Chemical Method of sterilization
- It involves the use of chemical agents to control the growth of microorganisms.
- Chemicals also are employed to prevent microbial growth in food, and certain chemicals are used to treat infectious diseases.
- Chemical agents can be employed for sterilization, disinfection, and antisepsis. All these chemical agents (e.g., alcohols, aldehydes, etc.) are described later in detail.
- The use of chemical agents to kill or inhibit the growth of microorganisms within host tissue is known as Chemotherapy.
- Some examples of chemical agents are; Halogens, Phenolics, Alcohols, Quaternary Ammonium Compounds, Aldehydes, Sterilizing Gases, Heavy Metals.
Characteristics of an ideal Chemical Antimicrobial agent or disinfectant
A chemical antimicrobial agent should contain the following characteristics;
- Antimicrobial activity: The capacity of the Antimicrobial agent to kill or inhibit microbes is the first requirement. The chemical should have a broad spectrum of antimicrobial activity at a low concentration.
- Solubility: The antimicrobial agent must be soluble in water or other solvents to the extent necessary for effective use.
- Stability: Changes in the substance upon standing should be minimal and should not result in significant loss of germicidal action.
- Non Toxicity to humans and other animals: Ideally, the compound should be lethal to microorganisms and non injurious to humans and other animals
- Homogeneity: The preparation must be uniform in composition so that active ingredients are present in each application. Pure chemicals are uniform, but mixtures of materials may lack homogeneity.
- Noncombination with extraneous organic material: Many disinfectants have an affinity for proteins or other organic material. When such disinfectants are used in situations where there is considerable organic material besides that of the microbial cells, little, if any, of the disinfectant will be available for action against the microorganisms.
- Toxicity to microorganisms at room or body temperatures: In using the compound, it should not be necessary to raise the temperature beyond that normally found in the environment where it is to be used.
- Capacity to penetrate: Unless the substance can penetrate through surfaces, its germicidal action is limited solely to the site of application. Sometimes, of course, surface action is all that is required.
- Nonorroding and nonstaining: It should not rust or otherwise disfigure metals nor stain or damage fabrics.
- Deodorizing ability: Deodorizing while disinfecting is a desirable attribute. Ideally, the disinfectant itself should either be odorless or have a pleasant smell.
- Detergent capacities: A disinfectant which is also a detergent (cleaning agent) accomplishes two objectives, and the cleansing action improves the effectiveness of the disinfectant.
- Availability: The compound must be available in large quantities at a reasonable price.
How to Select An Ideal Chemical Agent for Practical Application?
There are three major factors that need to be assessed in the process of selecting the most appropriate chemical agent for a specific practical application are:
1. Nature of the material to be treated
- The substance (Chemical Agent) selected must be compatible with the material to which it is applied.
- For example, a chemical agent used to disinfect contaminated utensils might be quite unsatisfactory for application to the skin; i.e., it might do serious injury to the tissue cells.
2. Types of microorganisms
The agent selected must be known to be effective against the type of organism to be destroyed. Chemical agents are not all equally effective against bacteria, fungi, viruses, and other microorganisms, because for the following reasons;
- Spores are more resistant than vegetative cells, that’s why the same chemical agent cannot be used to disinfect them, it will not work.
- Differences also exist between Gram-positive and Gram-negative bacteria: Escherichia coli is much more resistant to cationic disinfectants than Stophylococcus aureus.
- Differences in action also exist between strains of the same species.
3. Environmental conditions
- Environmental factors such as temperature, pH, time, concentration, and presence of extraneous organic material, may all have a bearing on the rate and efficiency of antimicrobial action.
- The successful use of an antimicrobial agent requires an understanding of the influence of these conditions on the particular agent, so it can be employed under the most favorable circumstances.
