What is Alternative pathway of the complement system ?
- The alternative pathway (AP) serves as a dominant component of the complement system, surpassing the classical and lectin pathways under normal physiological conditions. Unlike the other pathways, the AP is consistently active at a low level, wherein the hydrolysis of C3 to C3b occurs. This process, known as “tick over,” generates modest amounts of C3b, which can be further amplified if necessary.
- Playing a vital role in the innate immune system, the alternative pathway functions as a cascade reaction within the complement system, providing a natural defense against various infections. It acts as one of the three complement pathways responsible for opsonizing and eliminating pathogens.
- Activation of the alternative pathway occurs when the C3b protein directly binds to a microbe, initiating a series of events. Additionally, foreign biological and artificial surfaces, such as carbohydrates, lipids, proteins, and gas bubbles, can trigger this pathway. Moreover, damaged tissues are also capable of activating the alternative pathway, contributing to the immune response.
- The alternative pathway, with its unique ability to recognize and respond to diverse triggers, ensures the efficient elimination of pathogens and foreign materials from the body. By understanding the mechanisms and functions of this pathway, researchers and healthcare professionals can explore its potential applications in combating infections and enhancing immune responses.
Activators of the alternative pathway
Activators of the alternative pathway of complement activation include
- IgA,
- IgD,
- bacterial endotoxin,
- cobra venom factor,
- nephritic factor.
Steps of activation of alternative pathway
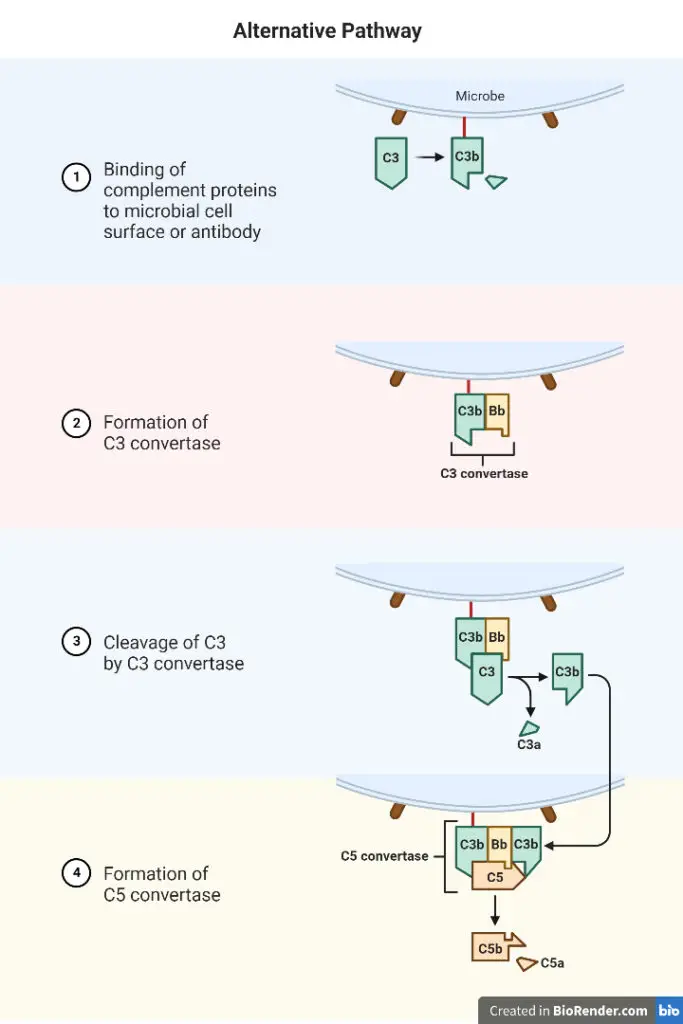
Four serum proteins comprise the initial component of the alternative pathway: C3b, factor B, factor D, and properdin.
1. C3bB complex Formation
- C3b and factor B combine to generate the C3bB complex. Mg2 stabilises the connection between C3b and factor B and is the only ion necessary for functional activation of the alternative pathway.
