What is Cellular Respiration?
Cellular respiration is a vital metabolic process that occurs within cells, enabling the conversion of energy stored in carbohydrates into energy carriers, most notably adenosine triphosphate (ATP).
Cellular respiration can occur in the presence or absence of oxygen. When oxygen is available, it is referred to as aerobic respiration. This process involves four main metabolic pathways: glycolysis, pyruvate oxidation, the Krebs cycle (also known as the citric acid cycle or the tricarboxylic acid cycle), and oxidative phosphorylation.
In the presence of oxygen, the process begins with glycolysis, which breaks down glucose into two molecules of pyruvate. These pyruvate molecules then enter the mitochondria, where they undergo oxidation, releasing carbon dioxide as a byproduct. The resulting acetyl-CoA molecules enter the Krebs cycle, generating additional carbon dioxide, ATP, and electron carriers (such as NADH and FADH2). The electron carriers then enter the electron transport chain, located in the inner mitochondrial membrane, where oxidative phosphorylation takes place. This final step produces a large amount of ATP through the utilization of oxygen.
In the absence of oxygen, known as anaerobic respiration, the process starts similarly with glycolysis. However, instead of proceeding through the remaining aerobic pathways, anaerobic respiration ends with the conversion of pyruvate into other compounds, such as lactate or ethanol. This allows the recycling of the electron carriers (NADH) back into their oxidized forms (NAD+), enabling glycolysis to continue.
Overall, the equation for cellular respiration can be represented as the reverse of the photosynthesis equation:
C6H12O6 + 6O2 -> 6CO2 + 6H2O
The energy released during cellular respiration, in the form of ATP and other energy carriers, fuels various anabolic processes within the cell. These processes require energy to synthesize complex molecules necessary for cellular growth, maintenance, and functioning.
In summary, cellular respiration is a fundamental process that enables cells to convert carbohydrates, such as sugars, starch, and glycogen, into usable energy. Through a series of interconnected metabolic pathways, the potential energy stored in the chemical bonds of these molecules is harnessed and transferred to energy carriers, which power other essential cellular processes.
Cellular Respiration Definition
Cellular respiration is the metabolic process by which cells convert carbohydrates into energy in the form of ATP.
Purpose of Cellular Respiration
The primary purpose of cellular respiration is to generate energy in the form of adenosine triphosphate (ATP) to power essential cellular processes. ATP serves as the “energy currency” of cells, providing the necessary fuel for various biological activities.
- Energy Production: Cells require a constant supply of energy to perform vital functions, including nutrient uptake, protein synthesis, DNA replication, and active transport across cell membranes. Cellular respiration breaks down organic molecules, such as sugars and fats, to release stored energy. Through a series of metabolic pathways, the energy is harnessed and converted into ATP. This process ensures a continuous supply of energy to support the diverse activities required for cell survival and function.
- ATP as an Energy Carrier: ATP serves as an immediate source of energy for cellular processes. When ATP is hydrolyzed, the high-energy phosphate bond is broken, releasing energy that can be utilized to drive chemical reactions and mechanical work within the cell. Enzymes and other proteins use ATP to power reactions, maintain ion gradients across membranes, contract muscle fibers, and transport molecules across cellular membranes.
- Long-Term Energy Storage: While ATP provides immediate energy, it is not suitable for long-term energy storage due to its instability. Instead, cells store energy in the form of sugars (e.g., glycogen in animals, starch in plants) and fats (triglycerides). Cellular respiration processes break down these stored molecules to produce ATP when needed. By converting stored energy into ATP, cells can access a readily available and versatile energy source to meet their energy demands.
- Oxygen Utilization: In aerobic respiration, oxygen serves as the final electron acceptor, enabling the efficient production of ATP. Oxygen is essential for the electron transport chain, which occurs in the mitochondria of eukaryotic cells. By utilizing oxygen, cells maximize the energy yield from glucose breakdown, generating a significant amount of ATP through oxidative phosphorylation. This process provides the necessary energy for complex organisms with higher energy demands.
- Adaptation to Different Conditions: Cellular respiration can adapt to varying environmental conditions. Aerobic respiration occurs in the presence of oxygen, while anaerobic respiration occurs in the absence of oxygen. Different organisms and cells can switch between these pathways depending on oxygen availability. Anaerobic respiration, such as fermentation, allows cells to generate ATP in oxygen-deprived conditions, albeit at a lower efficiency.
In summary, the purpose of cellular respiration is to produce ATP, the energy currency of cells, to sustain essential cellular processes and support life. By converting stored energy into ATP through various metabolic pathways, cells can ensure a continuous supply of energy for growth, maintenance, and the execution of vital biological functions.
The Location of Cellular Respiration
Cellular respiration occurs in specific cellular compartments, namely the cytosol and mitochondria. Different stages of cellular respiration take place in these distinct locations within the cell.
- Glycolysis: Glycolysis, the initial step of cellular respiration, takes place in the cytosol (cytoplasm) of the cell. This process involves the breakdown of glucose into two molecules of pyruvate. Glycolysis does not require the presence of oxygen and is common to both aerobic and anaerobic respiration.
- Pyruvate Oxidation and the Krebs Cycle: After glycolysis, if oxygen is available, pyruvate molecules are transported into the mitochondria. Within the mitochondria, pyruvate undergoes further processing in a series of reactions. Firstly, pyruvate is converted to acetyl-CoA in a process known as pyruvate oxidation. This occurs in the mitochondrial matrix. The acetyl-CoA then enters the Krebs cycle, also called the citric acid cycle or tricarboxylic acid cycle, which occurs in the mitochondrial matrix as well. In the Krebs cycle, acetyl-CoA is further broken down, releasing carbon dioxide and generating energy-rich molecules like NADH and FADH2.
- Oxidative Phosphorylation and the Electron Transport Chain: The final stage of cellular respiration, oxidative phosphorylation, occurs in the inner mitochondrial membrane. This process involves the electron transport chain, a series of protein complexes embedded in the inner membrane. NADH and FADH2, generated in previous steps, donate electrons to the electron transport chain. As electrons are passed along the chain, energy is released and used to pump protons (H+) from the mitochondrial matrix to the intermembrane space. This creates a proton gradient across the inner mitochondrial membrane. The flow of protons back into the matrix through ATP synthase drives the synthesis of ATP from ADP and inorganic phosphate (Pi). This process is called chemiosmosis.
The mitochondria, with their double membrane structure, are often referred to as the “powerhouses” of the cell because they play a central role in generating ATP through cellular respiration. The inner mitochondrial membrane houses the electron transport chain, while the mitochondrial matrix provides an environment for the Krebs cycle and other metabolic processes.
It’s important to note that if oxygen is unavailable, such as in anaerobic conditions, certain cells and organisms can carry out alternative pathways like fermentation to generate limited ATP.
In summary, cellular respiration occurs in different locations within the cell. Glycolysis takes place in the cytosol, while subsequent steps, including pyruvate oxidation, the Krebs cycle, and oxidative phosphorylation, occur in the mitochondria. This spatial organization allows for efficient energy production and the generation of ATP, which serves as a universal energy currency for cellular processes.
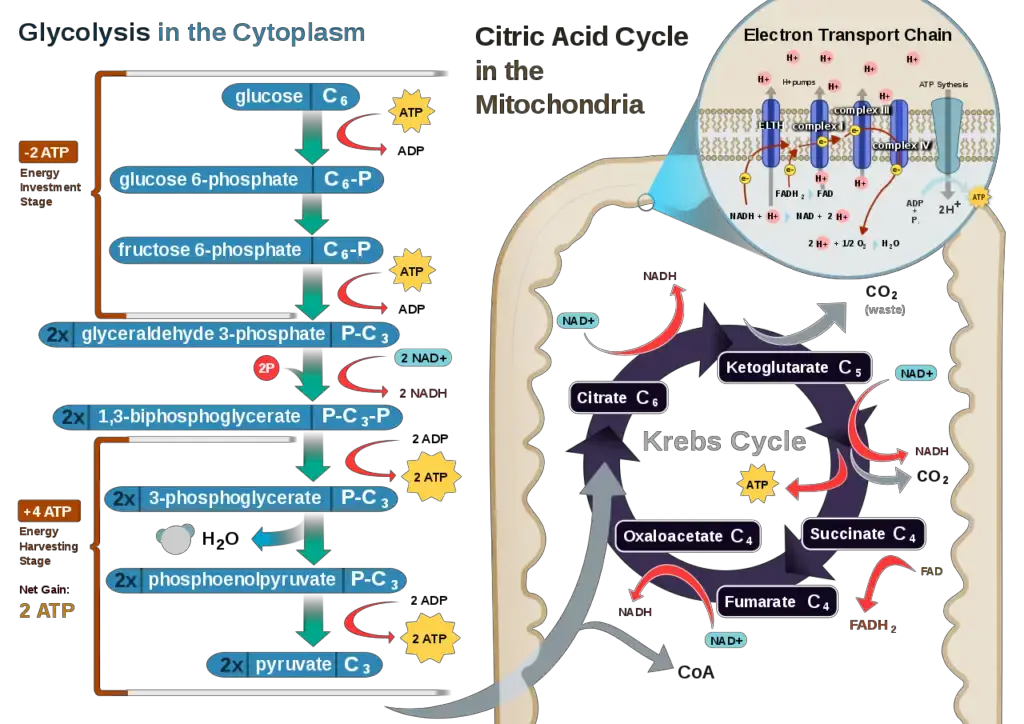
Role of Oxygen in Cellular Respiration
Oxygen plays a vital role in cellular respiration as the final electron acceptor in the process. Here are the key aspects of oxygen’s involvement:
- Electron Acceptor: During cellular respiration, the breakdown of organic molecules, such as glucose, releases high-energy electrons. These electrons are transferred through a series of enzymatic reactions, ultimately reaching the electron transport chain (ETC) located in the inner mitochondrial membrane. Oxygen serves as the final electron acceptor in the ETC. It accepts the low-energy electrons, along with protons (H+), to form water (H2O). This step is crucial for the completion of aerobic respiration.