Agents used for Chemical Sterilization
There are different methods of Chemical Sterilization. All of these are classified based on the material on which the agent will be applied. The Chemical methods of Sterilization are;
- Phenolic compounds
- Halogens
- Alcohols
- Aldehydes
- Gases
- Synthetic Detergents
- active agents
- oxidizing agents
- Dyes
- Heavy metals
- Quaternary Ammonium Compounds
- Acids and alkalis
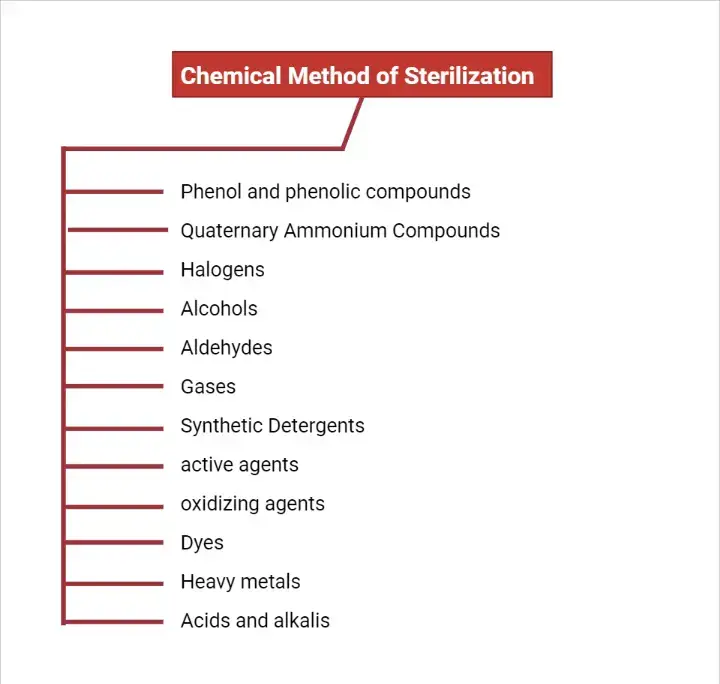
1. Phenol and phenolic compounds
- In 1867, Joseph Lister employed phenolic compounds to reduce the risk of infection during operations.
- Phenol was the first widely used antiseptic and disinfectant.
- Pure crystalline phenol is colorless.
- Phenolic compounds are the most widely used antiseptics and disinfectants in laboratories and hospitals worldwide.
- They are bactericidal or bacteriostatic and some are fungicidal also.
- They act by denaturing proteins and disrupting cell membranes.
- A 5% aqueous solution of phenol rapidly kills the vegetative cells of microorganisms spores are much more resistant.
- The antimicrobial activity of phenolics is reduced at an alkaline pH and by organic material.
- Low temperatures and the presence of soap also reduce the antimicrobial activity of phenolic compounds.
- One of the widely used phenolic derivatives is o-phenylphenol.
a. Mode of Action of phenolic compounds
- Depending upon the concentration of the phenolic compound it shows the following mode of action; disruption of cells, precipitation of cell protein, inactivation of enzymes, and leakage of amino acids from the cells.
- Although the specific mode of action is not clear, there is a consensus that the lethal effect is associated with physical damage to the membrane structures in the cell surface, which initiates further deterioration.
b. Practical Applications of phenolic compounds
- Aqueous solutions of from 2 to 5% can be employed to disinfect different materials such as sputum, urine, foces, and contaminated instruments or utensils.
- Derivatives of phenol are diluted in detergents or some other carrier and used in many commercial antiseptic and disinfectant preparations.
- A combination of compounds of o-phenylphenol with detergents results in products with good disinfectant as well as detergent properties.
c. Examples of phenolic compounds
- Phenol: It is powerful against vegetative forms of bacteria, Mycobacterium tuberculosis, and certain fungi. It is an excellent disinfectant for feces, blood, pus, sputum, etc. It has a low degree of activity as compared to other derivatives. It is not suitable for application to skin or mucous membranes.
- Cresol: Cresols are more germicidal and less poisonous than phenol but corrosive to living tissues. They are used for cleaning floors (1% solution), for disinfection of surgical instruments, and for disinfection of contaminated objects. Lysol is a solution of cresols in soap
- Halogenated diphenyl compounds: These compounds include hexachlorophene and chlorhexidine. They are highly effective against both Gram-positive and Gram-negative bacteria. They are used as skin antiseptics and for the cleaning of wound surfaces
- Hexachlorophene has been one of the most popular antiseptics because once applied it persists on the skin and reduces growth of skin bacteria for longer periods. However, it can cause brain damage and is now used in hospital nurseries only after a staphylococcal outbreak.
2. Alcohols as a Chemical Sterilizer
- Alcohol are among the most widely used disinfectants, antiseptics, and sanitizers.
- They are bactericidal and fungicidal but not sporicidal; some enveloped viruses are also destroyed.
- The two most popular alcohol germicides are ethanol and isopropanol.
- The germicidal power of alcohol progressively increases if the molecular weight of alcohol increases.