- In order to distinguish between the two complement activation routes, tests are frequently predicated on the selective chelation of Ca2 (to disrupt C1q, C1r, and C1s2) and the addition of sufficient Mg2 to allow activation of the alternate pathway.
2. C3bB Cleavage
- By another serum protein called factor D or C3 proactive convertase, the C3bB is divided into two fragments, Ba and Bb.
- Since factor D has never been isolated in its proenzyme form, it is often thought that it is activated as soon as it leaves the hepatocyte where it is generated.
- Ba is released into the medium, while Bb binds to C3b to create the C3bBb complex, which has C3 convertase activity.
3. Formation of C3bBb
- The C3bBb complex activates additional C3, which results in the creation of additional C3bBb, which is capable of activating C5 and the MAC.
- The C3bBb complex has a half-life of only 5 minutes, but upon binding with properdin, it produces the relatively heat-stable PC3bBb complex.
4. Formation of MAC
- Similarly to the traditional method, the alternative pathway continues from C3 to yield MAC as an end product.
Regulators of the Alternative Pathway
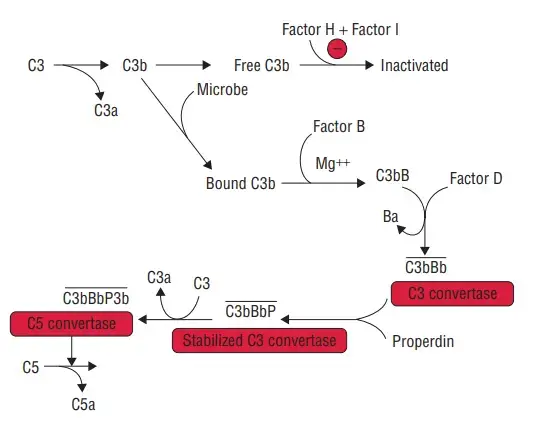
Regulation plays a crucial role in maintaining the delicate balance of the alternative pathway of complement activation. One of the key players in this regulatory network is C3b, which can bind to either host cells or pathogen surfaces in the plasma. However, to prevent unwanted complement activation on host cells, a variety of regulatory proteins come into action.
Two important regulators in this process are Complement Receptor 1 (CR1 or CD35) and Decay Accelerating Factor (DAF), also known as CD55. These proteins compete with Factor B in binding to C3b on the cell surface. Not only can they prevent the formation of a C3 convertase, but they can also remove Bb from an already formed C3bBb complex. By doing so, CR1 and DAF effectively disrupt the complement activation process on host cells.
Another regulator, known as Complement Factor I, plays a crucial role in preventing the formation of a C3 convertase. Factor I acts as a plasma protease that cleaves C3b into its inactive form, iC3b. However, Factor I requires a C3b-binding protein cofactor to exert its activity. Proteins such as Complement Factor H, CR1, or Membrane Cofactor of Proteolysis (MCP or CD46) serve as cofactors for Factor I. By binding to C3b, these cofactors facilitate the cleavage of C3b by Factor I, ensuring the inactivation of C3b and preventing the cascade of complement activation.
Complement Factor H, in particular, plays multiple roles in regulating the alternative pathway. It competes with Factor B for binding to C3b, inhibiting the formation of the C3 convertase. Additionally, it accelerates the decay of the C3 convertase, further halting the activation process. Moreover, Complement Factor H acts as a cofactor for Factor I-mediated cleavage of C3b, enhancing the inactivation of C3b. Notably, Complement Factor H exhibits a preference for binding to vertebrate cells due to its affinity for sialic acid residues. This preference allows Complement Factor H to provide preferential protection to host cells, safeguarding them from complement-mediated damage while targeting bacterial cells.
Another noteworthy regulator is CFHR5 (Complement Factor H-Related protein 5). CFHR5 acts as a cofactor for Factor I, aiding in the cleavage of C3b. Additionally, it possesses decay accelerating activity, which helps to prevent the excessive activation of the alternative pathway. CFHR5 also demonstrates a binding preference for C3b at host surfaces, further contributing to the regulation of complement activation on host cells.