- ATP Production: The flow of electrons along the electron transport chain leads to the establishment of a proton gradient across the inner mitochondrial membrane. As electrons move through the ETC, protons are actively pumped from the mitochondrial matrix into the intermembrane space. This creates an electrochemical gradient. When protons flow back into the matrix through ATP synthase, it drives the synthesis of adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and inorganic phosphate (Pi). Oxygen’s role as the final electron acceptor allows for the efficient production of ATP, the main energy currency of the cell.
- Efficiency of Energy Production: Oxygen’s high electronegativity makes it an ideal electron acceptor. By accepting the electrons, oxygen helps maintain the proper functioning of the electron transport chain and prevents an accumulation of excess electrons, which can be harmful to the cell. The presence of oxygen in aerobic respiration enables a more efficient extraction of energy from organic molecules, resulting in a higher yield of ATP production compared to anaerobic processes.
- Cellular Adaptation: The availability of oxygen influences the metabolic pathways utilized by cells. In the presence of oxygen, cells undergo aerobic respiration, which generates a substantial amount of ATP. This process allows for more sustained and efficient energy production, making it suitable for organisms with higher energy demands. In the absence of oxygen, cells may resort to anaerobic respiration or fermentation, which produce limited ATP and are less efficient. Some specialized cells, like muscle cells during intense exercise, may temporarily shift to anaerobic processes when oxygen supply is limited.
In summary, oxygen serves as the final electron acceptor in cellular respiration, allowing for the efficient extraction of energy from organic molecules and the production of ATP. Its role in the electron transport chain ensures the proper functioning of this critical process. The presence or absence of oxygen influences the metabolic pathways employed by cells, affecting their energy production capabilities.
Cellular Respiration Equation
Cellular respiration involves various equations that represent the breakdown of glucose and the production of energy in the form of ATP. These equations differ depending on the specific type of respiration being considered.
Aerobic Respiration Equation: The equation for aerobic respiration demonstrates the combination of glucose, oxygen, ADP (depleted ATP), and phosphate groups to produce carbon dioxide, water, and ATP:
C6H12O6 (glucose) + 6O2 + 36 ADP + 36 Pi → 6CO2 + 6H2O + 36 ATP
In this equation, glucose and oxygen react to release energy, resulting in the formation of carbon dioxide and water as byproducts. Simultaneously, ATP is produced, providing the cell with usable energy for various metabolic processes.
Lactic Acid Fermentation Equation: During lactic acid fermentation, one molecule of glucose is broken down to form two molecules of lactic acid. The energy stored in the bonds of glucose is transferred to ADP and phosphate groups to generate ATP:
C6H12O6 (glucose) + 2 ADP + 2 Pi → 2 CH3CHOHCOOH (lactic acid) + 2 ATP
Lactic acid fermentation occurs in certain microorganisms and muscle cells when oxygen is scarce. It allows for the continuation of ATP production, albeit at a lower efficiency compared to aerobic respiration.
Alcoholic Fermentation Equation: Alcohol fermentation, similar to lactic acid fermentation, occurs in the absence of oxygen. Instead of oxygen, a converted form of pyruvate acts as the final electron acceptor. This process results in the production of ethyl alcohol (found in alcoholic beverages), carbon dioxide, and ATP:
C6H12O6 (glucose) + 2 ADP + 2 Pi → 2 C2H5OH (ethyl alcohol) + 2 CO2 + 2 ATP
Yeast cells, commonly used in brewing and distillation processes, exhibit a high proficiency for alcoholic fermentation.
These equations illustrate the chemical transformations that take place during cellular respiration, highlighting the breakdown of glucose and the production of energy-rich ATP molecules. The specific equation utilized depends on the availability of oxygen and the metabolic requirements of the cell or organism.
What is the equation for cellular respiration?
The equation for cellular respiration, specifically aerobic respiration, can be represented as:
C6H12O6 (glucose) + 6O2 (oxygen) → 6CO2 (carbon dioxide) + 6H2O (water) + ATP (energy)
This equation shows the overall chemical reaction that occurs during the process of cellular respiration in the presence of oxygen. Glucose and oxygen are reactants that are consumed, while carbon dioxide, water, and ATP (adenosine triphosphate) are the products generated as a result of the series of biochemical reactions involved in cellular respiration. ATP is the energy currency of the cell and is produced through the various stages of cellular respiration.
Products of Cellular Respiration
Cellular respiration generates various products that play crucial roles in energy production and waste removal within cells. These products include:
- Adenosine Triphosphate (ATP): ATP is the primary product of cellular respiration. It is a high-energy molecule that stores and transfers energy within the cell. ATP serves as a universal currency of energy, providing the necessary fuel for various cellular processes, such as muscle contraction, active transport, and synthesis of macromolecules. The energy released during cellular respiration is captured and stored in the bonds of ATP, enabling it to be utilized throughout the cell.
- Carbon Dioxide (CO2): Carbon dioxide is a byproduct of cellular respiration. During respiration, glucose is broken down, and the carbon atoms within it are oxidized, resulting in the release of carbon dioxide. This waste product must be efficiently removed from the cell to prevent an accumulation that could lead to cellular dysfunction. Carbon dioxide can dissolve in water, forming carbonic acid, which can alter the pH of the cell. To maintain proper pH and prevent acidification, cells actively eliminate carbon dioxide.
- Water (H2O): Water is another product of cellular respiration, specifically in the final stage of aerobic respiration known as oxidative phosphorylation. During this process, electrons pass through the electron transport chain, and molecular oxygen acts as the final electron acceptor, combining with hydrogen ions to form water. This process helps maintain the balance of water within the cell and contributes to the overall hydration and homeostasis of the organism.
- Other Products: In addition to ATP, carbon dioxide, and water, different types of cellular respiration utilize various molecules as final electron acceptors. For example, in anaerobic respiration, the final electron acceptor may be molecules such as sulfate or nitrate, resulting in the production of sulfide or nitrogen gas, respectively. These alternative final products depend on the specific metabolic pathway and the availability of electron acceptors in the cell or surrounding environment.
The products of cellular respiration are essential for sustaining cellular activities and maintaining the overall energy balance within the organism. ATP provides the energy needed for vital cellular processes, while carbon dioxide and water are waste products that must be properly eliminated. Understanding these products and their roles in cellular respiration is fundamental to comprehending the energetic and metabolic functions of living organisms.
Types of Cellular Respiration
A. Aerobic Respiration
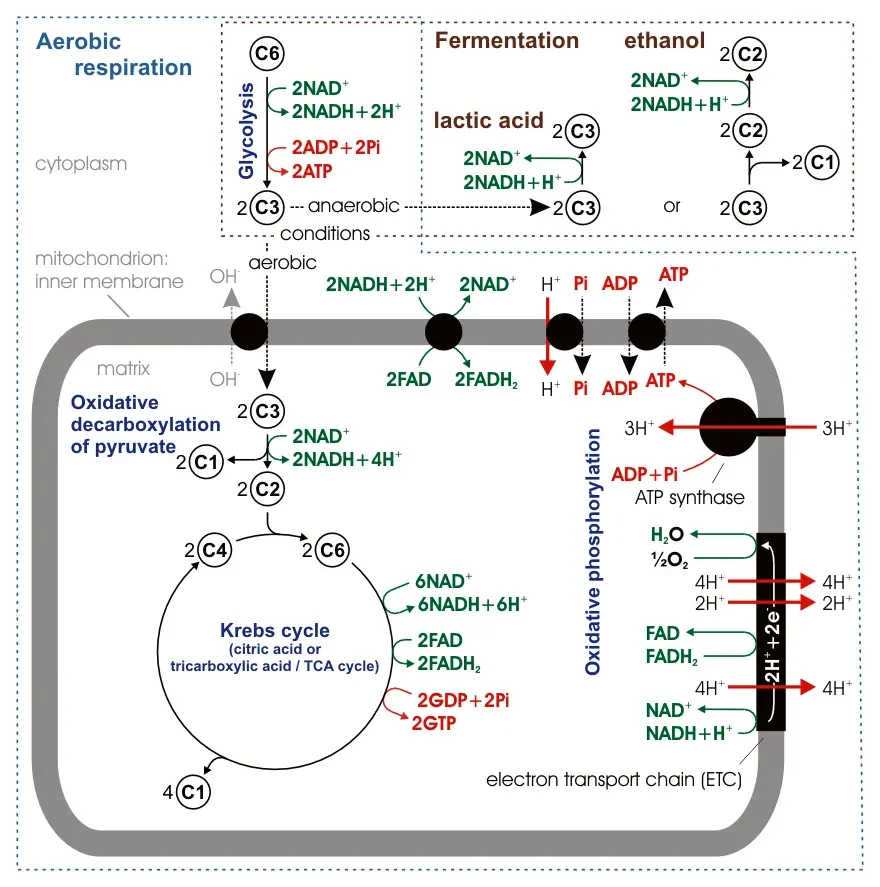
Aerobic respiration is a crucial metabolic process that occurs in most cells and organisms, utilizing oxygen as the final electron acceptor. It involves a series of interconnected pathways, including glycolysis, pyruvate decarboxylation, the Krebs cycle (also known as the citric acid cycle or tricarboxylic acid cycle), and oxidative phosphorylation. Here are the key features of aerobic respiration:
- Glycolysis: The process of aerobic respiration begins with glycolysis, which takes place in the cytosol of the cell. In glycolysis, glucose molecules are broken down into pyruvate. This step generates a small amount of ATP and NADH, a reduced form of nicotinamide adenine dinucleotide.