- Since alcohols of molecular weight higher than that of propyl alcohol are not miscible in all proportions with water, they are not commonly used in disinfectants.

a. Mode of Action of Alcohols
- Alcohols show their antimicrobial activity by denaturing the proteins of the microbial cells.
- Alcohols are also solvents for lipids, and hence they may damage lipid complexes in the cell membrane.
- They also act as a dehydrating agent.
b. Practical Applications of Alcohols
- Alcohol is effective in reducing the microbial flora of skin.
- Used for the disinfection of clinical oral thermometers.
- Alcohol concentrations above 60% are effective against viruses
c. Examples of Some Alcohols used as a chemical sterilizer
- Ethyl alcohol, CH3CH2OH, in concentrations between 50 and 90%, is effective against vegetative or non spore forming cells.
- For practical application a 70% concentration of alcohol is generally used.
- Methyl alcohol is less bactericidal as compared to ethyl alcohol; furthermore, it is highly poisonous. Even the fumes of this compound may produce permanent injury to the eyes, and is not generally employed for the destruction of microorganisms.
- The higher alcohols-propyl, butyl, amyl, and others are more germicidal than ethyl alcohol.
- Propyl and isopropyl alcohols in concentrations ranging from 40 to 80% are bactericidal for vegetative cells.
3. Halogens as a Chemical Sterilizer
- These agents are highly effective disinfectants and antiseptics, because they are microbicidal and not just microbiostatic.
- They are also sporicidal with longer exposure.
- Chlorine and iodine are the only two routinely used halogens because fluorine and bromine are dangerous to handle.
A. Iodine
- lodine is one of the oldest and most effective germicidal agents.
- Pure iodine is a bluish-black crystalline element having a metallic luster.
- It is only slightly soluble in water but readily soluble in alcohol and aqueous solutions of potassium or sodium iodide.
- It kills all types of microorganisms if optimum concentrations and exposure times are used.
- Iodine activity, unlike chlorine, is not as adversely affected by organic matter and pH.
a. Examples of Iodine preparations
The two primary iodine preparations are free iodine in solution and iodophors.
(i). Free iodine in solution
- Aqueous iodine contains 2% free iodine in solution and 2.4% sodium iodide.
- It is used as a topical antiseptic before surgery and also occasionally as a treatment for burnt and infected skin.
- A stronger iodine solution (5% iodine and 10% potassium iodide) is used primarily as a disinfectant for plastic items, rubber instruments, cutting blades, and thermometers.
- Iodine tincture is a 2% solution of iodine and sodium iodide in 70% alcohol that can be used in skin antisepsis. Because iodine can be extremely irritating to the skin and toxic when absorbed, strong aqueous solutions and tinctures (5–7%) are no longer considered safe for routine antisepsis.
- Iodine tablets are available for disinfecting water during emergencies or for destroying pathogens in impure water supplies.
(ii). Iodophors
- Iodophors are complexes of iodine and a neutral polymer, such as polyvinyl alcohol.
- This formulation permits the slow release of free iodine and increases its degree of penetration.
- These compounds have largely replaced free iodine solutions in medical antisepsis because they are less prone to staining or irritating tissues.
- Betadine, povidone, and isodine are the common iodophor compounds that contain 2–10% of available iodine. They are used to prepare skin and mucous membranes for surgery and in surgical hand scrubs.
- They are also used to treat burns and to disinfect equipment.
b. Mode of action of Iodine
- Iodine is an oxidizing agent, which irreversibly oxidizes and thus inactivates essential metabolic compounds such as proteins with sulfhydryl groups.
- It has also been suggested that the action may involve the halogenation of tyrosine units of enzymes and other cellular proteins requiring tyrosine for activity.
c. Practical Applications
- It is a highly effective bactericidal agent which is used against all kinds of bacteria.
- Iodine also possesses sporicidal activity; so, it is used to kill spores. However, the rate at which the spores are killed is markedly influenced by the conditions under which they are exposed, e.g., amount of organic material and extent of dehydration.
- In addition, it is highly fungicidal and is to some extent virucidal.
- Iodine solutions are chiefly used for the disinfection of skin.
- Iodine preparations are effective for other purposes, such as disinfection of water, disinfection of air iodine vapors, and sanitization of food utensils.
B. Chlorine and its compounds
- Chlorine, either in the form of gas or in certain chemical combinations, represents one of the most widely used disinfectants.
