tendons, cartilage, and oral mucosa. Collagen is a principal component in all these structures, contributing significantly to their strength and flexibility.
- Bone:
- Bone is composed of approximately 22-25% organic material, with 94-98% of this organic component being Type I collagen. This collagen interacts with mineral components, resulting in a robust structure that is harder than cartilage yet retains flexibility.
- Tendons and Ligaments:
- Tendons are primarily made of Type I collagen, which constitutes about 75% of their dry weight. This collagen is organized into elongated fibrils, allowing tendons to effectively transmit forces between muscles and bones. Ligaments also contain a significant amount of collagen, particularly Type I, contributing to their tensile strength.
- Cartilage:
- In contrast to bone and tendons, cartilage is mainly composed of Type II collagen. This type of collagen provides the necessary structural support and resilience to cartilage, which is crucial for joint function.
- Dentin:
- Dentin, a vital component of teeth, consists of 70% inorganic material, 20% organic material, and 10% water by weight. Of the organic material, around 20% is Type I collagen, which serves as a scaffold for mineralization, supporting the overall structure of the tooth.
- Pulp:
- The dental pulp is a loose connective tissue rich in Type I and Type III collagen, forming part of the extracellular matrix (ECM) along with the ground substance. The collagen content in pulp increases with age, contributing to the fibrotic changes observed over time.
- Cementum:
- Cementum, a calcified tissue covering the tooth roots, contains 45-50% hydroxyapatite and 50-55% organic material. Type I collagen predominates in this tissue, accompanied by Types III, V, VI, and XII in smaller proportions. The similarities in amino acid composition of collagen in dentin, alveolar bone, and cementum suggest a related function in tooth support.
- Periodontal Ligament (PDL):
- The PDL, which connects teeth to the surrounding alveolar bone, is primarily composed of Type I collagen (70%). Other types, including Type II, III, and XII, are also present. The collagen fibers in the PDL are arranged into distinct bundles known as principal fibers, allowing the ligament to adapt to functional changes.
- Oral Mucosa:
- The oral mucosa is structured with a stratified squamous epithelium and an underlying connective tissue layer called the lamina propria, which contains Type I and Type III collagen. The lamina propria provides structural support to the oral epithelium, allowing it to function effectively.
- Skin:
- Skin is predominantly composed of Type I collagen, which constitutes about 70% of its collagen content, while Type III collagen accounts for 10%. Other collagen types, such as IV, V, VI, and VII, are present in trace amounts. The collagen in the skin is critical for maintaining firmness and elasticity.
Stains for Collagen
Staining techniques are crucial for visualizing collagen within various tissues during histopathological evaluations. While hematoxylin and eosin (H&E) staining is commonly used to observe general tissue architecture, it may not adequately differentiate collagen fibers from other structures like keratin and muscle. Therefore, specialized stains are employed to specifically highlight collagen, enhancing the accuracy of tissue assessment.
- Hematoxylin and Eosin (H&E):
- This is the routine staining method in histopathology, effective for visualizing numerous tissue components under light microscopy. However, it often fails to distinctly differentiate collagen from other fibrous components.
- Specialized Collagen Stains:
- A variety of special stains are utilized to provide clearer visualization of collagen fibers. These stains can be categorized by the color they impart to collagen:
- Van Gieson Stain: Stains collagen red, providing a vivid contrast against other tissue components.
- Periodic Acid-Schiff (PAS): Along with Weigert’s resorcin-fuchsin and Wilder’s modification of Bielschowsky’s stain, these impart varying shades of pink to collagen fibers, facilitating their identification.
- Masson’s Trichrome Stain: Typically stains collagen blue or green, allowing for effective differentiation from muscle and other tissues.
- Lillie’s Modification of Masson’s Trichrome: Similar to Masson’s, it enhances the visualization of collagen in a blue hue.
- Goldner’s Trichrome: Also stains collagen blue or green, providing another option for highlighting these fibers.
- Martius Scarlet Blue Stain: Differentiates collagen fibers effectively through its distinctive color coding.
- Gomori’s Trichrome Stain: Another method that stains collagen blue or green, useful for highlighting connective tissue components.
- A variety of special stains are utilized to provide clearer visualization of collagen fibers. These stains can be categorized by the color they impart to collagen:
- Advanced Microscopy Techniques:
- In addition to standard light microscopy, polarizing microscopy offers enhanced capabilities to identify and analyze collagen fibers. This technique leverages the optical properties of collagen, making it easier to observe structural details.