Together, these regulators work harmoniously to maintain the balance of the alternative pathway. By interfering with key steps in the complement activation process, they ensure that host cells are protected from excessive complement-mediated damage, while still allowing the immune system to effectively target and eliminate pathogens.
- Complement Receptor 1 (CR1 or CD35): Competes with Factor B in binding to C3b on the cell surface.
- Decay Accelerating Factor (DAF or CD55): Competes with Factor B in binding to C3b and can remove Bb from an already formed C3bBb complex.
- Complement Factor I: Cleaves C3b into its inactive form, iC3b.
- C3b-binding protein cofactors: Required for the activity of Factor I. Includes Complement Factor H, CR1, or Membrane Cofactor of Proteolysis (MCP or CD46).
- Complement Factor H: Inhibits the formation of the C3 convertase by competing with Factor B for binding to C3b. It also accelerates the decay of the C3 convertase and acts as a cofactor for Factor I-mediated cleavage of C3b.
- CFHR5 (Complement Factor H-Related protein 5): Binds to C3b, acts as a cofactor for Factor I, possesses decay accelerating activity, and preferentially binds to C3b at host surfaces.
Alternative pathways to initiate alternative pathway
Three unique alternative route initiation pathways have been identified:
1. The alternative tickover pathway
- The mechanism that results in the spontaneous hydrolysis of C3 to C3(H2O) is known as the tickover pathway.
2. The alternative properdin-activated pathway
- Properdin, a serum protein, stimulates the creation of AP convertases. Monocytes, granulocytic cells, and T lymphocytes produce it.
- Under physiological settings, the C3Bb complex has a half-life of only 90 seconds, however properdin’s association with this complex makes it more stable.
- Properdin, in addition to preserving the stability of convertases, may potentially launch an alternate pathway.
- Properdin may now bind C3b and factor B when attached to components of microbial membranes in the presence of Mg2+.
3. The alternative protease-activated pathway
- C3 and C5 could be cleaved by blood coagulation pathway proteases such as thrombin and plasmin, releasing C3a and C5a, respectively.
- This method can boost complement cascade activation.
Deficiencies of the Alternative Pathway
Factors D, B and Properdin
- Factor D insufficiency is extremely uncommon and has only been documented in two families. Multiple members of both of these families have a history of severe infections. Factor B is a protein of the acute phase that increases during inflammation.
- One unverified report of this defect in humans exists. Properdin is the sole X-linked complement protein.
- Protein synthesis is performed by monocytes, granulocytic cells, and T-cells. Several mutant versions of the protein that diminish AP function have been found.
- A lack of properdin increases vulnerability to bacterial infections caused by the Neisseria genus.
- The most notable member of the group is N. Meningitis, the causative agent of a severe form of meningitis. Typical family histories include male ancestors who have experienced Neisseria illnesses or died from them.
Alternative Pathway Control Proteins
- Factor H deficiency is associated with a wide range of symptoms. Complete H deficit results in uncontrolled activation of the AP and C3 depletion.
- Due to low or missing amounts of C3, this form of factor H deficit manifests similarly to late-onset component deficiencies.
- Recent research has demonstrated the importance of this complement regulatory protein in regulating the health of a variety of tissues.
- In addition to bacterial infections, factor H loss or dysfunction and the subsequent dysregulation of the AP are associated with many kinds of kidney disease, including atypical Hemolytic Uremic Syndrome (aHUS), as well as age-related macular degeneration (AMD).
- These disorders are examples of malfunctioning control processes on the affected organs’ surfaces.
Applications of Alternative pathway
The alternative pathway of complement activation serves a range of crucial applications in the immune response against pathogen invasion and inflammation. Here are some key applications of the alternative pathway:
- Pathogen Monitoring: The alternative pathway operates under normal physiological conditions, constantly monitoring for potential pathogen invasion. This pathway acts as an early defense mechanism, quickly detecting the presence of pathogens and initiating an immune response.
- Infiltrating Cell Activation: Infiltrating immune cells, such as neutrophils, contribute to the activation of the alternative pathway. These cells bring in C3 and properdin, which are essential components for initiating the alternative pathway cascade. This activation helps recruit and amplify immune responses to combat invading pathogens effectively.