- The compressed gas in liquid form is almost universally employed for the purification of municipal water supplies.
- Chlorine kills not only bacterial cells and endospores but also fungi and viruses.
- Treatment of water with chlorine destroys many pathogenic vegetative microorganisms without unduly affecting its taste.
- Chlorination at a concentration of 0.6–1.0 part of chlorine per million parts of water makes water potable and safe to use.
a. Examples of Chlorine and its compounds
- There are available many compounds of chlorine which can be handled more conveniently than free chlorine and which, under proper conditions of use, are equally effective as disinfectants. One class of compounds in this category is the hypochlorites. Calcium hypochlorite, Ca(OCI)(also known as chlorinated lime), and sodium hypochlorite, NaOCI, are popular compounds.
- The chloramines represent another category of chlorine compounds used as disinfectants, sanitizing agents, or antiseptics
b. Mode of Actions of Chlorine and its compounds
- In solution, these compounds combine with water and release hypochlorous acid (HOCl), which oxidizes the sulfhydryl (S–H) group on the amino acid cysteine and interferes with disulfide (S–S) bridges on numerous enzymes. The resulting denaturation of the enzymes is permanent.
- The killing of microorganisms by chlorine and its compounds is also due in part to the direct combination of chlorine with proteins of the cell membranes and enzymes.
c. Practical Application of Chlorine and its compounds
- Chlorine compounds are very widely used to control microorganisms.
- They are also used in water treatment in the food industry, for domestic uses, and in medicine.
- Products containing calcium hypochlorite are used for sanitizing dairy equipment and eating utensils in restaurants.
- Solutions of sodium hypochlorite of a 1% concentration are used for personal hygiene and as a household disinfectant: higher concentrations of 5 to 12% are also employed as household bleaches and disinfectants and for use as sanitizing agents in dairy and food processing establishments.
- Chlorine compounds have been used to disinfect open wounds, to treat athlete’s feet, to treat other infections, and as a general disinfectant.
4. Heavy Metals and Their Compounds
- Most of the heavy metals, either alone or in certain compounds, exert a detrimental effect upon microorganisms.
- Soluble salts of mercury, silver, copper, arsenic, and other heavy metals have antibacterial activity, both bactericidal and bacteriostatic.
a. Examples of Heavy Metals and Their Compounds
- Mercury: Bactericidal in dilutions of 1:1,000: limited use because of corrosive action, high toxicity to animals, and reduction of effectiveness in presence of organic material: insoluble compounds, used in ointments as antiseptics.
- Silver: Consist of protein in combination with metallic silver or silver oxide (colloidal solution): bacteriostatic or bactericidal effect is a function of the free silver ions released from the combination; used as antiseptics, silver nitrate is the most widely used of these compounds, all of which are germicidal and employed as antiseptics in specific conditions; silver nitrate is bactericidal for most organisms at a dilution of 1:1.000; many states require that the eyes of newborns be treated with a few drops of 1% silvor nitrate solution to prevent ophthalmia neonatorum, a gonococcal infection of eyes.
- Copper: Much more effective against algae and molds than bacteria; 2 ppm in water sufficient to prevent algal growth: used in swimming pools and open water reservoirs: used in the form of Bordeaux mixture as a fungicide for prevention of certain plant diseases.
b. Mode of action of Heavy Metals and Their Compounds
- Heavy metals and their compounds act antimicrobial by combining with cellular proteins and inactivating them. For example, in the case of mercuric chloride the inhibition is directed at enzymes which contain the sulfhydryl grouping.
- High concentrations of salts of heavy metals like mercury, copper, and silver coagulate cytoplasmic proteins, resulting in damage or death to the cell.
5. Dye as a Chemical Sterilizer
There are two classes of dye that contain antimicrobial properties such as triphenylmethane and acridine dyes.
A. Triphenylmethane Dyes
- Some examples of Triphenylmethane Dyes are; malachite green, brilliant green, and crystal violet.
- Crystal violet will inhibit Gram-positive cocci at a dilution of 1:200,000 to 1:300,000; 10 times this concentration is required to inhibit Escherichia coli.
- Staphylococcus aureus is inhibited by malachite green at a concentration of 1:1,000,000; a concentration of about 1:30,000 is required to inhibit E. coli.
a. Mode of Action of Triphenylmethane Dyes
- The mode of action of triphenylmethane dyes is uncertain, but there is speculation that they exert their inhibitory effect by interfering with cellular oxidation processes.
b. Practical Applications of Triphenylmethane Dyes
- Certain selective media use these dyes (crystal violet, brilliant green or malachite green) at a low concentration (about 1:100,000) to inhibit the growth of Gram-positive bacteria.