- Electron Microscopy: This advanced method provides intricate details regarding the size, height, and shape of collagen fibrils, contributing to a deeper understanding of their structural organization.
- Transmission Electron Microscopy (TEM): Specifically useful for studying the protein and peptide composition of collagen, allowing researchers to investigate the molecular details of this essential protein.
Distribution, Structure, and Function of Different Collagen Types
Collagen is an essential protein in the body, forming the structural framework of various tissues. Its distribution, structure, and function vary significantly among different types, which can be classified into fibril-forming collagens and other specialized types. The primary types include I, II, III, V, and XI, along with several others like IX, XII, XIV, IV, X, and VIII.
Fibril-Forming Collagens: Types I, II, III, V, and XI
- Type I Collagen
- This is the most abundant collagen type, constituting over 90% of the organic mass in bone.
- It is integral to the structure of tendons, skin, ligaments, and cornea.
- The collagen type I molecule is typically a heterotrimer formed from two identical α1(I)-chains and one α2(I)-chain.
- In tissues such as skin and tendons, Type I collagen often associates with Type III collagen, while in bone, it combines with Type V collagen to provide tensile strength and structural integrity.
- Type II Collagen
- Characterized as the predominant collagen in hyaline cartilage, Type II collagen comprises homotrimers of three α1(II)-chains.
- It accounts for approximately 80% of the total collagen content in cartilage and is also found in the vitreous body and intervertebral discs.
- Type II collagen is crucial for maintaining the biomechanical properties of cartilage, including load-bearing capacity and resilience, often forming heterofibrils with Types IX and XI to regulate fibril diameter.
- Type III Collagen
- This collagen type consists of a homotrimer of three α1(III)-chains and is primarily found in tissues that also contain Type I collagen, except for bone.
- It is a key component of reticular fibers within the lungs, liver, and blood vessels, supporting the structural integrity of these tissues.
- Type III collagen also contributes to mixed fibrils with Type I collagen, particularly in elastic tissues.
- Types V and XI Collagen
- Both are formed as heterotrimers comprising different α-chains (α1, α2, α3).
- Type V collagen typically forms heterofibrils with Types I and III, contributing to the organic matrix of bone and the interstitial matrix of various organs.
- Type XI collagen largely codistributes with Type II collagen in articular cartilage and plays a role in cartilage structure and integrity.
- The unique properties of these types stem from the presence of large non-collagenous domains, which are thought to influence the assembly and stability of collagen fibrils.
FACIT Collagens: Types IX, XII, and XIV
- Type IX Collagen
- This heterotrimer consists of three distinct chains (α1(IX), α2(IX), α3(IX)) and is associated with Type II collagen in cartilage and the vitreous body.
- It stabilizes Type II collagen fibrils through covalent cross-links and interacts with proteoglycans, facilitating the organization of the extracellular matrix.
- Types XII and XIV Collagen
- Both types associate with Type I collagen in a variety of tissues, including skin and tendons.
- Their roles in these contexts, although similar, involve contributing to the structural and functional integrity of the extracellular matrix.
Short-Chain Collagens: Types X and VIII
- Type X Collagen
- This homotrimeric collagen plays a critical role in hypertrophic cartilage, particularly during fetal and juvenile development.
- It is involved in endochondral ossification and matrix calcification, highlighting its importance in skeletal development.
- Type VIII Collagen
- Similar in structure to Type X, Type VIII collagen is produced by endothelial cells and assembles into hexagonal lattices.
- It is predominantly found in the Descemet’s membrane of the cornea, indicating its specialized structural functions in vascular and corneal tissues.
Basement Membrane Collagen: Type IV
- Type IV Collagen
- This collagen type is a fundamental component of basement membranes, providing a stable matrix through interactions with laminins and nidogens.
- It features a flexible triple helix and is composed of six subunit chains (α1(IV) to α6(IV)), which form distinct heterotrimers crucial for tissue integrity.
- Type IV collagen is vital for the function of glomerular and alveolar basement membranes, with mutations in its subunits being associated with various diseases.
Functions of Collagens
Collagens are vital structural proteins that play numerous roles in the body, significantly contributing to the maintenance of tissue integrity and function. Their presence is essential in various organs and connective tissues, acting as a key component of the extracellular matrix (ECM).