- Pyruvate Decarboxylation: Following glycolysis, pyruvate molecules produced are transported into the mitochondrial matrix. Here, pyruvate undergoes decarboxylation, resulting in the formation of acetyl coenzyme A (acetyl-CoA). This step releases carbon dioxide and generates NADH. Acetyl-CoA then enters the next stage of aerobic respiration.
- Krebs Cycle: Acetyl-CoA derived from pyruvate decarboxylation enters the Krebs cycle, which occurs in the mitochondrial matrix. In this cycle, acetyl-CoA is further broken down, releasing carbon dioxide and generating energy-rich molecules such as NADH and flavin adenine dinucleotide (FADH2). These electron carriers play a crucial role in the subsequent stage of aerobic respiration.
- Oxidative Phosphorylation: The final stage of aerobic respiration is oxidative phosphorylation, which takes place in the inner mitochondrial membrane. NADH and FADH2, generated in previous steps, donate electrons to the electron transport chain embedded in the membrane. As electrons flow through the chain, protons are pumped across the membrane, creating a proton gradient. The final electron acceptor in this process is oxygen. Oxygen accepts electrons, along with protons, to form water. The flow of protons back into the mitochondrial matrix through ATP synthase drives the synthesis of adenosine triphosphate (ATP), the primary energy currency of the cell.
Overall, aerobic respiration is often referred to as the “complete” form of cellular respiration since it utilizes oxygen as the final electron acceptor, resulting in the most efficient production of ATP. The entire process can be summarized as the reverse of photosynthesis, as the energy released during aerobic respiration is captured and stored in the form of ATP, similar to how photosynthesis uses energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Aerobic respiration provides cells and organisms with a continuous supply of ATP, enabling them to perform essential cellular functions and meet their energy requirements.
B. Anaerobic Respiration
Often referred to as fermentation, anaerobic respiration occurs when oxygen is scarce or absent from cells. Beginning with glycolysis, glucose is transformed into pyruvate.
In contrast to aerobic respiration, however, the resultant pyruvate molecules are not transported to the mitochondria. They remain in the cytosol and participate in one of the subsequent reactions:
1. Lactic Acid Fermentation
Lactic acid fermentation is a metabolic process that occurs in both prokaryotes and eukaryotes, allowing cells to generate energy in the absence of oxygen. Here are the key characteristics of lactic acid fermentation:
- Conversion of Pyruvate to Lactic Acid: During lactic acid fermentation, pyruvate molecules produced during glycolysis accept electrons from the oxidation of NADH. This redox reaction is catalyzed by the enzyme lactate dehydrogenase. The reaction can be summarized as follows:
CH3COCOO- + NADH + H+ ⇌ CH3CH(OH)COO- + NAD+
In this reaction, pyruvate is reduced to lactic acid, while NAD+ is regenerated, allowing it to participate in further rounds of glycolysis. This regeneration of NAD+ is crucial to sustain the production of ATP through glycolysis.
- NAD+ Regeneration and Glycolysis: The regeneration of NAD+ by lactic acid fermentation ensures the continuous supply of this coenzyme to support glycolysis. Glycolysis is an anaerobic process that breaks down glucose into pyruvate, producing a small amount of ATP. Without the regeneration of NAD+, glycolysis would not be able to proceed, and ATP production would halt.
- Uses of Lactic Acid: Lactic acid produced during fermentation serves as a substrate for various metabolic pathways. In some organisms, lactic acid can be utilized in gluconeogenesis, a process that synthesizes glucose from non-carbohydrate sources. Gluconeogenesis allows the conversion of lactic acid back into glucose, which can be used as an energy source or for other cellular processes.
- Organisms and Environmental Adaptation: Lactic acid fermentation occurs in various organisms, including bacteria, fungi, and human muscle cells. Some bacteria, like Lactobacillus, are facultative anaerobes, capable of both aerobic respiration and lactic acid fermentation. Other organisms, such as certain prokaryotes, are obligate anaerobes, meaning they can only survive in the absence of oxygen. Additionally, there are aerotolerant anaerobes that can tolerate the presence of oxygen but do not require it for growth.
In summary, lactic acid fermentation is a metabolic pathway that allows cells to produce ATP in the absence of oxygen. By converting pyruvate to lactic acid, this process regenerates NAD+, ensuring the continued functioning of glycolysis. Lactic acid can be used in other metabolic pathways, and the ability to carry out lactic acid fermentation provides adaptability to different environmental conditions for various organisms.
2. Ethanol Fermentation
Ethanol fermentation is a metabolic process that occurs in certain organisms, such as yeast, plants, and some vertebrates. It allows for the conversion of pyruvate, derived from glycolysis, into ethanol. Here are the key aspects of ethanol fermentation:
- Cleavage of Pyruvate: After glycolysis, pyruvate molecules undergo decarboxylation in the presence of the enzyme pyruvate decarboxylase. This reaction, facilitated by vitamin B1 (thiamine) as a coenzyme, results in the cleavage of pyruvate, producing acetaldehyde and carbon dioxide:
CH3COCOO- + H+ ⇌ CH3COH + CO2
- Reduction of Acetaldehyde to Ethanol: In the second step of ethanol fermentation, acetaldehyde is reduced to ethanol. This reaction is catalyzed by the enzyme alcohol dehydrogenase. NADH, generated during glycolysis, donates electrons for the reduction reaction, leading to the regeneration of NAD+:
CH3COH + NADH + H+ ⇌ CH3CH2OH + NAD+
This process converts acetaldehyde into ethanol while replenishing the NAD+ pool. Ethanol serves as the end product of this fermentation pathway.
- Energy Yield and NAD+ Regeneration: Ethanol fermentation produces a net yield of two ATP molecules per glucose molecule. However, it is important to note that the energy output of ethanol fermentation is relatively low compared to aerobic respiration. The main purpose of ethanol fermentation is to regenerate NAD+ to sustain glycolysis, allowing for the continued production of ATP in the absence of oxygen.
- Organisms and Environmental Adaptation: Ethanol fermentation is commonly observed in organisms like yeast, which are facultative aerobes. Facultative aerobes can switch between aerobic respiration (in the presence of oxygen) and anaerobic fermentation (in the absence of oxygen) based on environmental conditions. Ethanol fermentation enables these organisms to generate energy when oxygen availability is limited.
- Applications: Ethanol fermentation is utilized in various industrial processes, such as the production of alcoholic beverages and biofuels. In the production of alcoholic beverages, yeast performs ethanol fermentation on the sugars present in the raw materials (e.g., grapes for wine, malted grains for beer). The ethanol produced contributes to the characteristic flavors and alcohol content of the final product. In biofuel production, microorganisms like yeast or bacteria are engineered to ferment sugars derived from biomass, such as corn or sugarcane, to produce ethanol as a renewable energy source.
In summary, ethanol fermentation is a metabolic pathway that allows for the conversion of pyruvate into ethanol. It occurs in certain organisms as an alternative to aerobic respiration when oxygen is limited. Ethanol fermentation replenishes the NAD+ pool and provides a limited energy yield, making it a valuable process in various applications, including the production of alcoholic beverages and biofuels.
3. Methanogenesis
Methanogenesis is a unique process carried out exclusively by anaerobic bacteria known as methanogens. These bacteria, belonging to the phylum Euryarchaeota, include various orders such as Methanobacteriales, Methanococcales, Methanomicrobiales, Methanopyrales, and Methanosarcinales. Methanogens thrive in oxygen-depleted environments, such as sediments, aquatic habitats, and the intestinal tracts of mammals. The process of methanogenesis involves three distinct pathways:
- Acetoclastic Methanogenesis: Acetoclastic methanogenesis is the most prevalent pathway, particularly carried out by Methanosarcina and Methanosarcinales. In this process, acetate is activated into acetyl-coenzyme A (acetyl-CoA), from which a methyl group is transferred into the central methanogenic pathway. Acetoclastic methanogens split acetate, resulting in the formation of carbon dioxide (CO2) and methane (CH4) as follows:
CH3COOH (Acetate) → CO2 (Carbon dioxide) + CH4 (Methane)
This pathway is commonly observed in freshwater sediments and is estimated to contribute to approximately two-thirds of the annual global methane formation from acetate.
- Methylotrophic Methanogenesis: Methylotrophic methanogenesis occurs when methanol or methylamines serve as the substrates instead of acetate. This pathway is found in environments such as marine sediments, where methylated compounds are present. Some acetoclastic methanosarcinales and certain members of the Methanomicrobiales can also utilize this pathway.
- Hydrogenotrophic Methanogenesis: Hydrogenotrophic methanogenesis is employed by all five orders of methanogens, including Methanobacteriales, Methanococcales, Methanomicrobiales, Methanopyrales, and Methanosarcinales. In this pathway, methanogens use hydrogen gas (H2) to reduce carbon dioxide (CO2), carbon monoxide (CO), or formate, leading to the production of methane (CH4) and water (H2O):
4H2 (Hydrogen) + CO2 (Carbon dioxide) → CH4 (Methane) + 2H2O (Water)
Unlike other forms of respiration that utilize an electron transport chain, methanogens rely on specific coenzymes to facilitate the methanogenesis process. Coenzyme F420 is involved in hydrogen activation, while coenzyme M plays a role in the terminal reduction of methyl (CH3) groups to methane.