- Susceptibility to various dyes can also be used for the identification of bacteria.
- Crystal violet has also been used as a fungicide. A concentration of 1:10,000 is lethal for Monilia and Torula, and a concentration of 1:1,000,000 is inhibitory.
B. Acridine Dyes
- Two examples of dyes derived from acridine are acriflavine and tryptoflavine.
- These compounds exhibit selective inhibition against bacteria, particularly staphylococci and gonococci.
- Gonococci are inhibited by tryptoflavine in dilutions of 1:10,000,000 to 1:50,000,000.
a. Practical Applications
- They are used to some extent for the treatment of burns and wounds and for ophthalmic application and bladder irrigation.
6. Synthetic Detergents or Surface active agents
- Surface active agents, such as detergents are the substances that alter energy relationship at interfaces producing a reduction in surface tension.
- Detergents are organic molecules that serve as wetting agents and emulsifiers because they have both polar hydrophilic and nonpolar hydrophobic ends. Due to their amphipathic nature, detergents solubilize and are very effective cleansing agents
a. Example of Synthetic Detergents
Surface active agents are of four types:
- Cationic surface active agents
- Anionic surface active agents
- Nonionic surface active agents
- Amphoteric or ampholytic compounds
(i). Cationic surface active agents
- The cationic detergents are effective disinfectants.
- Cationic detergents like benzalkonium chloride and cetylpyridinium chloride kill most bacteria but not M. tuberculosis, endospores, or viruses.
- They do have the advantages of being stable and nontoxic, but they are inactivated by hard water and soap.
- These are often used as skin antiseptics and also as disinfectants for disinfection of food utensils and small instruments.
- Quaternary ammonium compounds, such as cetrimide are the most popular cationic detergents.
- They act by disrupting microbial membranes and possibly by denaturing proteins
(ii). Anionic surface active agents
- These include soaps prepared either from saturated or unsaturated fatty acids, which act better at acidic pH.
- The soaps prepared from saturated fatty acids are more effective against Gram-negative organisms, whereas those prepared from unsaturated fatty acids are more active against Gram-positive bacilli and Neisseria.
(iii). Nonionic surface active agents
- These are nontoxic and some of them may even promote the growth of bacteria.
(iv). Amphoteric or ampholytic compounds
- These are active against a wide range of Gram-positive and Gram-negative bacteria and also against a few viruses. These are known as “Tego” compounds.
b. Mode of action of Synthetic Detergents
- Soaps reduce surface tension and thereby increase the wetting power of the water in which they are dissolved.
- Soapy water has the ability to emulsify and disperse oils and dirt.
- The microorganisms become enmeshed in the soap lather and are removed by the rinse water.
- Various chemicals have been incorporated into soaps to enhance their germicidal activity.
c. Application of Synthetic Detergents
- Synthetic Detergents are extensively used in laundry and dishwashing powders, shampoos, and other washing preparations.
- Some of them are used as bactericidal.
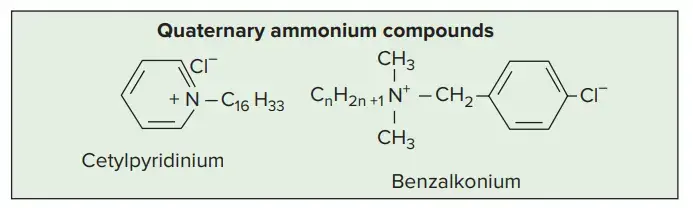
7. Quaternary Ammonium Compounds
- Quaternary ammonium compounds have broad spectrum antimicrobial activity and are effective disinfectants used for decontamination purposes.
- It has a low toxicity, high solubility in water, stability in solution, and noncorrosiveness.
- Most compounds of the germicidal cationic-detergent class are quaternary ammonium salts.
- The bactericidal power of the quaternaries is exceptionally high against Grampositive bacteria, and they are also quite active against Gram-negative organisms.
- Quaternaries have been shown to be fungicidal as well as destructive to certain of the pathogenic protozoa.
a. Mode of action of Quaternary Ammonium Compounds
- There are different Mode of action of Quaternary Ammonium Compounds, these are include denaturation of proteins, interference with glycolysis, and membrane damage.