- Acute Phase Response: Factor B, a component of the alternative pathway, is classified as an acute phase protein. During inflammation and infection, its levels increase significantly. This upregulation of Factor B is part of the acute phase response, where the immune system rapidly reacts to combat the invading pathogens.
- Mediators of Inflammation: The alternative pathway generates important mediators of inflammation, namely C5a and C3a. These molecules are known as anaphylatoxins and play vital roles in the inflammatory response. They can activate immune cells, induce vasodilation, increase vascular permeability, and attract other immune cells to the site of infection or injury, promoting an effective immune response.
- Cell Lysis and Inflammation: One of the outcomes of complement activation through the alternative pathway is the formation of the Membrane Attack Complex (MAC). MAC is capable of lysing target cells, including pathogens, by forming pores in their membranes. Additionally, MAC can contribute to inflammation by triggering the release of inflammatory mediators from targeted cells, further enhancing the immune response.
The alternative pathway’s applications highlight its importance in the immune system’s surveillance, initiation of immune responses, acute phase response, inflammation mediation, and elimination of pathogens. Understanding these applications provides insights into the diverse functions of the alternative pathway and its significance in maintaining immune homeostasis and defending against infections.
FAQ
What is the alternative pathway of the complement system?
The alternative pathway is one of the three pathways of the complement system, which is a part of the immune system. It is responsible for detecting and eliminating pathogens through a cascade of reactions.
How does the alternative pathway differ from the classical pathway?
The alternative pathway can be activated spontaneously in the absence of specific antibodies, unlike the classical pathway that requires antibody binding to pathogens. This makes the alternative pathway a more rapid and immediate response to pathogen invasion.
What initiates the alternative pathway?
The alternative pathway can be initiated by the presence of certain molecules on pathogen surfaces or by the spontaneous hydrolysis of the complement component C3.
What is the role of properdin in the alternative pathway?
Properdin is a key protein that stabilizes the C3 convertase complex, allowing the alternative pathway to proceed and amplify the complement cascade. It also helps to enhance the immune response against pathogens.
How does the alternative pathway contribute to inflammation?
The alternative pathway generates inflammatory mediators, such as C5a and C3a, which can activate immune cells, increase vascular permeability, and attract more immune cells to the site of infection. This leads to inflammation, a crucial component of the immune response.
What happens when the alternative pathway is dysregulated?
Dysregulation of the alternative pathway can lead to various diseases, including autoimmune disorders, kidney diseases, and infections. It can result in excessive complement activation or inadequate response, compromising immune defense or causing tissue damage.
How is the alternative pathway regulated to prevent host cell damage?
The alternative pathway is tightly regulated by several complement regulatory proteins. These proteins, such as Factor H, Complement Receptor 1 (CR1), and Decay Accelerating Factor (DAF), prevent complement activation on host cells and ensure that the immune response is directed towards pathogens.
Can the alternative pathway cause cell lysis?
Yes, the alternative pathway can cause cell lysis through the formation of the Membrane Attack Complex (MAC). MAC creates pores in the membranes of target cells, leading to their destruction.
Does the alternative pathway play a role in immune surveillance?
Yes, the alternative pathway plays a crucial role in immune surveillance. It continuously monitors for pathogen invasion and quickly activates an immune response when pathogens are detected.
Can the alternative pathway be targeted for therapeutic interventions?
Yes, targeting the alternative pathway has therapeutic potential. Modulating the complement system, including the alternative pathway, is being explored for the treatment of various diseases, such as autoimmune disorders, inflammatory conditions, and complement-mediated diseases.
References
- The alternative complement pathway revisited – PubMed (nih.gov)
- Owen JA et al (2013). Kuby Immunology. 7th edition. W.H. Freeman Company. New York
- https://www.sinobiological.com/research/complement-system/complement-activation-alternative-pathway
- https://www.creative-biolabs.com/complement-therapeutics/alternative-pathway.htm
- https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/complement-deficiencies
- https://microbenotes.com/alternative-pathway-of-the-complement-system/
- https://en.wikipedia.org/wiki/Alternative_complement_pathway