In summary, methanogenesis is a unique anaerobic process carried out by methanogenic bacteria. It involves three main pathways: acetoclastic methanogenesis, methylotrophic methanogenesis, and hydrogenotrophic methanogenesis. Methanogens utilize specific coenzymes and unique biochemical reactions to convert substrates such as acetate, methanol, or hydrogen into methane, which has significant implications for global biogeochemical cycles and various environmental ecosystems.
Four Stages in Cellular Respiration
1. Glycolysis
Glycolysis is a fundamental metabolic pathway that involves the breakdown of glucose to produce energy. It occurs in the cytosol of cells and serves as the initial step in both aerobic and anaerobic respiration. Here are the key details about glycolysis:
- Overview: The term “glycolysis” originates from the Greek words “glykos” meaning “sweet” and “lysis” meaning “to split,” reflecting its purpose of splitting glucose. Glycolysis is a conserved pathway found in most organisms, enabling the extraction of energy from glucose molecules.
- Phases of Glycolysis: Glycolysis can be divided into two main phases: the investment phase and the payoff phase.
- Investment Phase: In this phase, two ATP molecules are consumed to activate glucose for subsequent reactions. Glucose is initially phosphorylated by the enzyme hexokinase, forming glucose-6-phosphate. The molecule then undergoes further transformations to yield fructose-1,6-bisphosphate.
- Payoff Phase: During this phase, energy-rich molecules are generated. The enzyme phosphofructokinase plays a crucial role in catalyzing the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate. Subsequent reactions lead to the formation of two molecules of glyceraldehyde-3-phosphate (GAP). Finally, GAP is further metabolized to produce pyruvate, resulting in the generation of ATP and reduced coenzymes like NADH.
- Key Enzymes and Regulations: Several enzymes are involved in glycolysis, each catalyzing specific reactions. Hexokinase, phosphofructokinase, and pyruvate kinase are some of the key regulatory enzymes in glycolysis. The activity of these enzymes is tightly regulated by various factors, including the levels of ATP, ADP, and other metabolites. This regulation ensures that glycolysis operates efficiently based on the energy demands of the cell.
- Energy Yield: The overall energy yield of glycolysis is two ATP molecules, two NADH molecules, and two molecules of pyruvate per glucose molecule. While glycolysis itself is not a highly efficient pathway in terms of ATP production, it serves as the starting point for further energy extraction in subsequent stages of cellular respiration.
- Anaerobic and Aerobic Conditions: Glycolysis is an oxygen-independent process and can occur under both aerobic and anaerobic conditions. In the presence of oxygen, pyruvate produced during glycolysis enters the mitochondria for further oxidation in the Krebs cycle and oxidative phosphorylation. Under anaerobic conditions, pyruvate can be converted to lactate or ethanol through fermentation, regenerating NAD+ to sustain glycolysis in the absence of oxygen.
In summary, glycolysis is a vital metabolic pathway involved in the breakdown of glucose to produce energy in the form of ATP. It occurs in the cytosol of cells and serves as the starting point for both aerobic and anaerobic respiration. Glycolysis plays a fundamental role in cellular energy metabolism and is conserved across a wide range of organisms.
Steps of Glycolysis
Glycolysis is a complex metabolic pathway that involves ten distinct steps. Each step is catalyzed by a specific enzyme and contributes to the gradual breakdown of glucose to produce energy. Here are the detailed steps of glycolysis:
- Step 1: Hexokinase: Glucose enters the pathway and is phosphorylated by the enzyme hexokinase. This reaction requires the input of ATP and results in the formation of glucose-6-phosphate. The phosphorylation step traps glucose inside the cell and primes it for further metabolic reactions.
- Step 2: Phosphoglucose Isomerase: Glucose-6-phosphate undergoes an isomerization reaction catalyzed by the enzyme phosphoglucose isomerase. The molecule is converted into fructose-6-phosphate, which is an important intermediate in glycolysis.
- Step 3: Phosphofructokinase (PFK): Phosphofructokinase, an enzyme regulated by various factors, including ATP and AMP levels, phosphorylates fructose-6-phosphate. This step requires ATP and leads to the formation of fructose 1,6-bisphosphate. It is a key regulatory step in glycolysis.
- These first three steps of glycolysis are often referred to as the “investment phase” because they consume two molecules of ATP. This phase prepares the glucose molecule for subsequent breakdown and energy extraction.
- Step 4: Aldolase: Fructose 1,6-bisphosphate is cleaved into two three-carbon molecules: glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP). The enzyme aldolase facilitates this reaction.
- Step 5: Triosephosphate Isomerase: DHAP is converted into another molecule of GAP by the enzyme triosephosphate isomerase. Now, two molecules of GAP are available for further processing.
- Step 6: Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH): GAP is oxidized by the coenzyme nicotinamide adenine dinucleotide (NAD+), resulting in the production of NADH and a hydrogen atom. Simultaneously, the molecule is phosphorylated, forming 1,3-bisphosphoglycerate. The enzyme responsible for this step is glyceraldehyde-3-phosphate dehydrogenase.
- Step 7: Phosphoglycerate Kinase (PGK): 1,3-bisphosphoglycerate is converted into 3-phosphoglycerate by the enzyme phosphoglycerate kinase. In this process, a phosphate group is transferred to ADP, resulting in the synthesis of ATP. This step marks the first energy-generating step in glycolysis.
- Step 8: Phosphoglycerate Mutase: The position of the phosphate group in 3-phosphoglycerate is rearranged by the enzyme phosphoglycerate mutase. This conversion leads to the formation of 2-phosphoglycerate.
- Step 9: Enolase: 2-phosphoglycerate undergoes dehydration catalyzed by the enzyme enolase. Water is removed, resulting in the formation of phosphoenolpyruvate (PEP). Enolase plays a crucial role in this step by facilitating the rearrangement of the molecule.
- Step 10: Pyruvate Kinase: In the final step of glycolysis, pyruvate kinase transfers a phosphate group from PEP to ADP, producing ATP and pyruvate. This step generates additional ATP and leads to the formation of the end product, pyruvate.
In summary, glycolysis consists of ten enzymatic steps that progressively convert glucose to pyruvate, resulting in the production of ATP and reducing equivalents. The pathway serves as a vital energy-producing process in both aerobic and anaerobic conditions.
2. Pyruvate Oxidation
- Pyruvate oxidation is a crucial step in cellular respiration that follows glycolysis. After glycolysis, pyruvate molecules undergo further processing to generate energy and replenish the cellular NAD+ reserves.
- Depending on the availability of oxygen and the cellular NAD+/NADH ratio, pyruvate can follow different pathways. In anaerobic conditions or when oxygen is limited, pyruvate remains in the cytosol and undergoes anaerobic respiration. However, in the presence of oxygen and sufficient cellular NAD+, pyruvate is transported into the mitochondria to undergo aerobic respiration.
- In aerobic respiration, pyruvate enters the mitochondrial matrix, where it is transformed into acetyl-CoA through the action of the pyruvate dehydrogenase complex. This complex is composed of three enzymes: pyruvate dehydrogenase, dihydrolipoyl transacetylase, and dihydrolipoyl dehydrogenase. Each enzyme in the complex has its specific catalytic activity and requires coenzymes to function.
- The pyruvate dehydrogenase complex works in a coordinated manner, with each enzyme sequentially interacting with pyruvate to catalyze its conversion into acetyl-CoA. This process is known as substrate channeling, ensuring efficient transfer of intermediates between the enzyme active sites.
- The net reaction of pyruvate oxidation in aerobic respiration involves the oxidation of pyruvate, resulting in the production of acetyl-CoA, carbon dioxide, NADH, and a hydrogen ion.
- When combined with glycolysis and pyruvate oxidation, the overall cellular respiration equation for pyruvate decarboxylation includes the production of carbon dioxide, acetyl-CoA, ATP, NADH, and hydrogen ions.
- The enzymes in the pyruvate dehydrogenase complex require specific coenzymes for their catalytic activity. Pyruvate dehydrogenase relies on thiamine pyrophosphate (TPP or vitamin B1), dihydrolipoyl transacetylase utilizes lipoamide and coenzyme A (CoA), and dihydrolipoyl dehydrogenase requires flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NAD+).
- In summary, pyruvate oxidation plays a crucial role in aerobic respiration by converting pyruvate into acetyl-CoA, which serves as a key substrate for the subsequent Krebs cycle. This process generates energy in the form of ATP, produces carbon dioxide as a waste product, and replenishes the cellular NAD+ pool for continued glycolysis and energy production.
3. The Krebs Cycle
The Krebs cycle, also known as the citric acid cycle or tricarboxylic acid cycle, is a vital metabolic pathway in aerobic respiration. It occurs in the mitochondrial matrix and plays a central role in generating energy and providing precursors for biosynthesis.