- Experimental evidence suggests that the most likely site of the damage to the cell is the cytoplasmic membrane; the quaternaries alter the vital permeability features of this cell structure.
b. Practical Applications of Quaternary Ammonium Compounds
- Used as disinfectants and sanitizing agents.
- They are used as skin disinfectants, as a preservative in ophthalmic solutions, and in cosmetic preparations.
- Quaternaries are widely used for control of microorganisms on floors, walls, and other surfaces in hospitals, nursing homes, and other public places.
- They are used to sanitize food and beverage utensils in restaurants as well as surfaces and certain equipment in food processing plants.
- Other applications are to be found in the dairy, egg, and fishing industries to control microbial growth on surfaces of equipment and the environment in general.
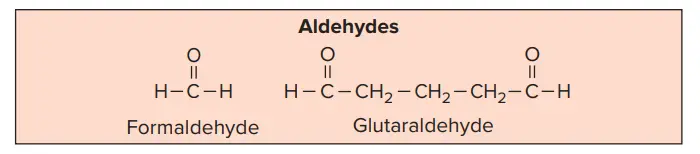
8. Aldehydes as a Chemical Sterilizer
- Formaldehyde and glutaraldehyde are the two most commonly used aldehydes that are used as disinfectants.
- They are highly reactive molecules that combine with nucleic and alkylating molecules.
- They are sporicidal and can also be used as chemical sterilants.
A. Formaldehyde
- Formaldehyde (HCHO) is the simplest compound in the aldehyde series.
- It is a gas that is stable only in high concentrations and at elevated temperatures.
- At room temperature it polymerizes, forming a solid substance.
- The important polymer is paraformaldehyde, a colorless substance which rapidly yields for maldehyde upon heating.
- Formaldehyde is also marketed in aqueous solution as formalin, which contains 37 to 40% formaldehyde.
- The fumes of formaldehyde are noxious: they are irritating to tissues and eyes.
a. Mode of action of Formaldehyde
- Formaldehyde is an extremely reactive chemical, It combines readily with vital organic nitrogen compounds such as proteins and nucleic acids.
- It is likely that the interaction of formaldehyde with these cellular substances accounts for its antimicrobial action.
b. Practical Application of Formaldehyde
- The solution of Formaldehyde is useful for sterilization of certain instruments.
- Formaldehyde in gaseous form can be used for disinfection and sterilization of enclosed areas. Formalin and paraformaldehyde are two principal sources of formaldehyde when it is used for gaseous disinfection. Vaporization of formaldehyde from either of these sources into an enclosed area for an adequate time will cause sterilization, vegetative cells being killed more quickly than spores.
- Used to Preserve fresh tissue specimens.
- Used to Destroy anthrax spores in hair and wool.
- Used to Prepare toxoids from toxins.
- Used to Sterilize bacterial vaccines.
- Used to Kill bacterial cultures and suspensions.
B. Glutaraldehyde
- Glutaraldehyde is a saturated dialdehyde.
- A 2% solution of this chemical agent exhibits a wide spectrum of antimicrobial activity.
- It is effective against vegetative bacteria, fungi, bacterial and fungal spores, and viruses.
- Glutaraldehyde usually disinfects objects within time frame of 10 minutes but may require as long as 12 hours to destroy all spores.
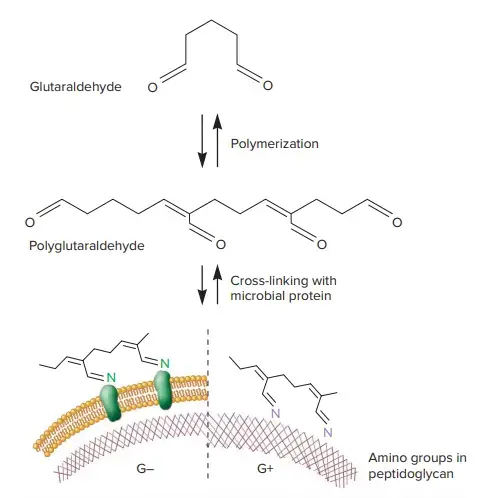
a. Mode of Action of Glutaraldehyde
Glutaraldehyde polymerizes and then interacts with amino acids in proteins (left in below image) or in peptidoglycan (right in below image). As a result, the proteins are alkylated and cross-linked to other proteins, which inactivates them. The amino groups in peptidoglycan are also alkylated and cross-linked, which prevents them from participating in other chemical reactions such as those involved in peptidoglycan synthesis.
b. Practical Application of Glutaraldehyde
- It is used in the medical field for sterilizing urological instruments, lensed instruments, respiratory therapy equipment, and other special equipment.