- Structural Support:
- Collagens provide the primary structural framework for parenchymal organs, forming the major component of the interstitial matrix and basement membranes. In connective tissues such as bone and cartilage, collagens serve as the functional backbone, offering strength and resilience.
- Cellular Microenvironment:
- The presence of collagens, particularly collagen VI, is crucial for establishing a defined pericellular microenvironment. This microenvironment supports cellular integrity and functionality, as evidenced in articular cartilage and potentially in bone.
- Cell-Cell and Cell-Matrix Interactions:
- Collagens facilitate interactions between cells and the ECM through specific receptors, including integrins, discoidin-domain receptors, glycoprotein VI, and specialized proteoglycan receptors. These interactions mediate various cellular functions, such as adhesion, differentiation, growth, and survival.
- Growth Factor and Cytokine Storage:
- Collagens play a significant role in the entrapment, local storage, and delivery of growth factors and cytokines. This is essential during organ development, wound healing, and tissue repair. For instance, collagen type I can bind decorin, indirectly modulating TGF-β action within tissues.
- Reservoirs of Growth Factors:
- In bone tissue, collagens act as reservoirs for insulin-like growth factors (IGF-I and IGF-II). The degradation of the collagen matrix by osteoclasts during bone remodeling releases these matrix-bound growth factors, stimulating osteoblastic activity and promoting new bone formation through paracrine signaling. Similar processes may occur in articular cartilage, where the degradation of collagen may activate chondrocytes through the release of bound growth factors.
- Transport Vehicles for Therapeutic Factors:
- Due to their ability to bind various growth factors and cytokines, collagens can serve as effective transport vehicles for the delivery of therapeutic factors. This property underscores their potential utility in medical and pharmaceutical applications.
- Regulation of Angiogenesis and Tumorigenesis:
- Recent studies have identified collagens, particularly non-collagenous fragments of collagens IV, XV, and XVIII, as influential in angiogenesis and tumorigenesis. These fragments, known as matricryptins, can affect various cellular responses and have garnered interest for their potential pharmaceutical applications.
Collagen-Related Disorders
Collagen-related disorders arise from abnormalities in collagen structure and function, often due to improper folding of collagen molecules or specific amino acid substitutions. These disorders can manifest in various forms, affecting different tissues and systems within the body. The following outlines several notable collagen-related disorders:
- Ehlers-Danlos Syndrome (EDS):
- Ehlers-Danlos syndrome encompasses a group of connective tissue disorders characterized by hyperelastic skin, joint hypermobility, and tissue fragility. This condition is associated with mutations affecting collagen synthesis or processing, particularly Type IV collagen, leading to weakened connective tissues and increased susceptibility to injury.
- Alport Syndrome:
- Alport syndrome is primarily linked to mutations in Type IV collagen, which is crucial for the structural integrity of basement membranes. This genetic disorder is characterized by progressive renal disease, hearing loss, and eye abnormalities, reflecting the widespread role of Type IV collagen in various tissues.
- Osteogenesis Imperfecta (OI):
- More commonly known as brittle bone disease, osteogenesis imperfecta results from defects in Type I collagen, which is essential for bone strength and integrity. Individuals with OI experience recurrent fractures, bone deformities, and in some cases, additional features such as blue sclera and dental issues. The severity of OI varies, depending on the specific mutations in the collagen genes.
- Chondrodysplasias:
- Chondrodysplasias refer to a group of skeletal disorders caused by abnormalities in cartilage and bone development, predominantly involving Type II collagen. These conditions can lead to disproportionate growth, resulting in skeletal deformities and impaired mobility.
- Atopic Dermatitis:
- Atopic dermatitis, also known as eczema, is a chronic inflammatory skin condition associated with abnormalities in Type III collagen, among other factors. The disorder is characterized by dry, itchy skin and can lead to significant discomfort and secondary infections due to skin barrier dysfunction.
- Gelse, K. (2003). Collagens—structure, function, and biosynthesis. Advanced Drug Delivery Reviews, 55(12), 1531–1546. doi:10.1016/j.addr.2003.08.002
- Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929-58. doi: 10.1146/annurev.biochem.77.032207.120833. PMID: 19344236; PMCID: PMC2846778.
- Wu M, Cronin K, Crane JS. Biochemistry, Collagen Synthesis. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507709/
- https://byjus.com/neet/what-is-collagen/
- https://jglobaloralhealth.org/collagen-structure-function-and-distribution-in-orodental-tissues/