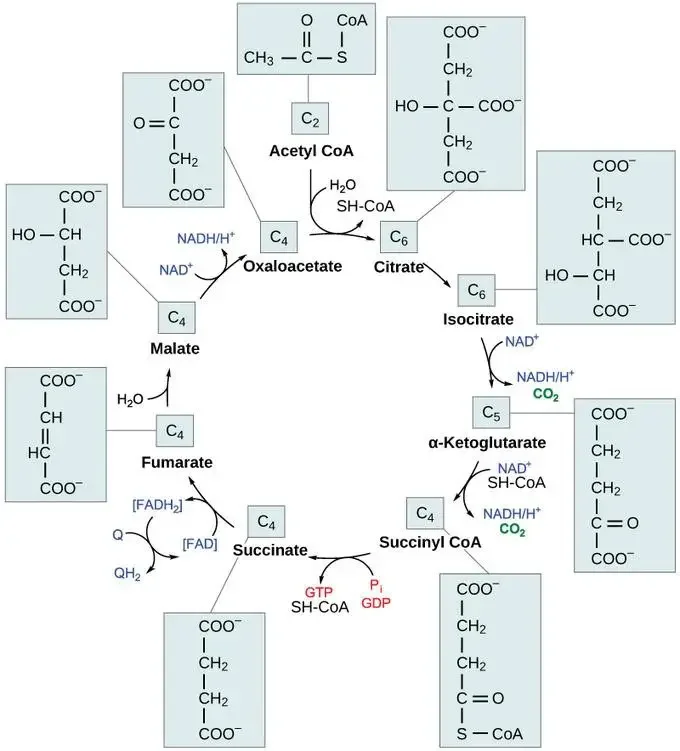
The cycle begins when acetyl-CoA, a two-carbon molecule derived from pyruvate through the transition reaction, combines with a four-carbon molecule called oxaloacetate. This reaction, catalyzed by citrate synthase, forms a six-carbon molecule called citric acid (or citrate) and releases coenzyme A (CoA).
In subsequent steps, citric acid undergoes a series of reactions that involve rearrangements and oxidations. Carbon dioxide is released, and energy carriers such as NADH and FADH2 are generated. The cycle produces three molecules of NADH, one molecule of FADH2, one molecule of GTP (which can be converted to ATP), and two molecules of carbon dioxide per cycle.
The reactions in the Krebs cycle can be summarized as follows:
- Acetyl-CoA combines with oxaloacetate to form citric acid.
- Citric acid undergoes rearrangement and oxidation reactions to produce α-ketoglutarate, releasing carbon dioxide and reducing NAD+ to NADH.
- α-ketoglutarate is further decarboxylated to produce succinyl-CoA, releasing another molecule of carbon dioxide and reducing NAD+ to NADH.
- Succinyl-CoA is converted to succinate, generating ATP or GTP through substrate-level phosphorylation.
- Succinate is oxidized to fumarate, reducing FAD to FADH2.
- Fumarate is hydrated to form L-malate.
- L-malate is oxidized to oxaloacetate, reducing NAD+ to NADH.
The net reaction of the Krebs cycle, when combined with previous stages of cellular respiration, shows the contribution of the cycle to the overall equation:
The eight steps of the Krebs cycle are summarized as:
CH3COCoA + 3NAD+ + FAD + ADP or GDP + Pi + 2H2O –> 2CO2 + 3NADH + 3H+ + FADH2 + ATP or GTP + CoASH
Combining with the previous stages, the contribution of the Krebs cycle to the cellular respiration equation is:
C6H12O6 + 10NAD+ + 2FAD + 4ADP (or 2ADP and 2GDP) + 4Pi + 6H2O —> 6CO2 + 10NADH + 10H+ + 2FADH2 + 4ATP (or 2ATP and 2GTP)
One molecule of glucose, after undergoing glycolysis and pyruvate oxidation, can produce two molecules of acetyl-CoA. Each acetyl-CoA molecule entering the Krebs cycle generates two molecules of carbon dioxide, three molecules of NADH, one molecule of FADH2, and one molecule of ATP or GTP.
The energy carriers, NADH and FADH2, generated in the Krebs cycle, play a crucial role in the electron transport chain and oxidative phosphorylation, where they donate electrons and contribute to the synthesis of ATP. The carbon dioxide produced is considered a waste product and cannot be used in other metabolic pathways.
In summary, the Krebs cycle is an essential part of aerobic respiration, generating energy-rich molecules (NADH, FADH2, and ATP/GTP) and carbon dioxide. It provides the necessary precursors for biosynthesis and serves as a crucial link between various metabolic pathways.
Steps of The Krebs Cycle
The Krebs cycle, also known as the citric acid cycle, is a series of eight steps that occur in the mitochondrial matrix. These steps involve various enzymatic reactions and result in the production of energy carriers and precursors for biosynthesis. Here are the key steps of the Krebs cycle:
- Step 1: Acetyl CoA combines with oxaloacetate, forming citrate. This reaction is catalyzed by citrate synthase.
- Step 2: Citrate is converted to isocitrate through a process involving the enzyme aconitase. This step includes the removal and addition of water.
- Step 3: Isocitrate is oxidized, leading to the formation of alpha-ketoglutarate. NAD+ acts as a coenzyme in this reaction, and a molecule of carbon dioxide is released. The enzyme responsible for this step is isocitrate dehydrogenase, which plays a vital role in regulating the speed of the Krebs cycle.
- Step 4: Alpha-ketoglutarate is oxidatively decarboxylated to form succinyl-CoA. This step is catalyzed by the enzyme alpha-ketoglutarate dehydrogenase. NADH is produced in this process, providing electrons for the respiratory chain.
- Step 5: Succinyl-CoA is converted to succinate through a series of reactions. Succinate thiokinase (or succinate synthase) is involved in this step, which results in the production of ATP or GTP. The coenzyme A in succinyl-CoA is replaced by a hydrogen phosphate ion, generating succinyl phosphate. This phosphate residue is then transferred to ADP or GDP, leading to the production of ATP or GTP and the formation of succinate.
- Step 6: Succinate is oxidized to fumarate by succinate dehydrogenase. This enzyme, bound to flavin adenine dinucleotide (FAD), removes two hydrogen atoms from succinate, forming FADH2. The energy released in this process is sufficient to reduce FAD. FADH2 remains bound to succinate dehydrogenase and transfers electrons directly to the electron transport chain, as it is located within the mitochondrial inner membrane.
- Step 7: Fumarate is hydrated, converting it into L-malate. This reaction is catalyzed by the enzyme fumarase.
- Step 8: L-malate is oxidized to oxaloacetate by malate dehydrogenase. NAD+ is reduced to NADH during this step, and another molecule of NADH is produced.
Overall, the Krebs cycle generates energy carriers such as NADH and FADH2, as well as carbon dioxide. These energy carriers play crucial roles in the electron transport chain, which is involved in oxidative phosphorylation and the synthesis of ATP. The cycle also provides precursor molecules for biosynthetic pathways, contributing to various cellular processes.
4. Electron Transport and Oxidative Phosphorylation
Electron transport and oxidative phosphorylation are the final stages of cellular respiration, where the electron carriers NADH and FADH2 play a crucial role. Let’s explore these processes in more detail:
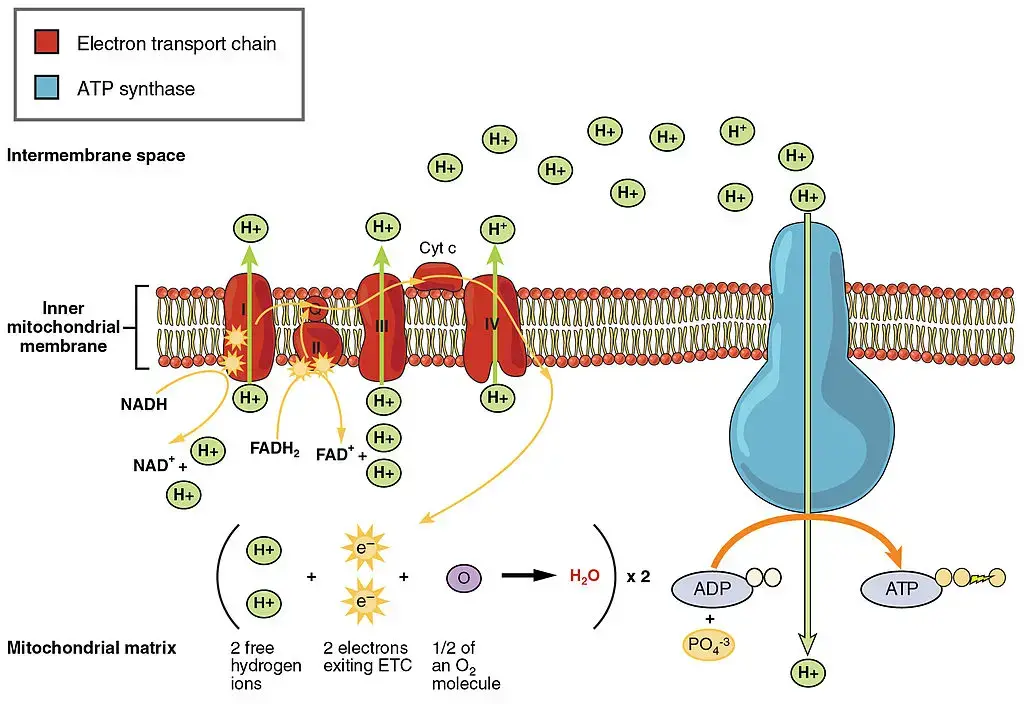
Electron Transport Chain (ETC): The electron transport chain is a series of proteins located in the inner mitochondrial membrane. Its primary function is to facilitate the transfer of electrons from NADH and FADH2 to molecular oxygen (O2), the final electron acceptor. As the electrons pass through the protein complexes of the electron transport chain, their energy is gradually released and used to pump protons (H+) from the mitochondrial matrix to the intermembrane space.