- It can be used for cleaning cystoscopes and bronchoscopes, corrugated rubber anesthetic tubes and face masks, plastic endotracheal tubes, metal instruments, and polythene tubing.
9. Gases Agents as a Chemical Sterilizer
- Various gaseous agents are used for sterilization of large volume of heat-sensitive disposable items and also instruments.
- Some examples of gaseous agents are Ethylene oxide, formaldehyde gas, and betapropiolactone.
a. Ethylene oxide
- Ethylene oxide is a colorless liquid used for gaseous sterilization.
- It is active against all kinds of bacteria, spores, and viruses.
- It kills all types of microorganisms by inhibiting proteins and nucleic acids.
- It is both microbicidal and sporicidal.
- It is a highly effective sterilizing agent because it rapidly penetrates packing materials, including plastic wraps.
- It is used to sterilize disposable plastic Petri dishes, sutures, syringes, heart-lung machine, respirators, and dental equipments.
- Ethylene oxide is highly inflammable and carcinogenic.
- Extensive aeration of the sterilized materials is necessary to remove residual ethylene oxide gas, which is toxic.
b. Formaldehyde gas
- The formaldehyde gas is used for (a) the fumigation of operation theaters, wards, sick rooms, and laboratories; and (b) the sterilization of instruments and heat-sensitive catheters, clothing and bedding, furniture, books, etc.
- The formaldehyde gas is produced by adding 150gm of potassium permanganate to 280 mL formalin in 1000cu ft of room volume.
- The room to be sterilized is completely closed and sealed at least for 48 hours after fumigation with formalin gas.
- Sterilization is achieved by condensation of gas on exposed surface.
- The gas is toxic when inhaled and is irritant to eye, hence its effect should be nullified by exposure to ammonia.
- It is highly inflammable and carcinogenic.
c. Beta-propiolactone
- Beta-propiolactone (BPL) is a condensation product of ketone and formaldehyde.
- It is active against all microorganisms and viruses.
- It is more efficient than formaldehyde for fumigation purpose.
- In the liquid form, it has been used to sterilize vaccines and sera.
- BPL destroys microorganisms more readily than ethylene oxide but does not penetrate materials well and may be carcinogenic. For these reasons, BPL has not been used as extensively as ethylene oxide.
- Recently, vapor-phase hydrogen peroxide has been used to decontaminate biological wastes.
A. Practical Application of Gases Agents
- Ethylene oxide used as an effective sterilizing agent for heat- and moisture-sensitive materials.
- The varieties of materials on which it is used include spices, biological preparations, soil, plastics, certain medical preparations, and contaminated laboratory equipment.
- It has been used in the space program by both the Americans and the Russians for decontaminating spacecraft components.
10.Acids and alkalis as Chemical Sterilizer
- Acids (such as sulfuric acid, nitric acid, hydrochloric acid, and benzoic acid) and alkalis (like potassium and sodium hydroxide and ammonium hydroxide) are germicidal in nature.
- They kill microorganisms by hydrolysis and altering the pH of the medium.
- They are rarely used as disinfectants.
- Organic acids are widely used in food preservation because they prevent spore germination and bacterial and fungal growth, and because they are generally regarded as safe to eat.
- Acetic acid, in the form of vinegar, is a pickling agent that inhibits bacterial growth.
- Propionic acid is commonly added into breads and cakes to retard molds; lactic acid is added to sauerkraut and olives to prevent growth of anaerobic bacteria, especially the clostridia; and benzoic and sorbic acids are added to beverages, syrups, and margarine to inhibit yeasts.
Factors Influencing Activity of Disinfectants
The following factors can influence the activity of Disinfectants;
- Temperature: Increase in temperature increases the efficiency of disinfectants.
- Type of microorganism: Vegetative cells are more susceptible than spores. Spores may be resistant to the action of disinfectants.
- Physiological state of the cell: Young and metabolically active cells are more sensitive than old dormant cells. Nongrowing cells may not be affected.
- Environment: The physical or chemical properties of the medium or substance influence rate as well as efficiency of disinfectants, e.g., pH of the medium and presence of extraneous materials.