The oxidation of NADH and FADH2 in the electron transport chain occurs in a stepwise manner, allowing for the controlled release of energy. This energy release is coupled with the pumping of protons, establishing an electrochemical gradient across the inner mitochondrial membrane.
Oxidative Phosphorylation: Oxidative phosphorylation is the process that utilizes the proton gradient generated by the electron transport chain to produce ATP. The protons in the intermembrane space seek to flow back into the mitochondrial matrix through ATP synthase, an enzyme complex embedded in the inner membrane. As the protons move through ATP synthase, their energy is harnessed to convert ADP (adenosine diphosphate) into ATP (adenosine triphosphate).
This coupling of proton flow and ATP synthesis is known as chemiosmosis. The movement of protons through ATP synthase drives the rotation of a molecular rotor within the enzyme, leading to the synthesis of ATP from ADP and inorganic phosphate (Pi). This process is referred to as oxidative phosphorylation because it couples ATP synthesis to the oxidation of electron carriers in the electron transport chain.
Overall, electron transport and oxidative phosphorylation work together to generate ATP, the primary energy currency of cells. The flow of electrons through the electron transport chain drives the establishment of a proton gradient, and the subsequent movement of protons through ATP synthase powers the synthesis of ATP. This final stage of cellular respiration completes the efficient conversion of energy stored in glucose to ATP, providing the necessary fuel for various cellular processes.
Electron transport sets up a proton gradient for oxidative phosphorylation
The ETC is composed of several electron-carrying proteins that assemble into four complexes in the inner mitochondrial membrane:
Complex I: NADH-CoQ Oxidoreductase
Complex I, also referred to as NADH-CoQ oxidoreductase, is a critical component of the electron transport chain (ETC) involved in oxidative phosphorylation. Let’s delve deeper into the unique characteristics and functions of Complex I:
- Composition and Electron Carriers: Complex I is composed of multiple protein subunits, including flavin mononucleotide (FMN), iron-sulfur clusters, and ubiquinone (UQ). FMN serves as the initial electron carrier in Complex I, accepting two electrons from NADH, which is generated during the previous stages of cellular respiration in the mitochondrial matrix. The electrons are then transferred to the iron-sulfur clusters within the complex.
- Function and Electron Transfer: The primary function of Complex I is to transfer electrons from NADH to ubiquinone (UQ), also known as coenzyme Q. NADH donates its electrons to FMN in Complex I, resulting in the reduction of FMN to FMNH2. From FMNH2, the electrons are sequentially passed along the iron-sulfur clusters within the complex until they reach ubiquinone.
- Ubiquinone plays a vital role as an electron carrier in the mitochondrial inner membrane. During electron transfer, ubiquinone undergoes a series of redox reactions, transitioning from its oxidized form (Ubiquinone, UQ) to a semiquinone intermediate (Ubisemiquinone, UQH) and eventually to its fully reduced form (Ubiquinol, UQH2). The reduced ubiquinol can then proceed to transfer the electrons to the next component of the electron transport chain.
- Proton Pumping and Proton Gradient Formation: As electrons flow through Complex I, energy is released, which is utilized to pump protons (H+) across the inner mitochondrial membrane, from the mitochondrial matrix to the intermembrane space. This proton pumping establishes an electrochemical gradient, with an excess of protons in the intermembrane space relative to the matrix. The proton gradient created by Complex I is a critical component of the overall proton motive force that drives ATP synthesis in the final stages of oxidative phosphorylation.
In summary, Complex I, or NADH-CoQ oxidoreductase, plays a vital role in the electron transport chain by transferring electrons from NADH to ubiquinone (UQ) while simultaneously pumping protons across the inner mitochondrial membrane. This complex acts as an essential link in the overall process of cellular respiration, contributing to the generation of ATP, the energy currency of the cell.
Complex II: Succinate-CoQ Oxidoreductase
Complex II, also known as Succinate-CoQ oxidoreductase, is the second complex in the electron transport chain (ETC). Let’s explore the unique characteristics and functions of Complex II:
- Enzyme and Electron Transfer: Complex II contains the enzyme succinate dehydrogenase, which plays a crucial role in the oxidation of succinate to fumarate. During this process, succinate is oxidized while the electron acceptor FAD (Flavin adenine dinucleotide) is reduced to FADH2. The electrons derived from succinate are then transferred to ubiquinone (UQ), also known as coenzyme Q, reducing it to ubiquinol (UQH2).
- Link to Metabolic Pathways: Complex II can also be associated with acyl-CoA dehydrogenase, which is involved in the catabolism of fatty acids through a process called β-oxidation. This alternative composition of the complex reflects its role in metabolizing fatty acids as an energy source.
- Proton Pumping and ATP Synthesis: Unlike other complexes in the electron transport chain, Complex II does not actively pump protons across the inner mitochondrial membrane. Therefore, it does not directly contribute to the generation of the proton gradient that drives ATP synthesis through oxidative phosphorylation.
Nevertheless, Complex II plays an important role in the overall electron flow and energy generation in cellular respiration. It functions as a bridge between the citric acid cycle (Krebs cycle) and the electron transport chain. By transferring electrons from succinate to ubiquinone, Complex II replenishes the pool of electron carriers and facilitates the flow of electrons through the subsequent complexes in the ETC.
In summary, Complex II, or Succinate-CoQ oxidoreductase, is an integral component of the electron transport chain. It oxidizes succinate to fumarate while reducing FAD to FADH2. This complex serves as a connecting point between the citric acid cycle and the electron transport chain and contributes to the overall flow of electrons and the regeneration of electron carriers. Although it does not actively pump protons or contribute to proton gradient formation, Complex II plays a crucial role in cellular respiration and energy production.
Complex III: Cytochrome bc1 Oxidoreductase
Complex III, also known as Cytochrome bc1 oxidoreductase, is a crucial component of the electron transport chain (ETC). Let’s explore the unique characteristics and functions of Complex III:
- Electron Transfer: Complex III receives electrons from ubiquinol (UQH2), which is generated by Complex I or Complex II in the previous steps of the electron transport chain. UQH2 carries these electrons to Complex III, where they undergo a series of redox reactions.
- Composition and Electron Flow: Complex III consists of cytochrome b and cytochrome c1 complexes, which contain important protein components such as the Rieske center and heme prosthetic groups. The Rieske center binds to UQH2, accepting one electron and transferring it to cytochrome c1. The electron is then passed to cytochrome c, which carries it to Complex IV.
- The Q Cycle: The transfer of electrons in Complex III occurs through a series of redox reactions known as the Q cycle. UQH2, carrying two electrons, undergoes oxidation and donates one electron to cytochrome c1. This electron is transferred to cytochrome c, while the remaining electron reduces cytochrome b and regenerates the oxidized form of ubiquinone (UQ).
- Proton Translocation: During the Q cycle, the transfer of electrons is coupled with the movement of protons across the inner mitochondrial membrane. Two protons are transferred to the intermembrane space when two electrons from UQH2 reduce cytochrome c1. Additionally, two protons from the matrix are transferred when each reduced cytochrome b donates its electron to regenerate UQ.
- Overall Function: Complex III serves as a critical site for electron transfer, generating a proton gradient across the inner mitochondrial membrane. This proton gradient is essential for ATP synthesis during oxidative phosphorylation. Complex III plays a crucial role in maintaining the flow of electrons and the generation of energy through the transfer of electrons to Complex IV via cytochrome c.
In summary, Complex III, or Cytochrome bc1 oxidoreductase, is a vital component of the electron transport chain. It receives electrons from UQH2 and transfers them through a series of redox reactions, known as the Q cycle, involving cytochrome b and cytochrome c1 complexes. This process leads to the transfer of electrons to Complex IV via cytochrome c and contributes to the maintenance of the proton gradient across the inner mitochondrial membrane. Complex III plays a key role in electron transfer and energy production in cellular respiration.
Complex IV: Cytochrome c Oxidase
Complex IV, also known as Cytochrome c oxidase, is the final complex of the electron transport chain (ETC) and plays a crucial role in the transfer of electrons and the generation of ATP. Let’s explore the unique features and functions of Complex IV:
- Composition and Redox Center: Complex IV consists of several key components, including cytochrome a, cytochrome a3, a copper atom (CuB), and a copper atom pair (CuA) center. These components form the redox center of the complex, which has the capacity to accommodate and transfer four electrons.
- Electron Donation and Oxygen Reduction: At Complex IV, the four electrons carried by cytochrome c from Complex III are donated to the redox center. The electrons flow through the redox center until they reach oxygen, which acts as the final electron acceptor in the electron transport chain. Oxygen can accept all four electrons, resulting in the formation of two water molecules.
- Proton Pumping and Proton Gradient: As the electrons are transferred through Complex IV, protons are simultaneously pumped from the mitochondrial matrix across the inner mitochondrial membrane to the intermembrane space. This proton pumping process contributes to the establishment of a proton gradient across the inner mitochondrial membrane.
- Generation of ATP: The accumulation of protons in the intermembrane space creates a proton gradient, which provides the driving force for ATP synthesis through oxidative phosphorylation. Protons flow back into the mitochondrial matrix through ATP synthase, leading to the phosphorylation of ADP to ATP.
- Overall Function: Complex IV serves as the final electron transfer point in the electron transport chain, accepting electrons from cytochrome c and transferring them to oxygen. This process not only generates water but also contributes to the generation of a proton gradient, which is essential for ATP production.
In summary, Complex IV, or Cytochrome c oxidase, is a critical component of the electron transport chain. It facilitates the transfer of electrons from cytochrome c to oxygen, leading to the formation of water and the establishment of a proton gradient. The proton gradient is utilized by ATP synthase to drive ATP synthesis. Complex IV plays a vital role in the final stage of cellular respiration, ensuring the efficient production of ATP for cellular energy needs.
ATP is produced by chemiosmosis in oxidative phosphorylation
ATP is produced through chemiosmosis in oxidative phosphorylation, a process that utilizes the proton motive force generated by the accumulation of protons in the intermembrane space of the mitochondria. Let’s explore the unique features and mechanism of ATP production through chemiosmosis:
- Proton Motive Force: The accumulation of protons in the intermembrane space creates a proton motive force, also known as an electrochemical gradient. This force is composed of both an electrical potential and a concentration gradient of protons across the inner mitochondrial membrane.
- ATP Synthase: ATP synthase, also referred to as Complex V, is an enzyme embedded in the inner mitochondrial membrane. It functions as a molecular motor and acts as a unidirectional proton pump. ATP synthase consists of two major components: the F0 component, embedded in the membrane and responsible for proton transport, and the F1 component, located in the mitochondrial matrix and responsible for ATP synthesis.
- Proton Flow and ATP Synthesis: As protons flow back into the mitochondrial matrix through ATP synthase, the electrochemical gradient collapses. This proton flow provides the potential energy required to drive the phosphorylation of ADP, resulting in the synthesis of ATP in the mitochondrial matrix. The ATP synthase uses this energy to couple the flow of protons with the production of ATP.
- Contributions of NADH and FADH2: During electron transport in the electron transport chain, NADH and FADH2 donate their electrons, which pass through complexes I, III, and IV. This electron flow leads to the pumping of protons and the establishment of the proton motive force. For every NADH molecule, it is estimated that the electron transfer results in the generation of three ATP molecules, while one FADH2 molecule contributes to the production of two ATP molecules.
- Overall ATP Production: When considering the contributions of NADH and FADH2 throughout cellular respiration, the ATP yield can be summarized. For each glucose molecule, the ATP production during oxidative phosphorylation can be expressed as 10 NADH + 2 FADH2 generating a total of 34 ATP molecules.
In combination with the ATP generated during glycolysis and the Krebs cycle, the overall ATP yield from cellular respiration is 38 ATP (or 36 ATP and 2 GTP) per glucose molecule.
the oxidative phosphorylation equation is:
2NADH + 2H+ + 2FADH2 + 2O2 + 10ADP + 10Pi –> 2NAD+ + 2FAD + 4H2O + 10ATP
Based on the cellular respiration equation summarized up to the Krebs cycle, one molecule of glucose produces ten NADH and two FADH2. Consequently, the equation for cellular respiration at the oxidative phosphorylation stage is:
10NADH + 10H+ + 2FADH2 + 6O2 + 34ADP + 34Pi –> 10NAD+ + 2FAD + 14H2O + 34ATP
From glycolysis to the Krebs cycle, cellular respiration has produced four ATP (or two ATP and two GTP). The cellular respiration equation with the contribution of oxidative phosphorylation is as follows:
C6H12O6 + 6O2 + 38ADP (or 36ADP + 2GDP) + 38Pi –> 6CO2 + 6H2O + 38ATP (or 36ATP and 2GTP)
In conclusion, ATP is produced through chemiosmosis in oxidative phosphorylation by utilizing the proton motive force generated by the accumulation of protons in the intermembrane space. ATP synthase harnesses the flow of protons to synthesize ATP from ADP and inorganic phosphate. The contributions of NADH and FADH2 play a vital role in the generation of ATP, resulting in an efficient production of cellular energy during cellular respiration.
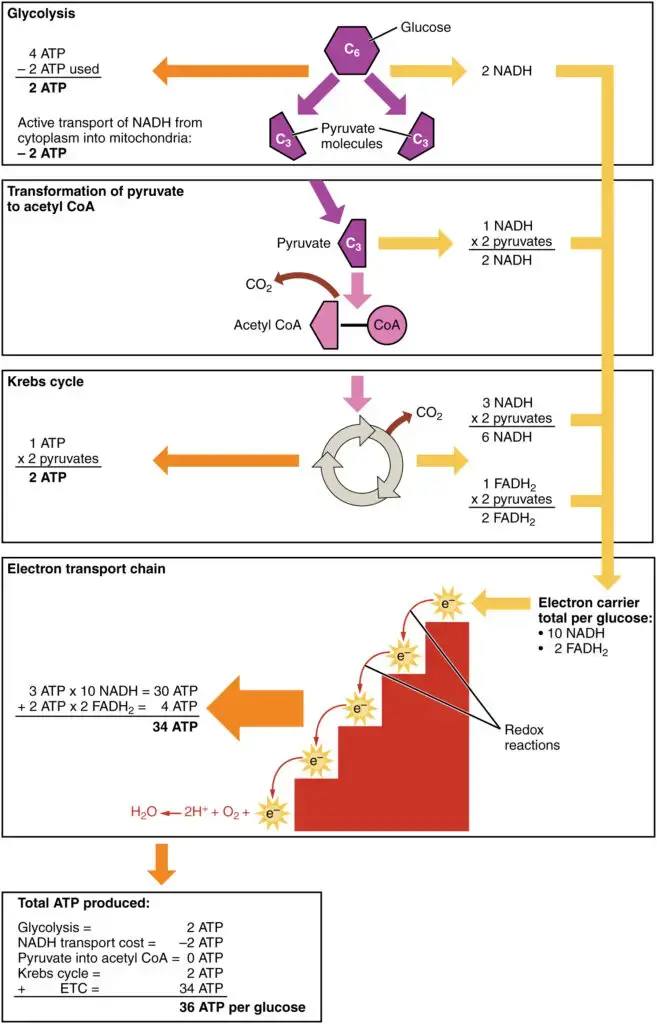
How Much ATP Produced in Cellular Respiration?
| Step | coenzyme yield | ATP yield | Source of ATP |
|---|---|---|---|
| Glycolysis preparatory phase | −2 | Phosphorylation of glucose and fructose 6-phosphate uses two ATP from the cytoplasm. | |
| Glycolysis pay-off phase | 4 | Substrate-level phosphorylation | |
| 2 NADH | 3 or 5 | Oxidative phosphorylation: Each NADH produces net 1.5 ATP (instead of usual 2.5) due to NADH transport over the mitochondrial membrane | |
| Oxidative decarboxylation of pyruvate | 2 NADH | 5 | Oxidative phosphorylation |
| Krebs cycle | 2 | Substrate-level phosphorylation | |
| 6 NADH | 15 | Oxidative phosphorylation | |
| 2 FADH2 | 3 | Oxidative phosphorylation | |
| Total yield | 30 or 32 ATP | From the complete oxidation of one glucose molecule to carbon dioxide and oxidation of all the reduced coenzymes. | |
During cellular respiration, the total ATP yield can vary depending on the specific conditions and the type of substrate being metabolized. The theoretical maximum yield of ATP is typically stated to be around 36-38 ATP molecules per molecule of glucose, but in practice, the actual yield is often lower due to various factors such as energy losses during transport processes.
Recent research suggests that the ATP yield during aerobic respiration is lower than previously believed, with estimates ranging from 30 to 32 ATP molecules per molecule of glucose. This revised estimation is based on several factors:
- ATP:NADH+H+ and ATP:FADH2 ratios: The stoichiometry of ATP production during oxidative phosphorylation appears to be 2.5 ATP per NADH+H+ and 1.5 ATP per FADH2, rather than the previously assumed 3 ATP and 2 ATP, respectively. Determining the exact ratios in this process is challenging due to various factors involved.
- ATP synthase and proton exchange: ATP synthase, the enzyme responsible for ATP synthesis, produces 1 ATP molecule per 3 protons (H+). However, the exchange of ATP from the mitochondrial matrix for ADP and Pi in the cytosol consumes 1 H+ per ATP due to the regeneration of the transmembrane potential. As a result, the net ratio is 1 ATP to 4 H+.
- Proton transfer by the electron transport chain: The proton pumps of the mitochondrial electron transport chain transfer protons across the inner membrane. It is estimated that 10 H+ are transferred per NADH+H+ or 6 H+ per FADH2.
Based on these considerations, the final stoichiometry can be summarized as follows:
- 1 NADH+H+ is associated with 10 H+ and yields approximately 2.5 ATP.
- 1 FADH2 is associated with 6 H+ and yields approximately 1.5 ATP.
The ratio of ATP to NADH+H+ from glycolysis during oxidative phosphorylation is approximately 1.5 ATP, assuming that hydrogen atoms (2H++2e-) are transferred from cytosolic NADH+H+ to mitochondrial FAD through the glycerol phosphate shuttle. In the case of the malate-aspartate shuttle, which transfers hydrogen atoms from cytosolic NADH+H+ to mitochondrial NAD+, the ratio is approximately 2.5 ATP.
It is important to note that these values are subject to ongoing research and may be refined further as new information becomes available. The complexities of ATP production and the involvement of various shuttle mechanisms contribute to the variability in ATP yields. Nevertheless, the revised estimate of 30 to 32 ATP molecules per molecule of glucose provides a more accurate representation of ATP production during aerobic respiration.
This understanding of ATP yield is crucial for comprehending the energy generation and utilization processes within cells and organisms, shedding light on the efficiency and overall functioning of cellular respiration.
Here is a breakdown of the ATP yield during cellular respiration:
- Glycolysis: During the preparatory phase of glycolysis, two ATP molecules are consumed. However, during the pay-off phase, four ATP molecules are produced through substrate-level phosphorylation. This results in a net gain of two ATP molecules.
- Pyruvate oxidation: When pyruvate is oxidized in the mitochondria, two NADH molecules are generated, which can yield a total of 5 ATP molecules during oxidative phosphorylation.
- Krebs cycle: During the Krebs cycle, for each glucose molecule, two ATP molecules are generated through substrate-level phosphorylation. Additionally, six NADH molecules and two FADH2 molecules are produced, which can yield a total of 15 ATP and 3 ATP, respectively, during oxidative phosphorylation.
Considering the above steps, the total ATP yield from one molecule of glucose in the presence of oxygen can be estimated to be around 30-32 ATP molecules. This estimate takes into account the losses and inefficiencies in the transport processes and the stoichiometry of ATP synthesis.
It’s important to note that the actual ATP yield may vary in different organisms and under different conditions. Factors such as the efficiency of the electron transport chain, the activity of transport proteins, and variations in shuttle mechanisms can influence the final ATP production.
In contrast, during fermentation processes such as lactic acid or ethanol fermentation, only two ATP molecules are generated directly from glycolysis. In these pathways, pyruvate is not further metabolized in the mitochondria, resulting in a lower ATP yield compared to aerobic respiration.
Overall, cellular respiration plays a vital role in producing ATP, the energy currency of the cell. The ATP generated through this process is essential for various cellular functions, including metabolism, growth, movement, and signaling.
ATP per molecule of glucose
When one molecule of glucose is fully oxidized through cellular respiration, the total ATP yield can be estimated based on the different stages of ATP production. Here is a breakdown of the ATP yield per molecule of glucose:
- Substrate-level phosphorylation:
- Glycolysis: 2 ATP molecules are directly produced through substrate-level phosphorylation.
- Krebs cycle: 2 ATP molecules (or GTP molecules, which can be converted to ATP) are generated through substrate-level phosphorylation.
- Oxidative phosphorylation:
- Glycolysis: 2 NADH+H+ molecules are produced, which can yield either 1.5 ATP or 2.5 ATP, depending on the shuttle mechanism involved (glycerol phosphate shuttle or malate-aspartate shuttle).
- Oxidative decarboxylation of pyruvate: 2 NADH+H+ molecules are generated, contributing to 5 ATP.
- Krebs cycle: 6 NADH+H+ molecules produce 15 ATP, and 2 FADH2 molecules produce 3 ATP during oxidative phosphorylation.
Taking all of these factors into account, the total ATP yield can be calculated as follows:
ATP from substrate-level phosphorylation: 4 ATP ATP from oxidative phosphorylation:
- NADH+H+ from glycolysis: 2 × 1.5 ATP (or 2 × 2.5 ATP)
- NADH+H+ from oxidative decarboxylation of pyruvate: 2 × 2.5 ATP
- NADH+H+ from Krebs cycle: 6 × 2.5 ATP
- FADH2 from Krebs cycle: 2 × 1.5 ATP
Therefore, the total ATP yield per molecule of glucose can be estimated as 30 ATP (or 32 ATP if the malate-aspartate shuttle is utilized).
It is important to note that these figures are subject to further refinement as new structural details become available. The specific ATP yield can vary depending on the shuttle mechanisms involved and the characteristics of the ATP synthase complex. The efficiency of ATP synthesis also depends on the proton translocation and the number of c subunits in the Fo c-ring. Experimental results indicate slight variations from the theoretical values.
In contrast, in fermentation processes such as ethanol or lactic acid fermentation, only 2 ATP molecules are produced directly from glycolysis, as pyruvate is not further metabolized in the mitochondria. Instead, it is reduced to ethanol or lactic acid in the cytoplasm.
Overall, the ATP yield in cellular respiration is essential for providing the energy needed for various cellular functions and processes.
Importance of Cellular Respiration
Cellular respiration plays a crucial role in the growth, well-being, and survival of organisms. It is essential for various physiological processes and has both medical and industrial significance. Let’s explore the importance of cellular respiration in more detail:
Medical Significance:
- Glucose Metabolism: Deficiencies in key enzymes of cellular respiration, such as pyruvate carboxylase and succinate dehydrogenase, can disrupt glucose metabolism. These deficiencies can lead to a wide range of clinical symptoms, including abnormal blood glucose levels, metabolic acidosis, developmental delays, and other metabolic disorders.
- Oxidative Phosphorylation Diseases: Mutations in genes involved in oxidative phosphorylation, the final stage of cellular respiration, can result in neurodegenerative diseases such as Leigh syndrome, NARP syndrome, and DDON syndrome. These conditions highlight the critical role of cellular respiration in maintaining proper energy production in neuronal tissues.
Industrial Applications:
- Lactate Fermentation: Anaerobic respiration in lactic bacteria, such as Lactobacillus and Streptococcus, is the basis for food and beverage preservation and processing. Fermentation by these bacteria is responsible for the production of yogurt, cheese, and other fermented dairy products. Additionally, sourdough starters, which contain lactic bacteria and yeasts, undergo anaerobic respiration during the fermentation process, resulting in the production of carbon dioxide and lactic acid, crucial for the texture and flavor of sourdough bread.
- Ethanol Fermentation: Yeasts and other ethanol-producing microorganisms utilize anaerobic respiration, specifically ethanol fermentation, to convert carbohydrate-rich materials like honey, fruits, grains, and tubers into ethanol. This process is not only utilized in the production of biofuels but also contributes to the manufacturing of alcoholic beverages and byproducts such as spent yeast, protein concentrates, and industrial enzymes.
In summary, cellular respiration is of paramount importance for the normal functioning and survival of organisms. Deficiencies in cellular respiration can lead to various medical conditions, while an understanding of its processes has practical applications in food processing, fermentation, biofuel production, and other industrial sectors. By studying and comprehending cellular respiration, we can gain insights into the complexities of life and develop strategies to address medical challenges and meet industrial needs.
FAQ
What is cellular respiration?
Cellular respiration is the metabolic process by which cells convert nutrients, such as glucose, into usable energy in the form of adenosine triphosphate (ATP). It occurs in the mitochondria of eukaryotic cells and involves a series of biochemical reactions.
What are the main stages of cellular respiration?
Cellular respiration consists of three main stages: glycolysis, the Krebs cycle (or citric acid cycle), and oxidative phosphorylation (or electron transport chain). These stages involve a series of biochemical reactions that gradually break down glucose and produce ATP.
Where does cellular respiration occur?
Cellular respiration occurs in the mitochondria of eukaryotic cells. Glycolysis takes place in the cytoplasm, while the Krebs cycle and oxidative phosphorylation occur in the mitochondrial matrix and inner membrane, respectively.
What is the net ATP yield of cellular respiration?
The net ATP yield of cellular respiration varies depending on the specific organism and conditions. In aerobic respiration, the theoretical maximum ATP yield is around 30-32 ATP molecules per glucose molecule. However, the actual yield may be lower due to various factors, such as transport processes and inefficiencies in ATP production.
What is the role of oxygen in cellular respiration?
Oxygen plays a critical role in cellular respiration as the final electron acceptor in the electron transport chain during oxidative phosphorylation. It enables the efficient production of ATP by facilitating the transfer of electrons and the generation of a proton gradient.
Can cells perform cellular respiration without oxygen?
Yes, cells can carry out cellular respiration in the absence of oxygen through a process called anaerobic respiration or fermentation. This occurs in the cytoplasm and involves the partial breakdown of glucose, leading to the production of ATP and either lactic acid or ethanol, depending on the organism.
What is the relationship between photosynthesis and cellular respiration?
Photosynthesis and cellular respiration are interconnected processes in living organisms. Photosynthesis occurs in plants, algae, and some bacteria, where it converts sunlight, carbon dioxide, and water into glucose and oxygen. The glucose produced during photosynthesis is then used as a fuel source for cellular respiration, where it is broken down to release energy.
How does exercise affect cellular respiration?
During exercise, cells require increased energy to support muscle contractions and other physiological processes. As a result, cellular respiration ramps up to meet this demand, leading to an increased consumption of oxygen and glucose. The increased oxygen uptake helps supply the necessary energy through aerobic respiration.
Are there any disorders or diseases related to cellular respiration?
Various disorders and diseases can affect cellular respiration. Examples include mitochondrial diseases, where defects in the mitochondria impair the production of ATP, and respiratory diseases like chronic obstructive pulmonary disease (COPD), which can limit oxygen uptake and disrupt cellular respiration.
Why is cellular respiration important?
Cellular respiration is vital for organisms as it provides the energy needed to perform various cellular functions and sustain life. It allows cells to generate ATP, which serves as the primary energy currency for biochemical reactions.
References
- https://conductscience.com/cellular-respiration-equation-steps-types-and-importance/
- https://www.biologyonline.com/dictionary/cellular-respiration
- https://biologydictionary.net/cellular-respiration/#cellular-respiration-equation
- https://humanbiology.pressbooks.tru.ca/chapter/4-10-cellular-respiration/
- https://www.khanacademy.org/science/ap-biology/cellular-energetics/cellular-respiration-ap/a/steps-of-cellular-respiration
- https://www.osmosis.org/answers/cellular-respiration
- https://courses.lumenlearning.com/suny-wmopen-biology1/chapter/cellular-respiration/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.